INTRODUCTION
Soils contaminated with potentially toxic elements (PTEs) constitute a risk to human health and to biota. At low contents some PTEs, such as copper, chromium, molybdenum, selenium, and zinc are essential for the functioning of human health and for the growth of plants and animals. However, at higher contents they become toxic and cause adverse effects (Li et al. 2014, 2015). Other elements such as thallium, arsenic, cadmium, mercury and lead are toxic even at low contents (Mingot et al. 2011, Smith et al. 2011, Yang et al. 2015, Mendoza et al. 2017, Cui et al. 2018, Cruz-Hernández et al. 2018, Pelfrêne and Douay 2018).
A soil is considered contaminated with PTEs when their total contents exceed the natural background levels (SEMARNAT 2007). However, it is currently agreed upon that a real ecological risk occurs only when the elements are in bioavailable forms, i.e., when the chemical species present can enter living organisms and cause adverse health effects (Mendoza et al. 2017). The most bioavailable forms of PTEs are obviously the water-soluble chemical species because they can enter the organism directly through the consumption of polluted water. However, other less available chemical forms may also pose high threats to organisms because of the chemical reactions that occur upon their entry into or their interactions with the organisms (Santana-Silva 2016). The different chemical forms of PTEs depend on many factors and geochemical conditions, such as pH, their mineralogical occurrence or associations, the environmental redox status, and the availability of complexing agents (Cruz-Hernández et al. 2018).
One route of exposure of environmental contaminants in humans and animals is by oral ingestion (Luo et al. 2012). Intake can occur through the consumption of soil directly or indirectly through vegetables, animals and drinking water that are contaminated by soil dust containing these elements, causing potentially harmful health effects (Li et al. 2016b, Fujimori et al. 2017). Soil intake is a major problem in communities that live in areas adjacent to mining-metallurgical wastes, because of the relatively high contents of PTEs.
The bioavailability (BV) of PTEs in soils is determined by different methods that involve feeding the living organisms of interest with the contaminated material, and then, at the appropriate times, sampling fluids or tissues from within the organisms, or from their excretions, such as urine. This makes the procedures quite costly, complicated, and cumbersome, and has prompted widespread attempts at simulating chemically (in vitro) as closely as possible the digestive processes that occur, in such a way as to obtain equal results to those in BV tests. A suite of simpler chemical-based (in vitro) extraction tests applicable to contaminated soils has emerged to simulate the BV process and have been called bioaccessibility (BA) tests (Ruby et al. 1999, Carrizales et al. 2006, Mendoza et al. 2017).
One of the most popular BA methods is the Solubility and Bioavailability Research Consortium (SBRC) test, originally devised to determine the BA of lead, but now extended to other PTEs. This test consists of simulating the extraction of contaminants in the gastric phase, at pH = 1.5, using a 0.04 M glycine buffer (Drexler and Brattin 2007); it is the method adopted by the Official Mexican Standard NOM-147-SEMARNAT/SSA1-2004 (SEMARNAT 2007) to evaluate BA in contaminated soils.
The gastric SBRC BA test has proven to correlate highly with the BV measured in vivo (Drexler and Brattin 2007) and, therefore, it is this simpler method that is extremely useful to estimate with great accuracy the BV of several PTEs, which is too laborious and expensive and requires the sacrifice of living beings. However, since this BA test is still a complex method that requires equipment and reagents specifically designed for it, the goal of the present research was to determine if it is possible to perform an even simpler extraction test, applicable in a widespread manner in environmental analysis laboratories that could correlate strongly with this chosen reference BA test, considering HCl is the main extracting agent in the gastric phase.
Several simple extraction methods have been evaluated to determine the content of metals extracted from the soil and to estimate bioaccesibility and phytoavailability (Madrid et al. 2008, Cao et al. 2009, Rodrigues et al. 2013, Li et al. 2017). Inorganic acids have been used in urban soils, but have not been tested in those affected by mining-metallurgical activities [0.43 M HNO3 (Rodrigues et al. 2013)], showing high correlations with the SBET method [Simple Bioaccessibility Extraction Test (Kim et al. 2002, USEPA 2008)], which consists of soil e xtractions with a 0.4 M glycine solution adjusted to pH 1.50 ± 0.05 (with HCl), for assessing the BA of Cd, Cu, Pb, and Zn) [r values ranging from 0.97 (for Cd) to 0.99 (for Cu, Pb and Zn)]. As and Tl were not tested. Also organic acid and complexing agents have been used [acetic acid and EDTA (Waterlot et al. 2017)], yielding relatively good correlations (r ˃ 0.94) with the gastric and gastrointestinal phases. Neutral salts have been used as well [CaCl2 (Rodrigues et al. 2013), NaNO3 (Pueyo et al. 2004), MgCl2 (Takeda et al. 2006)], yielding accurate results (normally < 10 %) for all metals (Cu, Zn, Pb and Cd), taking into account the low concentrations of metals extracted. The extraction efficiency of metals obtained with each salt was slightly different, and all three methods provided equivalent information, where the relative mobility of the trace metals (Cd > Zn > Cu > Pb) was correctly predicted in the studied soils.
