Introduction
Fruits and nuts are healthy food for human nutrition, thus the Food and Agriculture Organization (FAO) declared 2021 as the International Year of Fruits and Vegetables (Avila-Quezada and Rai, 2021). The pecan tree Carya illinoinensis is the most important deciduous fruit tree in Mexico, with 144 567 ha planted. 88 834 ha are established in the state of Chihuahua (SIAP, 2020). Zinc (Zn) is a key element for plants nutrition, since many Zn-dependent enzymes have an important role in the metabolism of proteins, sugars (photosynthesis and conversion of starch to sugars), carbohydrates and auxins (Nandal and Solanki, 2021).
In face of zinc deficiency, the activity of carbonic anhydrase (CA) and superoxide dismutase (SOD) is reduced, since the availability of Zn have a positive correlation between their activities and Zn efficiency of the plant (Mathpal, Srivastava, Shankhdhar, and Shankhdhar, 2015; Singh, Shukla, Behera, and Tiwari, 2019).
The enzyme SOD takes part in the detoxification of active oxygen species in crops and converts superoxide into H2O2 and O2 (Miller, Suzuki, Ciftci, and Mittler, 2010), while the enzyme CA catalyzes the conversion of CO2 to HCO3, which is employed by the enzyme phosphoenolpyruvate carboxylase in the initial carboxylation reaction of photosynthesis. Zn-activated enzymes intervene in carbohydrate metabolism, in the integrity of the cell membrane, in protein synthesis, and in the regulation of auxin synthesis (Hafeez, Khanif, and Saleem, 2013).
Conversely, Zn deficiency alters the plant metabolism and produce symptoms in the leaves such as intervenous chlorosis (Arce, Storey, and Lyons, 1992), as well as growth retardation, small leaves and intense oxidative degradation (aging premature tissue) (Kirkby and Römheld, 2007) (Figure 1). In addition, there is evidence that Zn promotes the integrity of the cell membrane, which results in the protection of the plant from attack by root pathogens (Kirkby and Römheld, 2007; Bell and Dell, 2008; Hafeez et al., 2013). Moreover, factors such type of soil, pH and moisture affect the availability of zinc in soil (Singh et al., 2019).
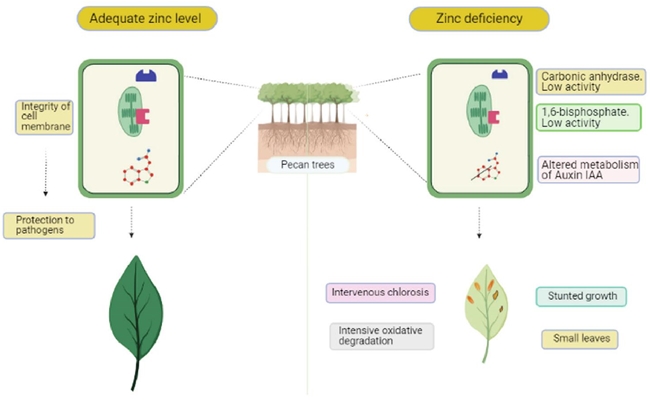
Figure 1: Under zinc deficiency, the carbonic anhydrase activity is reduced, as well as the 1,6-bisphosphate activity. Therefore, Zn deficiency causes symptoms such as intervenous chlorosis (Arce et al., 1991), growth retardation, small leaves and intense oxidative degradation (aging premature tissue) (Kirkby and Römheld, 2007). In addition, Zn promotes the integrity of the cell membrane, which derives in protection against root pathogens (Kirkby and Römheld, 2007; Bell and Dell, 2008).
Zinc-inefficient plants respond to external zinc fertilization (Singh et al., 2019). Various fertilization methods have proven to be effective such as biofortification and the application of chelated elements (Das, Singh, Kumar, and Kumar, 2019; Ciscomani-Larios et al., 2021). Foliar fertilization is a commonly used technology in pecan trees. Foliar spraying is nor a sustainable management because it pollutes the environment, it is laborious due to the use of supplies and equipment, and because it requires to be applied at night. In addition to this, the continuous traffic of machinery causes soil compaction (Fenn, Malstrom, Riley, and Horst, 1990; Herrera, 2008) limiting the availability of zinc (López, Gómez, and Rodríguez, 2014) and causing compaction-related damage. Most of the pecan trees in northern Mexico and the southern United States are established in calcareous soils, where the alkaline pH strongly reduces the availability of zinc for the tree (Núñez, 20091). This suggests that the difficulty of the tree to supply Zn is not only a problem of chemical unavailability of the nutrient in the soil, but also of inefficiency in root uptake (Smith and Storey, 1976).
