Introduction
Arsenic water pollution is an environmental health problem in Bangladesh, India, Mongolia, China, Croatia, Austria, New Zealand, Afghanistan, Mali, Zambia, Thailand, Taiwan, Argentina, Chile, El Salvador, Peru, Nicaragua and Mexico, among other countries.1,2 The available information on the number of the exposed population and the burden of arsenic related diseases in Mexico is limited. However, according to the World Health Organization (WHO), an indicator of the magnitude of high-risk exposure is the percentage of individuals exposed to concentrations above 10 μg/L in water, and having more than 6.4 μg/L in urine.3,4 In Mexico, it has been estimated that between 400 000 and 2 million people are exposed to iAs concentrations in drinking water above the maximum limit (25 μg/L) established by the current Mexican regulation (NOM 127-SSA1-1994-2000), which is 2.5 times higher than the maximum WHO limit.5,6 Therefore, the magnitude of this problem is serious, since the number of people exposed to concentrations >10 μg/L is even larger.
Inorganic arsenic (iAs) is widely distributed in the Earth’s crust, usually combined with oxygen, chlorine and sulfur. Inorganic arsenic contaminates the soil, air and water through wind-borne dust and also enters the water through runoff and leaching, join particles and/or sediment carried by watercourses. Some fish and crustaceans absorb iAs and transform it into organic forms, such as arsenobetaine.7
Ingested iAs is metabolized through reduction-oxidation and methylation reactions. The most soluble metabolites that are eliminated in the urine are the dimethylated forms -dimethylarsinous acid [DMA+3] and dimethylarsinic acid [DMA+5], in proportions of 60 to 70%-, followed by the monomethylated forms -monomethylarsonous acid [MMA+3] and monomethylarsonic acid [MMA+5], in proportions of 10 to 20%-, and a fraction that remains as iAs (10-30%).8
Chronic exposure to iAs through drinking water has been associated with adverse health effects, such as cancer in various organs, diabetes, and cardiovascular diseases.9,10 The development of these diseases depends not only on the dose of iAs exposure, but also on the individual capacity to metabolize it, which in turn is related to other factors such as age, sex, body mass index, smoking habit, the consumption of certain nutrients, and genetic susceptibility.11,12
The studies of our research group suggest that iAs methylation capacity is better in women who had greater consumption of methionine, choline, folate, vitamin B12, zinc, selenium, vitamin C13and some flavonoids,14 as well as in the carriers of certain polymorphisms in genes involved in one-carbon metabolism (FOLH1 c.223T> C, MTR c.2756A> G, MTHFD1 c.1958G> A).15 Additionally, we have identified that women with less iAs methylation capacity are at risk of developing breast cancer.16
The objectives of this study in women from northern Mexico are: 1) to describe arsenic contamination through its concentrations in urine and water; 2) to describe interindividual metabolism variations; 3) to identify sociodemographic characteristics associated with urinary total arsenic (TAs).
Materials and methods
For this report, which is part of a case-control study of the exposure to environmental factors and breast cancer, there was available information on the urinary concentrations of TAs and iAs metabolites of 1 028 healthy women (control group). The women were 20 years or older, living in one of the following Mexican states: Chihuahua, Coahuila, Durango, Nuevo Leon and Sonora, during the 2007-2009 period. Recruitment procedures were described in detail elsewhere.16 Briefly, women were identified through the Master Sample Framework used for the National Health Surveys, which contains basic geostatistical units comprising groups of households. Randomly eligible women were identified at their homes. The response rate was 99.7% (1028/1031). The project was approved by the Ethics Committee of the National Institute of Public Health (Instituto Nacional de Salud Pública, INSP).
After signing the informed consent form, the participants provided sociodemographic and lifestyle information during a direct interview in their homes. Likewise, they donated a sample of the first morning urine in sterile polypropylene containers. The samples were kept at -70 °C until analysis.