Mingot et al. (2011) evaluated three extraction methods to determine the bioaccessibility of As in soils of a recreational area in Madrid. The methods used were PBET, SBRC and HCl-extraction. The extraction conditions with HCl were at a temperature of 37 ºC, under stirring for one hour with a concentration of 0.07 M HCl and a sample mass(g) to volume (mL) ratio of 1: 1000. In this study, a relatively low correlation (r = 0.776) was found between the SBRC method and HCl for the extraction of As.
Dodd et al. (2013) determined bioaccesibility in dust samples and soil standards. Gastric BA was evaluated using the SBRC test and a modification of the EN-71 protocol (European Standard Toy Safety Protocol EN-71, European Commitee for Standarization 1995) (0.07 M HCl solution at 37 ºC, stirring for two hours, a sample mass(g) to volume (mL) ratio of 1: 2000, which is the key modification of the EN-71 Toy Safety protocol). Correlation coefficients between the SBRC test and the modified protocol EN-71 for As, Cd, Cu, Pb, Ni and Zn, ranged between 0.85 and 0.92.
In the present work we evaluated a simple extraction method using HCl at a fixed pH of 1.5 and compared it to the gastric SBRC BA method for Tl, As, Pb, Zn, Cu and Cd in a series of samples originating from mining wastes and from soils contaminated with metallurgical wastes, from two selected areas of Mexico. The goal was to investigate the possibility of improving considerably the correlations found from past reports by carefully controlling the following experimental parameters: the soil: solution ratio and stirring time, pH, a very small pore size of the filtration membrane, and the temperature. Although the composition of the human gastrointestinal tract is complex, the primary component from the point of view of PTE extraction is HCl and, therefore, simple extractions with HCl may strongly correlate with those of the SBRC method at the same pH value and controlled experimental conditions that ensure measuring only dissolved concentrations (which in turn correlates quite well with BV values). The specific PTE speciation was not discussed or analyzed, except in the few cases where important differences were found between the two methods.
MATERIALS AND METHODS
Study area and sample collection
The areas studied in Mexico correspond to the North-Center of the country: Metallurgical District of “San Luis Potosí” (SLP) (Fig. 1a), and the South-Center: soils and mine tailings of the “Taxco” (TX) mining area in the state of Guerrero (Fig. 1b and c). Nine superficial samples were analyzed from the old SLP copper and arsenic plants, and 18 samples of six mine tailings vertical profiles and four superficial soil samples contaminated by such tailings from TX. The TX samples came from three tailings impoundments, which correspond to different degrees of oxidation: aged (oxidized) tailings from “La Concha” showed brown colors, and fresh (reduced) tailings from “Foster” showed a gray color; while tailings from “El Fraile” showed both types of tailings. Table SI (Supporting Information) summarizes the location and description of the selected samples.
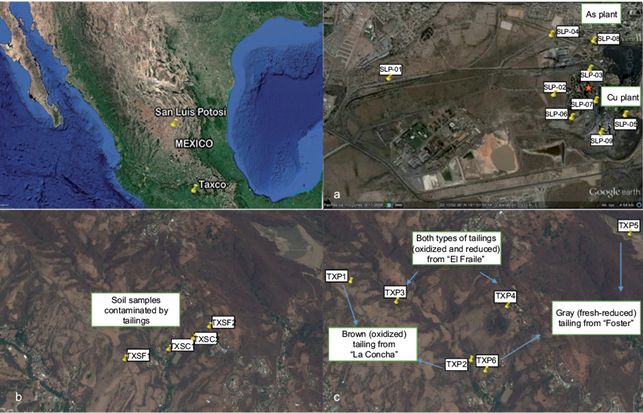
Fig. 1 Google Earth images of San Luis Potosí and Taxco study areas showing the locations of mining wastes, soils and metallurgical residues collected from a) San Luis Potosí metallurgical plant, b) Taxco soils, and c) Taxco mine tailings
Approximately two kilograms of sample were collected for each sampling point, by simple random sampling (USEPA 1993). In the laboratory, all samples were dried at 40 ºC for 72 h after weakly dispersing the soils horizontally in trays, then homogenized, sieved (2 mm mesh) and transferred to plastic bags prior to analysis.