Pecan tree demands a considerable amount of zinc (Heerema et al., 2017). Zinc application is generally supplied with inorganic salts such as zinc sulfate (ZnSO4). The edaphic contribution of Zn in pecan trees with alkaline soil by means of ZnSO4 and adjuvants to acidify the soil has been very inconsistent and costly (Worley, Harmon, and Carter, 1972; Fenn et al., 1990).
Nevertheless, the chelated form of Zn, such as Zn ethylene diamine tetra-acetic acid (EDTA) is another source that supplies enough Zn to the plant. This form does not interact with soil components since the central metal ion (Zn2+) is surrounded by chelate ligands. EDTA is one of the strongest synthetic chelating agents, indeed, it contributes greatly to the movement of Zn in the soil under highly irrigated conditions (Suganya, Saravanan, and Manivannan, 2020). This makes it a viable option due to its stability in calcareous soils, making it more available to plants (Heerema et al., 2017; Suganya et al., 2020).
It has been shown that the edaphic contribution of Zn chelated with carboxylic acids is effective in pecan trees in production (Heerema et al., 2017), whose growth and fruiting are the same as with Zn applied to the foliage. In orchards with drip irrigation, the contribution of nutrients is made in a very localized area of the root, which makes fertigation more effective (Worley, Daniel, Dutcher, Harrison, and Mullinix, 1995). In this technology, the key is the concentration of the product in the middle of the drip area of the trees.
In addition, non-tillage promotes ectomycorrhization (Tarango and Olivas, 2018). Previous studies have shown that the absorption of Zn depends on the degree of ectomycorrhization (Tarango, Macías, Alarcón, and Pérez, 2004). In this context, the objective of this work was to evaluate the production of pecan trees with edaphic contribution of chelated zinc through drip irrigation. This will offer growers an economical, ecological and sustainable option that allows them to improve yield.
Materials and Methods
The study was carried out in the "La Promesa" orchard, in Conchos county, Chihuahua, México from 2018 to 2020. The orchard has an area of 22 ha, with 12-14-year-old trees of the Western variety grafted on Creole pattern from seed. The trees are planted at 13 × 13 m in a staggered pattern, on sandy and calcareous crumb soil. In addition, the physical and chemical properties of 0-20 cm of the soil in the experimental area were as follow: soil texture, crumbly light sand composed by sand 65.21%, silt 17.08% clay 17.71%; pH 7.5; organic matter 1%; Ca 3912.5 mg kg‑1 (moderately high); Zn 1.34 mg kg-1 (moderately low).
Drip irrigation with a hose on each side of the row of trees, with 32 drippers per tree is established in the orchard. The irrigation scheme is detailed in Table 1. By treatment, five homogeneous trees were chosen based on the cross-sectional area of the trunk and the volume of the crown. Also, the soil in which they are found is uniform in physical and chemical characteristics. In a completely randomized experimental design, the established treatments were the following:
1.- Foliar zinc. Six nocturnal sprays during the vegetative cycle described in Table 2.
2.- Edaphic zinc (Carboxy Zn® Innovak Global) via drip irrigation + a microbial consortium (Rhizo TX® Innovak Global): Rhizo TX® six applications described in Table 3. The dose of zinc applied was 12, 14 and 15 kg ha-1 per successive year of the study, that is, 12 kg ha-1 in 2018, 14 kg ha-1 in 2019 and 15 kg ha‑1 in 2020. The dose was increasing due to the annual increase in the size of the tree. The commercial source of zinc was Carboxy Zn®. In treatment 2 there was a strong incidence of Texas root rot caused by the fungus Phymatotrichopsis omnivora. To manage the disease, the commercial product Rhizo TX® based on Trichoderma viride, Streptomyces lydicus, Bacillus subtilis, Bacillus megaterium and Penicillium bilaii was applied. This was applied in doses of 4 kg ha-1 each year; 2 kg in April, and 2 kg in June. Therefore, given the appearance of Texas root rot and the application of the microbial consortium, the microbiological profile was determined in the two treatments.