The concentrations (μg/L) of the species arsenite (As+3), arsenate (As+5), monomethylarsonic acid (MMA+5), dimethylarsinic acid (DMA+5), and arsenobetaine (AsB) were determined by high performance liquid chromatography inductively coupled to mass spectrometry (HPLC-ICP-MS), according to the methodology described by Gilbert-Diamond and colleagues (2011).17 Samples that were below the limit of detection (LOD), were imputed with the LOD value (AsB: 0.08, As+3: 0.15, As+5: 0.41, MMA+5: 0.12, DMA+5: 0.12 μg/L) divided by the square root of two (LOD/√2).18 The percentage of samples that had values below the LOD was 24.3% for AsB,19.3% for As+3, 56.3% for As+5, 1.9% for MMA+5, and 0.5% for DMA+5. The urinary creatinine concentration (mg/dL) was determined with a commercial kit based on an enzymatic method (Randox, Antrim County, UK). The following coefficients of variation were obtained: MMA+5= 8%, DMA+5= 9%, As+3= 8%, and creatinine= 2.8%. The concentration of iAs was obtained from the sum of As+3+ As+5. Total arsenic in urine was obtained from the sum of iAs + MMA+5+ DMA+5. The percentages of iAs, MMA+5and DMA+5were calculated with respect to TAs.
The regression model proposed by Calderón and colleagues (1999)19 was used to estimate the concentrations of iAs in water from the concentrations of TAs determined in urine. This model was obtained from the determination of iAs in tap water and TAs in urine in five consecutive days among 100 residents in the USA: TAs in urine [µg/mg creatinine]= 10-2.57x (iAs in drinking water [µg/L])0.63.
Urinary TAs concentration (in µg/L and µg/g creatinine) and estimated iAs in water had no normal distribution; therefore, they were characterized with the median and the 10th and 90th percentiles, and were compared between categories of the sociodemographic variables with the Kruskal-Wallis test.
To evaluate individual differences in iAs metabolism, the percentages of iAs metabolites were plotted according to the urinary concentration of TAs (in µg/g creatinine). The percentages of iAs metabolites and TAs concentration were transformed to their natural logarithm and their relationship was assessed by simple linear regression. Likewise, TAs concentration (in µg/L) was normalized and its association with the variables of interest (age, schooling, state of residency) was evaluated including in the model creatinine as an independent variable. The validation of the models was carried out through the evaluation of the residuals’ normality with the Shapiro-Wilk test, and the visual evaluation of patterns, by plotting the residuals dispersion vs. the predicted values dispersion. All analyses were performed with the statistical package Stata 13 (Stata Corp, College Station, TX, USA).
Results
Most women in the study were between 40-69 years old, and had 1 to 9 years of schooling, and a residence time in the study area of 40 to 69 years. In the total population, the concentration of TAs in urine varied from p10=3.41 to p90=56.93 μg/L, and creatinine ranged between p10=6.48 and p90=100.04 μg/g. Women in the state of Chihuahua had significantly higher concentrations of TAs in urine and iAs in water, compared to those in Nuevo Leon. Around 74% of the women had a concentration of TAs >6.4 μg/L (biomonitoring equivalent, BE), among them, women living in the state of Nuevo Leon had the lowest percentage (53%) that exceeded the BE, in contrast to 96% of those living in the state of Chihuahua. Values of iAs in drinking water >10 μg/L were estimated in 64.11% of women, while 40.86% had >25 μg/L (table I).
Table I: Observed total arsenic and estimated inorganic arsenic according to selected sociodemographic characteristics in women from northern Mexico, 2007-2009
|
Variables |
n |
TAs observed in urine (µg/L) |
TAs observed in urine (µg/g creatinine) |
Percentage >6.4 µg/L* |
iAs estimated in water (µg/L) |
Percentage >10 µg/L‡ |
Percentage >25 µg/L§ |