The metallurgical study area is located in the western part of the city of San Luis Potosí in North-Central Mexico. Mining activities in this area began in April 1892. Three metallurgical plants that produced copper, arsenic and lead operated on this site. When the metallurgical activities began, this site was not populated, but with the passage of time towns adjacent to the site began to develop. There is currently a residential area to the East of today’s inactive metallurgical plants (Villalobos et al. 2010), which carried out a pyro-metallurgical process by raising the temperatures from 1000 to 1300 ºC of metallic sulfide concentrates of As, Cu and Pb, under controlled oxidizing conditions to obtain metals of commercial value. More than a century of industrial activities in this metallurgical site resulted in the generation of waste, which dispersed to the surroundings, causing soil contamination with PTEs (Villalobos et al. 2010), and representing a potential risk to human health. The main residues generated in the plants were vitreous slag and atmospheric dusts. There are two deposits of slag South of the old facilities of the copper plant, which form mounds of compact material, one corresponds to slag of the copper plant, and the other a vitrified waste that was obtained in the furnaces of the old lead plant that operated until 1952.
The mining district of Taxco is located in South-Central Mexico, in the city of Taxco de Alarcón, in the state of Guerrero. The study area has a mining tradition that dates back to the Colonial era (XV century), from which waste materials have been accumulating in mine tailings impoundments. The tailings were placed for a long time on the banks of rivers and in areas that over the years have been surrounded by human settlements (Armienta et al. 2003). The mineralization for mining purposes appears mainly in hydrothermal veins, spare minerals and stockworks housed in limestone, slate and shale.
Chemical and mineralogical analysis
All samples were analyzed to determine total contents of Tl, As, Pb, Cu, Zn and Cd and other major elements (Fe, Mn, Ca) through digestions of the finely ground and 60 mesh-sieved samples (particle size less than 250 μm) with a mixture of HNO3: HCl: HF (3: 1: 1), using an ultrawave ECR microwave oven operating at 260 ºC for 40 min. After digestion the total content of the metals studied were analyzed by inductively coupled plasma atomic emission spectrometry (ICP-AES) using a Perkin Elmer Optima 8300 unit. When element contents were below the detection limit of this technique analyses were carried out by inductively coupled plasma mass spectrometry (ICP-MS) using an iCAP Thermo Scientific instrument. The precision and accuracy of the digestion procedure and the analytical methods were checked using standard reference materials (CRM 2709a and Sandy Soil C). Also, these standards were used to calibrate for total Tl, As, Pb, Cu, Zn and Cd contents obtaining recovery percentages of up to 105 %
The mineralogy of all samples was determined via X-ray diffraction (XRD) using an Empyrean diffractometer equipped with a fine focus Cu tube, nickel filter, and PixCel 3D detector operating at 40 mA and 45 kV at the Instituto de Geología, UNAM. For this, samples were ground with an agate pestle and mortar to <75 μm and mounted in back-side aluminum holders. The analyses were carried out on randomly oriented samples by the step scan method using the measurement range (2θ) of 5 to 70º with an integration time of 40 s and step size of 0. 003º. Phase identification was made with PDF-2 and ICSD databases and semi-quantification was made using version 4.5 of HighScore Plus software and RIR (Reference Intensity Ratio) method.
The determination of the physical and chemical properties was carried out in duplicate on the < 100 µm size fraction: soil pH and electrical conductivity (EC) were measured in 1: 5 (weight/volume) and following the procedure described in the method (ISO 10390 2005).
Determination of the bioaccessible (BA) fraction
Two methods (described in detail below) were used to determine this fraction: the SBRC test (Drexler and Brattin 2007) was compared with the simple extraction proposed here using HCl at pH 1.5. For these procedures sample particle sizes of less than 250 μm were separated (sieved) because it is the representative size range that adheres to the hands of humans (Ruby et al. 1996).
Solubility and bioavailability research consortium (SBRC)
The SBRC test simulates the stomach digestion in terms of digestive juice composition, temperature, pH and transit time. To achieve this, weights of 0.500 g of soil or tailings, with particle size < 250 μm, were transferred to high density polyethylene containers and 50 mL of a 0.4 M glycine solution was measured and transferred to each. The pH was adjusted to 1.5 ± 0.1 with concentrated HCl [37 % Sigma Aldrich (Poznan, PL]. To reach this pH in the presence of glycine the final concentrations of HCl added to the different samples varied between 0.3 and 0.4 M. Subsequently, the bottles were placed in an orbital shaker to perform the extractions of PTEs with temperature control of 37 ± 2 ºC, with stirring (orbital) speed of 28 ± 2 rpm for one hour (± 1 min). The pH value was measured at 5, 10, 15 and 30 minutes, readjusting if necessary (depending on the type of samples).