Table 1: Historical water consumption per month of young Western pecan trees (12-14 years) in a buried drip system in south-central Chihuahua.
Month |
Liters of water per day tree -1 |
Irrigation per day (hours) |
March1 |
180 |
2.5 |
April2 |
300 |
4.2 |
April3 |
350 |
5 |
May2 |
400 |
5.6 |
June2 |
500 |
7* |
July2 |
600 |
8.5* |
August2 |
500 |
7* |
August3 |
460 |
6.5* |
September2 |
375 |
5.3 |
September3 |
300 |
4.2 |
October2 |
180 |
2.5 |
Winter irrigation |
2nd December fortnight = 8 h 2nd January fortnight = 8 h 2nd February fortnight = 8 h |
1 On March 15. 2 First fortnight of the month. 3 Second fortnight of the month. * Due to lack of water on these moths, only a 6h irrigation per day was carried out.
Table 2: Western pecan trees fertilization program.
Number of applications / dates |
||
Doses 100 L-1 water |
Dose ha-1 |
|
|
1.- At a length of one inch on the shoot 03 April |
250 g foliar ZnSO4 500 g urea |
2 kg edaphic Zn 1 kg Cupper sulfate (CuSO₄) 500 g Urea 15 ml Nitric acid (Density = 1.33)* |
|
2.- After 10 days Mid-April |
300 g foliar ZnSO4 150 g Cupper sulfate (CuSO₄) 250 g Potassium nitrate (KNO3) 500 g urea |
2 kg edaphic Zinc 2 kg RhizoTX® 3 kg Magnesium sulfate (MgSO4) 500 g urea |
|
3.- After 15 days 03 May |
300 g foliar ZnSO4 500 g MgSO4 250 g KNO3 500 g urea |
2 kg edaphic Zn 1 kg CuSO₄ 500 g urea |
|
4.- After 15 days Mid-May |
250 g foliar ZnSO4 500 g MgSO4 250 g KNO3 250 g urea |
2 kg edaphic Zn 3 kg MgSO4 250 g urea |
|
5.- After 15 days 01 June |
250 g foliar ZnSO4 250 g FeSO4 250 g KNO3 250 g urea |
2 kg edaphic Zn 250 g urea |
|
6.- After 15 days Mid-June |
250 g foliar ZnSO4 250 g urea |
2 kg edaphic Zn 2 kg RhizoTX® 3 kg MgSO4 250 g urea |
Treatment 1 = foliar zinc sulfate (ZnSO4). Treatment 2 = chelated zinc added to soil in drip irrigation. *Included on every application to adjust the pH to 5.
Table 3: Microbial population of rhizospheric soil of Western pecan trees after three years of zinc fertilization and microbial inoculant supply.
Treatment |
Bacteria |
Fungi |
Actinomycetes |
|
- - - - - - - - - - CFU g-1 of soil - - - - - - - - - - - |
% |
|||
Foliar zinc |
1.58 E + 05 b |
1.08 E + 03 b |
1.85 E + 04 a |
40.7 b |
Edaphic Zn via drip irrigation + microbial consortium |
3.81 E + 05 a |
5.07 E + 03 a |
7.67 E + 03 a |
69.9 a |
P ≤ 0.05 |
0.015 |
0.086 |
0.193 |
0.028 |
CFU g-1 = colony forming units per gram; EM = ectomycorrhizal colonization. Treatments with different letters show different statistical significance (P ≤ 0.05).
Both treatments were fertilized to the soil with the formula 45-05-10 g NPK for each centimeter of trunk diameter, divided into seven applications according to the tree phenology (Tarango, 2012), applied via drip irrigation. The sources urea, phosphoric acid and potassium sulfate were used. Each treatment consisted of 5 repetitions (trees) per treatment.