||||||
|
Median |
p10 |
p90 |
Median |
p10 |
p90 |
Median |
p10 |
p90 |
|||||
|
Age (years) | |||||||||||||
|
20-29 |
17 |
21.68# |
10.07 |
63.39 |
19.98 |
5.38 |
66.61 |
100 |
17.18 |
2.31 |
108.62 |
58.82 |
41.18 |
|
30-39 |
120 |
14.56 |
3.54 |
96.22 |
16.9 |
5.85 |
77 |
80.83 |
12.24 |
2.62 |
135.55 |
55.83 |
41.67 |
|
40-49 |
241 |
13.34 |
4.95 |
46.79 |
21.08 |
6.97 |
111.45 |
82.57 |
18.09 |
3.43 |
238.37 |
66.8 |
39.42 |
|
50-59 |
318 |
11.55 |
3.19 |
62.67 |
21.64 |
6.77 |
104.6 |
70.12 |
19.28 |
3.09 |
213.77 |
65.72 |
42.77 |
|
60-69 |
196 |
10.22 |
3.6 |
54.83 |
17.9 |
6.06 |
105.74 |
68.88 |
14.53 |
2.74 |
219.96 |
62.76 |
39.8 |
|
70-79 |
113 |
9.66 |
2.59 |
34.07 |
20 |
7.04 |
83.11 |
63.72 |
17 |
3.24 |
152.3 |
64.6 |
38.94 |
|
80-89 |
23 |
10.83 |
2.23 |
68.72 |
24.74 |
5.53 |
104.26 |
60.87 |
23.38 |
2.43 |
215.29 |
69.57 |
43.48 |
|
Schooling (years) | |||||||||||||
|
0 |
87 |
15.97 |
3.84 |
73.35 |
25.12& |
9.47 |
163.36 |
78.16 |
24.50& |
5.47 |
427.34 |
78.16 |
48.28 |
|
1-9 |
827 |
11.87 |
3.31 |
56.37 |
20.46 |
6.46 |
100.86 |
73.15 |
17.75 |
2.95 |
204.67 |
64.09 |
41.6 |
|
19-12 |
52 |
12.68 |
4.03 |
34.02 |
13.32 |
5.38 |
44.59 |
78.85 |
9.22 |
2.31 |
58.37 |
48.08 |
28.85 |
|
>12 |
62 |
10.48 |
3.93 |
38.48 |
16.32 |
5.94 |
41.98 |
69.35 |
12.36 |
2.64 |
53.68 |
58.06 |
30.65 |
|
Residence time (years) | |||||||||||||
|
20-29 |
17 |
21.68# |
10.07 |
63.39 |
19.98 |
5.38 |
66.61 |
100 |
17.18 |
2.31 |
108.62 |
58.82 |
41.18 |
|
30-39 |
116 |
14.1 |
3.35 |
94.3 |
15.89 |
5.79 |
75.91 |
80.17 |
11.49 |
2.55 |
132.61 |
54.31 |
40.52 |
|
40-49 |
247 |
14.13 |
5.07 |
53.78 |
22.16 |
6.97 |
100.3 |
83.8 |
19.96 |
3.43 |
202.94 |
68.42 |
40.89 |
|
50-59 |
310 |
11.51 |
3.18 |
61.9 |
21.14 |
6.74 |
105.57 |
70 |
18.43 |
3.07 |
219.43 |
65.16 |
41.94 |
|
60-69 |
200 |
9.95 |
3.6 |
48.74 |
17.9 |
6.41 |
101.15 |
67 |
14.53 |
2.95 |
205.47 |
63 |
39.5 |
|
70-79 |
114 |
9.81 |
2.56 |
37.38 |
19.72 |
6.9 |
94.22 |
64.91 |
16.72 |
3.18 |
183.57 |
63.16 |
39.47 |
|
80-89 |
24 |
11.97 |
2.23 |
68.72 |
24.75 |
5.53 |
104.26 |
62.5 |
23.63 |
2.43 |
215.29 |
70.83 |
45.83 |
|
Residence state | |||||||||||||
|
Nuevo Leon |
296 |
6.84& |
2.44 |
16.74 |
10.83& |
5.03 |
34.75 |
53.38 |
6.50& |
1.99 |
39.54 |
37.5 |
14.19 |
|
Sonora |
251 |
13.68 |
4.44 |
38.48 |
22.63 |
8.7 |
73.8 |
78.49 |
20.6 |
4.79 |
125.95 |
75.3 |
43.03 |
|
Coahuila≠ |
307 |
15 |
3.58 |
98.45 |
25.03 |
6.33 |
169.1 |
76.55 |
23.95 |
2.89 |
450.47 |
64.17 |
49.51 |
|
Chihuahua |
174 |
24.05 |
8.84 |
69.29 |
35.06 |
15.39 |
111.7 |
95.98 |
40.77 |
11.6 |
239.17 |
93.1 |
67.82 |
|
All |
1 028 |
12.1 |
3.41 |
56.93 |
20.2 |
6.48 |
100.04 |
73.64 |
17.25 |
3.04 |
202.12 |
64.11 |
40.86 |
* Hays SM, Aylward LL, Gagné M, Nong A, Krishnan K. Biomonitoring Equivalents for inorganic arsenic.4
‡World Health Organization. Exposure to Arsenic: A Major Public Health Concern.3
§Norma Oficial Mexicana NOM-127-SSA1-1994-20006
#Kruskal-Wallis test p<0.001 among categories of the same variable
&Kruskal-Wallis test p<0.004 among categories of the same variable
≠Includes 69 women of northern Durango
Figure 1 illustrates the large variations in iAs methylation capacity observed in this population. For example, one of two women with relatively low urinary TAs concentrations (~ 14 μg/L) exhibited 69% iAs with 17.9% DMA (woman 1), while the other had 10% iAs and 75% DMA (woman 2); in both cases the percentages of MMA were similar. On the other hand, one of two women with higher concentrations of TAs (248 μg/L) had 50.6% iAs and 40% DMA (woman 3), while the other exhibited 8% iAs and 82% DMA (woman 4), with similar percentages of MMA. The percentage of iAs was negatively associated with the concentration (μg/L) of urinary TAs (β = -0.152, p<0.001), while the percentages of MMA and DMA were positively associated (β = 0.039, p= 0.001 and β = 0.038, p<0.001, respectively) (see the foot of figure 1).