Hydrochloric acid-extractable fraction
A modified version of the European Standard Toy Safety Protocol EN-71 (European Committee for Standardization 1995) proposed by Rasmussen et al. (2008) was used to determine HCl-extractable PTEs contents. Various dilutions of concentrated HCl [37 % from Sigma Aldrich (Poznan, PL)] were used to adjust the samples to pH = 1.5.
Weights of 0.500 g of soil or tailings, with particle size < 250 μm, were transferred to a wide-mouth polyethylene container. 50 mL of the appropriate dilution of HCl (with final concentrations between 0.03 M and 0.1 M) was measured and transferred to the container yielding a sample mass to fluid ratio of 1:100 (g:mL), which is the modification of the EN-71 Toy Safety protocol and of the method by Dodd et al. (2013) (1: 2000). The samples were placed on an oscillating shaker at 300 rpm at room temperature for one hour (± 1 min). The pH of the extractant solution was evaluated at 5, 10, 15 and 30 minutes and readjusted so that the pH of the solution did not vary from the range of 1.5 ± 0.1.
The PTEs contents from both extractions were determined by ICP-AES, except for thallium, by ICP-MS. The reproducibility of the measurements was evaluated by analyzing all samples and a blank consisting of 50 mL of 0.4 M glycine solution at pH 1.5 or 50 mL of 0.03 M HCl solution at pH 1.5 in triplicate. The results were considered satisfactory when the coefficient of variation of these measurements was less than 10 %.
Absolute PTEs gastric BA was calculated by dividing the extracted content (termed SBRC-G and HCl-G) by the total soil PTE content (Eq. 1).
Where: In vitro PTE = PTE (mg/kg) extracted from soil following either gastric phase extraction (SBRC-G or HCl-G), and Total PTE (mg/kg) present in the contaminated soil.
Filtration method
In the literature different methods of separation of the dissolved (BA) fraction are reported. Among the most common are the use of centrifugation and microfiltration (pore size 0.45 μm) (Ruby et al. 1996, Mingot et al. 2011, Laird et al. 2015, Li et al. 2015, Zong et al. 2016).
In this research work the use of membranes with different pore sizes was evaluated to separate the dissolved from the solid (colloidal) phase: pore size 0.45 μm, corresponding to microfiltration, and 0.05 μm, corresponding to nanofiltration. It is expected that by reducing the pore size in the filtration, this will simulate better the PTEs absorption processes in the intestinal phase.
Therefore, the aqueous concentrations of PTEs were obtained after nanofiltration of the extracts by using Amicon ultrafilters (Amicon Ultra-15 10K, Millipore, MA) containing porous cellulose membranes with a pore size of 0.05 μm.
Statistical analysis
All determinations were made in triplicate. To evaluate the variability of the results in the quantifications of the total contents and in the extractions performed, the coefficients of variation (CV) were calculated from the averages and standard deviations for each sample. A CV of less than 10% was accepted. The Pearson correlation analysis was performed by comparing the results of the physical and chemical parameters, with the total contents of PTE in soil particles of less than < 250 μm and in the gastric phase BA extracts. The correlation coefficient values were compared with those found in tables with a significance of 1 and 5 %, with n-2 degrees of freedom (Rollinson 1993). Significance values greater than those found in tables for 1 % were considered as strongly correlated.
Comparisons between both BA methods were performed using linear regressions.
RESULTS AND DISCUSSION
Soils properties and total contents of major elements and PTEs
Table I shows the characterization results of all analyzed samples: pH, electrical conductivity (EC), total contents of major elements and PTEs analyzed.