The variables evaluated were fruit shoot length, terminal leaf area (estimate of AFE = specific leaf area), zinc leaf concentration, yield, nut size and percentage of almond. The EFA was estimated with the equation Y = 1.56 + 0.62 X (r2 = 0.98), where Y = leaf area in cm2 and X = length by width of the leaflet in cm (Medina, 1993).
The leaflets collected for leaf analysis were washed with a 15% acetic acid solution and dried in a solar dryer. The leaves were subjected to wet digestion with an acid mix (HNO3/HClO4) by atomic absorption spectrometry.
Evaluation of total bacteria and fungi
The concentration of bacteria and fungi CFU g-1 of soil was determined with the techniques established by Official Mexican Standards NOM-111-SSA1-1994 (1995), NOM-092-SSA1-1994 (1995), and NOM-110-SSA1-1994 (1995). The ectomycorrhizae percent was evaluated since it has been reported that Zn absorption depends largely on the degree of ectomycorrhization of the tree root (Tarango et al., 2004). Sixty feeder roots equal to 50 cm of roots per tree were sampled in each of the 10 trees to determine the percentage of ectomycorrhizal with a stereomicroscope. The following equation was used: colonization percentage = of mycorrhizae/total number of roots.
Results and Discussion
Shoot growth
The optimum minimum fruiting shoot growth for adult Western pecan trees is 15 cm (McEachern, 1985). In this study, the vigor of the sprout was not affected by the way the zinc was supplied to the pecan trees, and in all cases, it exceeded the minimum optimal value. Although on average, the edaphic zinc treatment increased the shoot length 4.5% with respect to foliar fertilization (Figure 2).
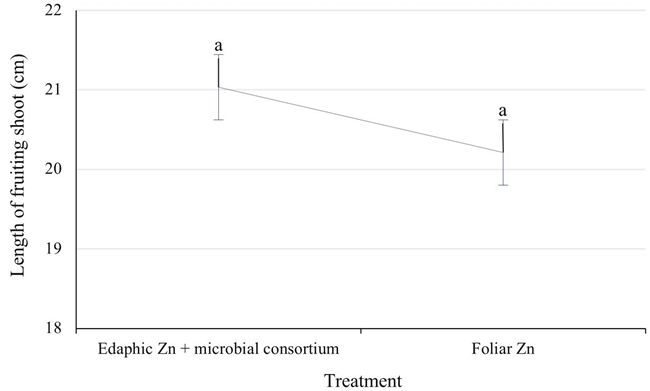
Figure 2: Mean values of fruiting shoot length (cm) of Western pecan trees under two zinc fertilization treatments. Error bars indicate standard deviation. Treatments with different letters show different statistical significance (P ≤ 0.05).
The specific leaf area (EFA) is a very sensitive variable in studies of nutrition, pruning and irrigation in pecan trees, and has a direct relationship with nut production (Tarango, 2017). The EFA was always higher in pecan trees with edaphic zinc in the three years of evaluation. The average increase was 6% with respect to the commercial technology of spraying zinc to the foliage (Figure 3). Edaphic zinc must be absorbed by the root and transported to the aerial part. The transport of metal ions in plants consists of the absorption of the solution from the soil by the root. Zn is predominantly acquired and transported as Zn2+ (divalent). Zn ions can also bind with root exudates such as malate, citrate, oxalate, and other low molecular weight organic acids that help it move into the rhizosphere (Ajeesh Krishna, Maharajan, Victor Roch, Ignacimuthu, and Antony Ceasar, 2020).
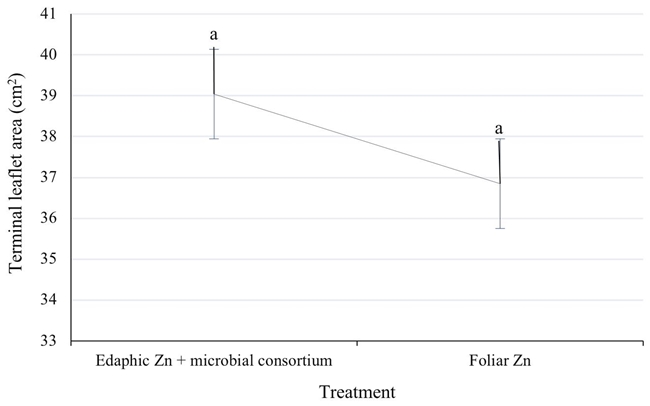
Figure 3: Mean values of terminal leaflet area (cm2) of Western pecan trees under two zinc fertilization treatments. Error bars indicate standard deviation. Treatments with different letters present different statistical significance (P ≤ 0.05).