The main determinant of TAs concentration was the state of residence. In addition, age and schooling were negative and significantly associated with the concentration of urinary TAs, while the concentration of creatinine in urine was positive and significantly associated, adjusting for the percentage of iAs metabolites (table II).
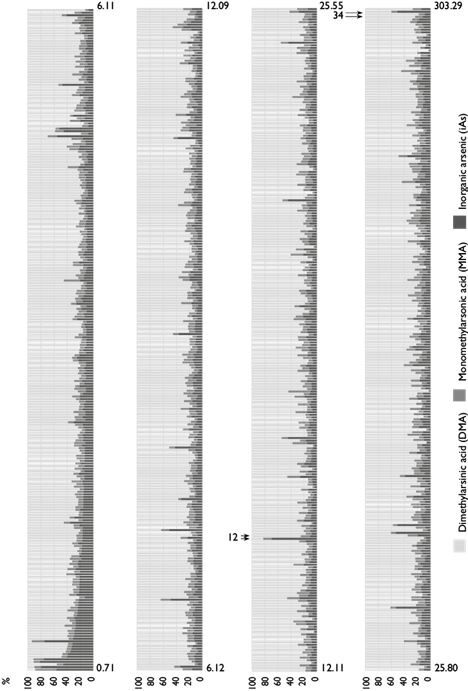
Figure 1: Inorganic arsenic (iAs) metabolites’ individual percentage distribution according to urinary total arsenic (TAs) concentration (each bar corresponds to one individual) in women from northern Mexico, 2007-2009. One of two women with relatively low urinary TAs concentrations (~ 14 μg/L) exhibited 69% iAs with 17.9% DMA (woman 1), while the other had 10% iAs and 75% DMA (woman 2); in both cases, the percentages of MMA were similar. One of two women with higher concentrations of TAs (248 μg/L) had 50.6% iAs and 40% DMA (woman 3), while the other exhibited 8% iAs and 82% DMA (woman 4), with similar percentages of MMA. Coefficients of regression between urinary TAs (µg/L) and %iAs: ß = -0.152, p<0.001; %MMA: ß = 0.039, p = 0.001, and %DMA: ß = 0.038, p<0.001
Table II: Association of urinary TAs concentration (µg/L) with selected characteristics in women from northern Mexico, 2007-2009
|
Variable |
β |
95%CI |
|
Age (years) |
-0.29* |
-0.54, -0.04 |
|
Schooling (total years) |
-0.02 |
-0.04, -0.00 |
|
Residence state | ||
|
Nuevo Leon |
Ref |
|
|
Sonora |
0.68 |
0.53, 0.82 |
|
Coahuila‡ |
0.90 |
0.76, 1.04 |
|
Chihuahua |
1.18 |
1.02, 1.34 |
|
Urinary creatinine (mg/dL) |
0.49* |
0.43, 0.55 |
* For log-transformed variable
‡Includes 69 women of northern Durango
Discussion
The results showed that 74% of women in this population had urinary concentrations of TAs (iAs + MMA + DMA) above the BE (6.4 μg/L). Biomonitoring Equivalents are defined as the concentration or range of concentrations of a chemical or its metabolites in a biological medium (blood, urine, or other) that is consistent with an existing health-based exposure guidance value such as a reference dose (RfD) or Tolerable Daily Intake (TDI).4
The concentration of TAs in urine was negative and significantly associated with age, which was consistent with the results of similar studies that reported negative but not significant trends.20,21 In the present study, the main source of iAs exposure for the population may be drinking water. Since water consumption decreases with age,22,23 it is reasonable to find a negative association between TAs and age.