TABLE I PHYSICAL AND CHEMICAL PROPERTIES OF SOILS AND TOTAL CONCENTRATION OF MAJOR ELEMENTS AND OF POTENTIAL TOXIC ELEMENTS (PTEs), SEPARATED BY LOCATION
| Samples | Total Concentration | ||||||||||
| mg/kg | |||||||||||
| pH | Electrical Conductivity (µS/cm) |
Fe (%) | Ca (%) | Mn (%) | Tl | As | Pb | Cu | Zn | Cd | |
| Soils from the metallurgical site. From SLP | |||||||||||
| SLP01 | 6.81 | 710 | 1.98 | 0.511 | 0.054 | 4.12 | 869 | 1.06×103 | 926 | 6.74×103 | 167 |
| SLP02 | 4.82 | 1.00×103 | 3.91 | 0.512 | 0.052 | 4.22 | 1.06×104 | 1.11×104 | 1.98×103 | 3.18×103 | 264 |
| SLP03 | 5.73 | 310 | 6.90 | 0.690 | 0.042 | 1.56 | 7.34×103 | 1.17×104 | 2.96×104 | 9.18×103 | 406 |
| SLP04 | 5.31 | 1.7×103 | 3.54 | 1.11 | 0.134 | 3.08 | 3.69×104 | 7.98×103 | 3.43×103 | 5.68×103 | 594 |
| SLP05 | 6.60 | 290 | 5.37 | 1.38 | 0.060 | 2.83 | 1.24×104 | 1.08×104 | 1.97×104 | 9.57×103 | 1.31×103 |
| SLP06 | 10.1 | 2.00×104 | 10.1 | 2.83 | 0.520 | 11.9 | 3.29×104 | 1.88×104 | 9.69×103 | 4.60×104 | 2.26×103 |
| SLP07 | 6.22 | 2.40×103 | 12.0 | 1.40 | 0.180 | 3.25 | 1.62×103 | 5.14×103 | 2.61×104 | 1.74×103 | 3.26×103 |
| SLP08 | 6.81 | 200 | 2.37 | 0.612 | 0.130 | 7.11 | 1.64×103 | 6.04×103 | 7.95×103 | 1.85×104 | 98.0 |
| SLP09 | 6.01 | 5.40×103 | 8.00 | 0.791 | 0.026 | 46.7 | 6.00×105 | 2.12×105 | 4.87×104 | 2.66×105 | 3.93×104 |
| Soils from mine waste sites (TX) | |||||||||||
| “La Concha” tailings impoundment (brown- oxidized tailings) | |||||||||||
| TXP1a | 6.20 | 119 | 19.6 | 8.32 | 1.41 | 1.46 | 2.29×103 | 2.00×104 | 864 | 3.94×104 | 274 |
| TXP1b | 6.30 | 83.1 | 21.7 | 8.41 | 1.34 | 1.68 | 2.38×103 | 2.22×104 | 1.12×103 | 4.82×104 | 435 |
| TXP1c | 6.70 | 114 | 35.7 | 7.00 | 2.32 | 1.87 | 4.10×103 | 4.05×104 | 1.96×103 | 5.80×104 | 544 |
| TXP2a | 6.90 | 61.3 | 21.0 | 10.0 | 1.28 | 1.19 | 2.03×103 | 2.04×104 | 1.23×103 | 8.28×104 | 643 |
| TXP2b | 6.70 | 88.3 | 23.3 | 11.1 | 1.38 | 1.38 | 2.65×103 | 2.85×104 | 1.56×103 | 7.54×104 | 793 |
| TXP2c | 6.80 | 162 | 18.4 | 7.30 | 1.15 | 1.41 | 2.28×103 | 2.84×104 | 1.33×103 | 7.21×104 | 557 |
| “El Fraile” tailings impoundment (varied tailings) | |||||||||||
| TXP3a | 7.00 | 156 | 2.60 | 9.22 | 0.021 | 0.400 | 122 | 147 | 59.0 | 212 | < D.L |
| TXP3b | 6.50 | 965 | 8.40 | 2.38 | 0.022 | 1.34 | 2.03×103 | 3.45×103 | 160 | 885 | < D.L |
| TXP3c | 4.40 | 1.90×103 | 4.96 | 2.69 | 0.075 | 0.750 | 919 | 1.93×103 | 989 | 4.62×103 | 41 |
| TXP4a | 7.00 | 145 | 3.00 | 7.13 | 0.050 | 0.490 | 193 | 388 | 101 | 625 | < D.L |
| TXP4b | 4.70 | 317 | 7.60 | 0.490 | 0.042 | 0.840 | 1.22×103 | 2.08×103 | 100 | 742 | < D.L |
| TXP4c | 5.40 | 1.13×103 | 8.80 | 1.92 | 0.640 | 0.690 | 1.50×103 | 1.90×103 | 317 | 1.58×104 | 131 |
| “Foster” tailings impoundment (gray- fresh reduced tailings) | |||||||||||
| TXP5a | 6.30 | 834 | 15.0 | 10.3 | 0.860 | 1.10 | 843 | 588 | 112 | 4.71×103 | 45.0 |
| TXP5b | 6.30 | 988 | 15.6 | 10.2 | 0.890 | 1.00 | 925 | 524 | 136 | 4.21×103 | 57.0 |
| TXP5c | 6.40 | 911 | 20.6 | 8.12 | 1.00 | 1.29 | 795 | 350 | 168 | 7.66×103 | 101 |
| TXP6a | 6.50 | 600 | 20.0 | 9.26 | 0.930 | 1.20 | 842 | 313 | 87 | 2.80×103 | 36.0 |
| TXP6b | 6.50 | 627 | 16.3 | 10.4 | 0.942 | 1.20 | 660 | 314 | 118 | 2.56×103 | 38.0 |
| TXP6c | 6.60 | 523 | 18.3 | 10.8 | 1.04 | 1.30 | 956 | 385 | 124 | 3.09×103 | 43.0 |
| Soil samples near “La Concha” impoundment | |||||||||||
| TXSC1 | 6.10 | 138 | 14.3 | 6.14 | 0.63 | 0.740 | 2.18×103 | 1.01×104 | 537 | 3.84×104 | 333 |
| TXSC2 | 6.40 | 78.4 | 3.51 | 4.09 | 0.08 | 0.810 | 187 | 1.29×103 | 111 | 3.84×103 | 30.0 |
| Soil samples near “El Fraile” impoundment | |||||||||||