Then, from the root, the metal ions will be supplied to the various organs of the plant. These functions are performed by specific transporter proteins that regulate intercellular and intracellular transport. Several genes code for Zn transporter proteins or regulate their expression at the transcriptional, post-transcriptional, and translational levels (Gupta, Ram, and Kumar, 2016). The minimum adequate EFA for Western pecan trees in production is 28 cm2 (Kilby, 1985); value that is exceeded in this study in both treatments for three years. This result because the treated trees are young in production with selective thinning pruning, but above all to the efficient provision of Zn with both application methods. It was visually observed that pecan trees fertilized with edaphic zinc exhibited healthy foliar condition, even in the Wichita variety, which is very demanding of this nutrient (Heerema et al., 2017).
Nutrimental state
Particularly in calcareous soils, the contribution of zinc is reflected in its foliar concentration and in the growth of shoots and leaves (Kilby, 1985). In the three years of study, the treatment of zinc sprinkled on the foliage had the highest foliar concentration of the nutrient, 86% more than treatment with edaphic zinc (Figure 4). The foliar zinc concentration in the soil zinc treatment was very low, only 22 mg kg-1 on average, according to the accepted standards of 50 to 100 mg kg-1 (Walworth, Pond, Núñez, and Kilby, 2008). Reports on foliar concentrations of Zn for good commercial pecan production are highly variable. In this regard, studies with soil contribution of Zn have shown that a foliar concentration of 20 - 58 mg kg-1 is sufficient for pecan trees to grow and produce well, in addition to the fact that the leaves do not exhibit deficiency symptoms (Núñez, 20091).
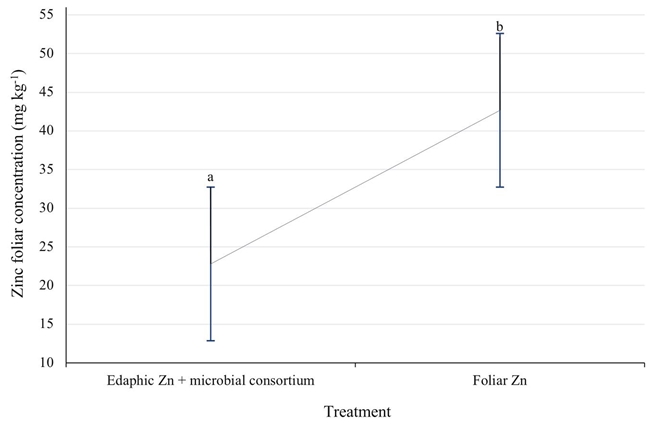
Figure 4: Zinc foliar concentration (mg kg-1) in Western pecan trees under two zinc fertilization treatments. Error bars indicate standard deviation. Treatments with different letters present different statistical significance (P ≤ 0.05).
Other authors suggest amounts of foliar Zn of 40 - 60 mg kg-1 (Smith, Walworth, Comeau, Heerema, and Sherman, 2021). Moreover, Heerema et al. (2017) suggested much lower amounts; 14 - 22 mg kg-1 as an adequate level of Zn to maximize production in commercial pecan orchards.
This consideration is important, since for decades it has been argued that much of the zinc considered sufficient is actually part of the residual deposit on the leaf surface, rather than metabolic Zn (Smith and Storey, 1976). In addition, it has been shown that the absorption of Zn by the pecan tree leaf is very low, of the total contributed by a sprayed solution, only 1% is absorbed by the young leaflets (April and May) and 0.2% by the mature ones (Storey, 1985). In this study, the trees treated with edaphic zinc and with 22 mg kg-1 of foliar zinc exhibited a slight presence of leaflets with marginal wavy lines, a symptom that was more common in pecan trees treated with zinc to the foliage and with 42 mg kg-1 of foliar zinc.