TAs in urine was also negatively associated with schooling, which is considered a dimension of the socioeconomic level.24 Studies conducted in different parts of the world have obtained similar results, for example, in Bangladesh, it was observed that the socioeconomic level, measured through the possession of goods, was negatively associated with arsenic exposure.25 In Mexico, a study in which a socioeconomic index was estimated based on household conditions and family assets, found that children with lower socioeconomic status had higher urinary TAs concentrations.26 It is likely that people with more education have a better risk perception of iAs exposure through drinking water and may act to reduce it by consuming more bottled water, which implies a greater economic cost than consuming tap water. In our study population, 58.17% of participants reported that their main source of consumption was bottled water and 41.15% tap water (data not shown). However, in this regard, there is no information about the iAs exposure risk perception in the study area, which would be useful to eventually develop programs for exposure prevention and control.
It has been suggested that the concentrations of TAs should be expressed per g of creatinine, to adjust for variations in urinary dilution.27 However, the concentration of creatinine correlates with some arsenic related variables such as age and consumption of foods.28,29 In our study, for example, we found that creatinine correlated significantly with age and with riboflavin, niacin, folate, zinc and selenium intake (information not shown). Therefore, dividing by creatinine may not be the best way to adjust for urine dilution. Others have proposed to include creatinine as a covariable in the multivariate models; since this as an ongoing debate,30 we decided to present the results in both manners.
Subjects with similar TAs concentrations showed different proportions of metabolites (MMA and DMA). The individual ability to methylate depends on age, sex, body mass index, smoking habit, nutrient intake and genetic susceptibility, among other factors.11,12 The importance of this variability lies in the fact that the toxic effects of iAs exposure have shown differences associated with the urinary metabolite excretion profile. An increase in %MMA has been associated mainly with cancer and cardiovascular diseases, while the increase in %DMA has been linked to diabetes and metabolic syndrome.10 Therefore, urinary TAs is not a good risk marker for the development of specific diseases. However, due to the higher cost involved in determining the metabolic profile, compared to the quantification of TAs, its use in population studies represents an economic challenge.
The observed levels of urinary TAs allowed us to estimate that approximately 65% of women in the study region would be exposed to concentrations of iAs in water over the limit (10 μg/L) recommended by the WHO,3 assuming that all women consumed similar volumes of water. However, our estimate has limitations, since we are extrapolating information from a model calculated in a population with lower TAs than ours (Calderón and colleagues: ~0.005 to ~0.50 µg As/mg creatinine; our study: 0.001 to 1.68 µg As/mg creatinine), and thus we can have greater inaccuracy.
In Mexico, the current limit for the concentration of iAs in water for human consumption under the NOM 127-SSA1-1994-2000 (NOM) is 25 μg/L. This NOM has been revised (PROY NOM 250-SSA1-2014) to reduce iAs to 10 μg/L, however, to date, it remains as a draft.31 Although the implementation of the revised NOM is necessary to reduce the exposure in these populations, the great challenge is to have the technology for removing iAs from drinking water in the contaminated areas of the country with levels above 10 μg/L, as well as to strengthen monitoring and evaluation programs.32
Information on the levels of iAs in drinking water is more abundant in the northwest and the center-north of the country, and the concentrations show great variations.33 Coahuila, Durango and Chihuahua stand out for their high concentrations compared to Nuevo Leon; however, in the latter state, 37% of individuals might be drinking water with concentrations above the limit established by the WHO (10 μg/L). As for the rest of the country, what little information is available suggests the presence of high concentrations of iAs in groundwater; such is the case of Colima, Chiapas and Yucatan, where concentrations ≥25 μg/L have been found.31,34 If the aforementioned international limits were adopted, it is likely that iAs exposure would emerge as a more extensive problem in Mexico, and updated information would be required to verify it.
In conclusion, this work confirms that there is a significant number of women with levels that exceed the limits of biological exposure and who exhibit wide variations in the metabolism of iAs, and suggests that iAs exposure is an environmental health problem in Mexico that requires prevention and control.











 nueva página del texto (beta)
nueva página del texto (beta)