| TXSF1 | 6.60 | 46.4 | 15.4 | 0.602 | 0.300 | 0.500 | 1.88×103 | 4.21×103 | 344 | 1.47×103 | 20.0 |
| TXSF2 | 6.62 | 62.6 | 7.85 | 1.77 | 0.072 | 0.480 | 899 | 5.56×103 | 92.0 | 1.35×103 | < D.L |
< D.L Below detection limit
In the soils of SLP, variable pH values between 4.82 and 10.1 were found. Sample SLP06 showed a high pH value (= 10.1) and EC value (2.00×104 μS/cm), suggesting contamination by alkaline residues. The EC interval was also very variable, from 200 to 2.00×104 μS/cm, values belonging to SLP08 and SLP06, respectively. However, the pH of the sample with the next highest EC value of 5.40×103, was only 6.01 (SLP09). Therefore, if the EC is better indicative of external contamination by waste, it is clear that not all residues necessarily contain alkaline components.
Taxco (TX) samples yielded an average pH of 6.40 ± 0.66 (n = 22) considering the soils and tailings together. The highest pH values were found in TXP3a and TXP4a with a value of 7.00 for both. The lowest value was registered for TXP3c with a pH of 4.40, suggesting some acid mine drainage (AMD) formation.
All samples of tailings and soils from “La Concha” (brown = oxidized) showed near-neutral pH values (6.10-6.90), which suggests the presence of acid-neutralizing minerals (Romero and Gutiérrez 2010). They all showed low EC, in a range of 83.0-162 μS/cm, which indicates that the sulfates of soluble metals formed during oxidation of sulfides in tailings have been leached out of the system.
In general, when descending in the profiles of “El Fraile” mine tailings, the pH begins at neutral values and becomes rapidly more acidic and the EC increases, indicating that AMD is being formed but is transported downwards. Somehow, neutralization is happening only at the surface and not in deeper horizons, but it is not clear what the reason or mechanism is.
The soils adjacent to the “El Fraile” tailings impoundment had pH values close to neutral (pH = 6.60 and 6.20) and the EC was lower than the tailings samples (46.0 and 62.0 μs/cm).
In the “Foster” tailings impoundment, the average pH of the samples was very close to neutral, ~ 6.40. The electrical conductivity of the samples was found in a range between 600 and 988 μS/cm.
The total Fe contents in the two locations are quite high, but considerably higher for TX than for SLP (15.5 ± 8.15 % and 5.37 ± 3.50 % respectively). In the case of TX, the values in tailings are much higher (more than double on average) than in soils, which is a reflection of the high initial content of pyrite (FeS2) in waste tailings from mines. In TX soils, the values are also greater than those of SLP, probably from contamination from tailings.
The contents of total Ca in TX (7.71 ± 3.59 %) are seven times higher than in SLP (0.79 ± 0.74 %) suggesting higher calcite and/or gypsum contents in TX. Total Mn in TX (0.875 ± 0.616 %) was also sixteen times higher than in SLP (0.06 ± 0.15 %).
The amorphous minerals of Mn(III) and (IV) provide active sites on their surfaces and internal structures on which PTEs cations can be sorbed (Villalobos et al. 2005, Peacock and Moon 2012, Villalobos et al. 2014, Voegelin et al. 2015, Atkins et al. 2016, Wick et al. 2018). A high correlation was found between the total contents of Tl and Mn (r = 0.89, p < 0.01, not considering the sample SLP09), suggesting a strong association between these metals (Cruz-Hernández et al. 2018). The rest of PTEs did not show correlations with Mn.
A very important correlation between total Mn and Fe was found in TX soils (r = 0.94, p < 0.01), which reveals their common origin.