Production
The yield of adult pecan trees is dependent on the nutritional reserves of the previous year, although the nutritional contribution of the current year also affects fruiting (Smith, 1991; Wood, 1991). The foliar concentration of 15 to 29 mg kg-1 of zinc in the pecan trees treated with edaphic zinc had a yield equal to the foliar fertilized ones, including 3% more nuts in an average of three years (Figure 5). That is, the contribution of chelated zinc to the soil through drip irrigation adequately supports the fruiting of the pecan trees. The production standard for 12 - 14 years old pecan trees is 23 to 28 kg per tree. Therefore, the technology proposed in this study exceeds the production of nuts per tree, with an average of 29 kg tree-1 in three years. Assuming that the trees in 2020 were 15 years old, the production standard is 34.5 kg tree-1. Therefore, both treatments exceeded the average production. Even the proposed technology outperformed it by one more kg per tree.
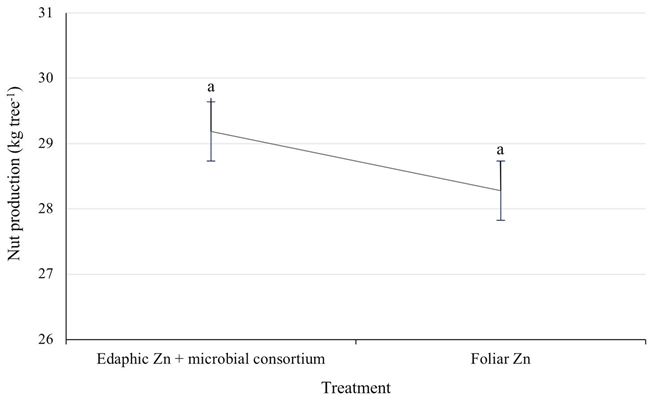
Figure 5: Nut production (kg tree-1) in Western pecan trees under two zinc fertilization treatments. Error bars indicate standard deviation. Treatments with different letters present different statistical significance (P ≤ 0.05).
As a reference, Tarango and Olivas (2018) reported that in 16-19-year-old pecan trees fertilized with 280 g year-1 of edaphic zinc, when the leaf had 24 mg kg-1 of Zn, the growth of the shoot was reduced by 11.5% and the production of nuts 7.9%, and when the foliage had 32 mg kg-1 of Zn, the growth decreased 5.7% and the yield 1.7%, compared to foliar fertilization. The foregoing suggests that the contribution of zinc to the soil, with the scheme in Table 2, is slightly more effective by drip fertigation than it is by localized contribution to the soil and sprinkler irrigation.
In soils with low Zn concentration, the high affinity Zn transporter system is more active, at molecular level ZIP and IRT transporters promote mobilize Zn2+ ions through cell membranes into the cytoplasm. After that, the Zn2+ ions pass through the Casparian strip, the endodermis, and the xylem parenchyma cells that are subsequently loaded into the xylem (Ajeesh Krishna et al., 2020). Therefore, understanding element movement is essential for proper pecan trees management to increase production.
Quality
There is generally a direct relationship between sprout vigor, leaf area and nut quality, and an inverse relationship with yield (Worley et al., 1995; Tarango, 2017). In the three years of study, the size of the nut was consistently greater in the treatment with zinc to the soil, 5.8% more on average (Figure 6). Both treatments exceed the standard of 6.5 g nut-1 for the Western variety (Herrera, 2008).
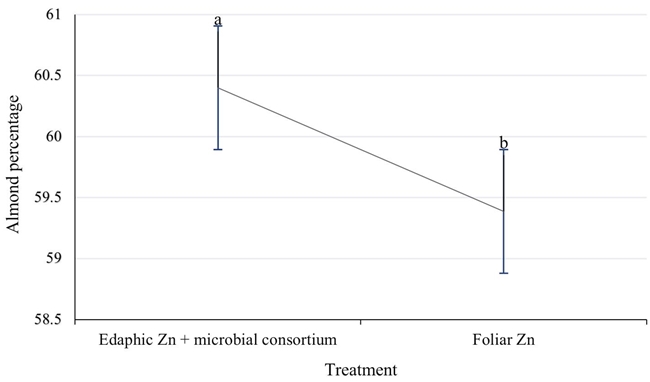
Figure 6: Almond percentage in Western pecan trees under two zinc fertilization treatments. Error bars indicate standard deviation. Treatments with different letters present different statistical significance (P ≤ 0.05).