The Mexican standard (NOM-147-SEMARNAT/SSA1-2004) (SEMARNAT 2007) defines as limits of total Tl for non-contaminated soils a value below 5.20 mg/kg for residential and agricultural areas, and a value below 67.0 mg/kg for industrial use; for As 22.0 mg/kg and 260 mg/kg, respectively; for Pb 400 mg/kg and 800 mg/kg, respectively; and for Cd 37.0 mg/kg and 450 mg/kg, respectively.
The samples collected have higher concentrations of the regulated elements As, Cd and Pb. In the case of Tl, the reported values are below the norm. There are other standards such as Decree 18/2015 of the Junta de Andalucía in Spain that set lower limits: lower than 0.23 mg/kg for residential and agricultural areas, and a value lower than 2.30 mg/kg for industrial use in other areas.
Mineralogical characterization
The results derived from the XRD analysis are shown in Table SII.
In SLP the mineralogical composition is highly variable. There are major components of most of the primary mineral such as quartz, feldspar and plagioclase and minor secondary iron oxide minerals such as hematite and goethite in samples SLP01, SLP02 and SLP08. In samples SLP03, SLP04 and SLP07 the presence of mica and secondary minerals (neoformed or not) such as gypsum and jarosite as minor components is noteworthy (Cruz-Hernández et al. 2018).
In TX the mineralogical composition through all profiles is quite similar and consists of primary minerals of iron such as pyrite. In addition, there is quartz and pyroxene of diopside type. As secondary minerals it contains gypsum and calcite. On the one hand the presence of gypsum is a signal of a certain oxidation degree, but the presence of remnant calcite ensures neutralization of the protons formed during sulfide oxidation, which in turn slows down the oxidative process. The semi-quantitative analysis also shows high mineral contents of the micas, and potassic feldspars groups as well as crystalline iron oxides such as goethite. On the other hand, samples without pyrite contained jarosite. Mica was identified in all samples. In soils of TX, quartz was found as the predominant mineral. In general, jarosite, calcite, feldspar and mica are the most abundant mineral phases present.
Determination of the BA fraction
Comparison of filtration methods
To compare the two filtration methods, four soil and tailing samples were selected to perform the single extraction, filtering through each of the two membranes. The results obtained are shown in figure 2.
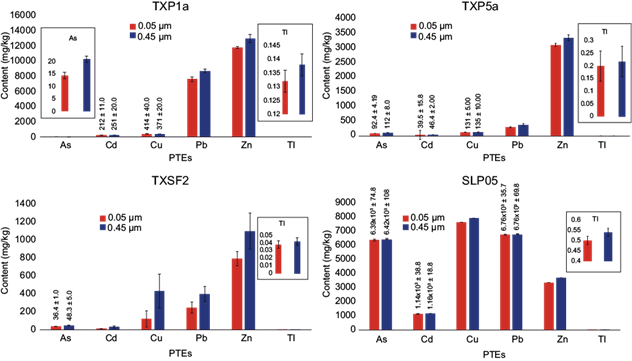
Fig. 2 Potential toxic elements (PTEs) contents after a simple extraction with hydrochloric acid (HCl) at pH 1.5 for one hour with filtration through two membranes of different pore size: 0.05 μm and 0.45 μm. Error bars indicate standard error
Although for some elements no significant differences were found between the two filtering methods (p < 0.05), in many others the differences were highly significant (Fig. 2). Two very relevant observations can be made for all cases of significant differences: (1) The contents measured in the filtrates through 0.45 μm pore membranes were always higher than those through 0.05 μm pores. This suggests that colloids smaller than 450 nm that can pass through the first membrane contribute in the measurement by ICP-AES of the total “dissolved” element in a significant way, and (2) The standard deviation of the data with the membrane of 0.45 μm was always considerably higher than that with the membrane of 0.05 μm, which suggests that the presence of these suspended colloids causes greater variability in the measurements by this technique, as compared to the almost purely dissolved phase from the filtrate through the 0.05 μm pore.
These results justified the widespread use of the 0.05 μm pore membranes in all subsequent BA extractions.
In this study, the use of dialysis membranes with a considerably smaller pore size and closer to that expected in intestinal tissues was also evaluated. However, the use of this type of filtration requires a longer time for complete ion diffusion across the membrane (3.5 hours), which clearly surpasses the one hour established of the extraction method. In the digestion process the dissolution status of the minerals present is far from an equilibrium state at the imposed pH of 1.5 and one hour, in which it may be, in fact, in a state of fast dissolution kinetics. For this reason, maintaining the same exact extraction time is crucial for obtaining reproducible results. Therefore, the dialysis membrane filtration method could not be applied and the use of 0.05 μm membranes was decided to be sufficient to have the best possible separation of the dissolved phase.
Gastric BA of PTEs
General aspects
Table II shows the gastric BA contents of PTEs by the two methods studied.