Likewise, the almond percentage was favored by the contribution of zinc to the soil, 1% more on average (Figure 6), although both treatments exceed the standard of 56% almond for the Western variety (Herrera, 2008). Said quality variables were higher in the trees with zinc to the soil, surely favored by the greater sprout vigor and more leaf area, as has been reported to occur (Worley et al., 1995). That is, zinc in the soil first encourages vegetative growth, which later translates into larger and more almond-like nuts.
Microbiological profile
The inclusion of the microbial consortium can modify the response to edaphic zinc and is an influence point for the treatment. Although it was not measured, it was observed that the commercial product reduced the advance of P. omnivora, which suggests a microbiological improvement associated with microbial competition. After three years of applying edaphic Zn via drip irrigation + microbial consortium, the microbial population increased in the rhizospheric soil of the trees (Table 3).
The contribution of edaphic Zn via drip irrigation + microbial consortium, led to the increase of microbial populations; bacteria increased 2.4 times and 4.6 times in fungi, but actinomycetes decreased 2.4 times. This is explained because the commercial product applied is a microbial inoculant, which also stimulates the production of radical exudates. The root exudates promote microbial colonization in the rhizosphere (Vivanco, Rodríguez, Medina, and Velázquez, 2010).
Moreover, a notable response was the increase in root mycorrhizal colonization in the trees with the edaphic Zn via drip irrigation, 42% higher than the control. The applied product does not contain mycorrhizal inoculum, thus, it is assumed that ectomycorrhizae are found naturally in the orchard. This result is very interesting, since the pecan tree is an ectomycorrhizal-dependent tree (Brison, 1976). In addition, the substantial increase in microbes in rhizospheric soil may have slowed down the advance of Texas root rot, and may have contributed to improving the vegetative vigor (measured with the length of the fruiting shoot), and the quality of the nut of the treated pecan trees.
Many efforts adding biocontrol microorganisms have been made to minimize the effect of phytopathogens and to stimulate plant growth, and thus to achieve food security (Guigón-López et al., 2010; Companioni-González, Domínguez, and García, 2019; Hernández-Montiel, Droby, González, Duran, and Avila, 2021). These efforts are based on the fact that the beneficial organisms commonly found in plants rhizosphere including plant growth-promoting rhizobacteria (PGPR), nitrogen-fixing bacteria and mycorrhizal fungi (Mendes, Garbeva, and Raaijmakers, 2013; Madrid-Delgado et al., 2021). Future studies should focus on the analysis of antioxidant activity and phenolic compounds (Ciscomani-Larios et al., 2021) in the pecan trees treatments with edaphic and foliar zinc.
Conclusions
The chelated zinc added in the drip irrigation allowed the shoot (21 vs. 20.1 cm) and the leaf (39 vs. 36.8 cm2) to grow slightly better than with zinc applied to the foliage. The new sustainable technology proposed in this study which includes 12 to 15 kg ha-1 of soil chelated zinc and a microbial consortium applied in drip irrigation, allowed an increase in yield of 1 kg of nut per tree after three years of application. In addition, the chelated zinc (Zn) treatment applied in drip irrigation avoided night-time spraying. The contribution of edaphic Zn via drip irrigation and a microbial consortium substantially promoted the microbial population of the rhizospheric soil in bacterial populations with 3.8 E + 05 CFU g-1 compared to 1.58 E + 05 CFU g-1 in the foliar zinc treatment, also the populations of fungi were favored with concentrations of 5.07 E + 03 and 1.08 E + 03 CFU g-1 respectively. In addition, this treatment promoted natural ectomycorrhizal colonization of the root with 69.9%. This management in pecan orchards offers producers an economic, ecological and sustainable option that allows them to improve yield.











 nueva página del texto (beta)
nueva página del texto (beta)