TABLE II GASTRIC BIOACCESSIBILITY POTENTIAL TOXIC ELEMENTS CONTENTS (mg/kg) BY THE SOLUBILITY AND BIOAVAILABILITY RESEARCH CONSORTIUM (SBRC) METHOD (WITH GLYCINE + HYDROCHLORIC ACID) AND BY SIMPLE HYDROCHLORIC ACID EXTRACTIONS, BOTH AT pH = 1.5 ± 0.1. THE POTENTIAL TOXIC ELEMENTS BIOACCESSIBILITY PERCENTAGES ARE SHOWN IN PARENTHESES
| Samples | Tl | As | Pb | Cu | Zn | Cd | |||||||||||
| SBRC-G | HCL-G | SBRC-G | HCl-G | SBRC-G | HCL-G | SBRC-G | HCl-G | SBRC-G | HCl-G | SBRC-G | HCl-G | ||||||
| SLP01 | 0.250(6.07) | 0.150(3.64) | 527(60.6) | 432(49.7) | 788(74.5) | 572(54.1) | 591(63.8) | 517(55.9) | 4.65×103(68.9) | 3.59×103(53.3) | 176(106) | 157(94.3) | |||||
| SLP02 | 1.00(23.7) | 0.700(16.6) | 2.01×103(19.3) | 1.57×103 (14.8) | 4.20×103(37.9) | 3.06×103(27.6) | 521(26.4) | 419(21.2) | 313(9.80) | 333(10.5) | 102(38.6) | 85.0(32.3) | |||||
| SLP03 | 0.120(7.69) | 0.100(6.41) | 3.73×103(50.8) | 2.93×103(40.0) | 9.80×103(84.1) | 8.04×103(69.0) | 5.62×103(19.0) | 4.58×103(15.5) | 1.06×103(11.5) | 973(10.6) | 253(62.5) | 216(53.2) | |||||
| SLP04 | 1.19(38.6) | 1.16(37.7) | 1.70×104(46.0) | 1.43×104(38.9) | 6.59×103(82.5) | 4.40×103(65.1) | 1.08×103(31.5) | 1.07×103(31.1) | 1.90×103(33.3) | 1.87×103(33.0) | 364(61.3) | 326(54.9) | |||||
| SLP05 | 0.620(21.9) | 0.490(17.3) | 7.29×103(58.8) | 6.01×103(48.5) | 8.51×103(78.6) | 6.35×103(58.6) | 9.05×103(45.9) | 7.44×103(37.7) | 4.48×103(46.8) | 3.45×103(36.0) | 1.17×103(89.3) | 1.10×103(85.0) | |||||
| SLP06 | 6.40(53.8) | 7.20(60.5) | 1.51×104(29.1) | 2.17×104(41.7) | 2.07×104(63.0) | 1.64×104(49.7) | 8.95×103(39.2) | 9.15×103(40.1) | 5.17×104(45.5) | 5.00×104(43.9) | 1.68×103(100) | 1.69×103(101) | |||||
| SLP07 | 2.65(81.5) | 2.70(83.1) | 9.53×103(47.7) | 8.57×103(43.0) | 1.19×104(63.5) | 4.26×103(22.7) | 1.30×104(29.3) | 1.25×104(28.1) | 1.29×104(39.1) | 1.36×103(41.0) | 2.74×103(105) | 2.90×103(111) | |||||
| SLP08 | 5.20(73.1) | 4.60(64.7) | 1.16×103(70.5) | 1.10×103(67.4) | 2.66×103(88.8) | 2.63×103(87.8) | 2.80×103(46.3) | 2.62×103(43.4) | 976(52.8) | 952(51.4) | 86.0(87.1) | 94.0(95.9) | |||||
| SLP09 | 40.5(86.7) | 40.0(85.6) | 1.36×104(22.7) | 1.31×104(21.8) | 5.15×104(24.3) | 2.09×104(9.80) | 1.91×103(3.90) | 1.39×103(2.90) | 5.23×104(19.7) | 5.44×104(20.5) | 3.07×104(78.1) | 3.14×104(80.0) | |||||
| TXP1a* | 0.140(8.33) | 0.129(8.84) | 11.0(0.50) | 5.00(0.20) | 7.73×103(38.7) | 7.16×103(35.8) | 221(25.6) | 204(23.6) | 2.08×104(52.8) | 2.02×104(51.3) | 215(77.6) | 313(78.3) | |||||
| TXP1b | 0.150(8.02) | 0.147(8.75) | 7.00(0.30) | < D.L | 9.01×103(40.5) | 8.33×103(37.5) | 261(23.3) | 242(19.6) | 1.41×104(29.3) | 1.42×104(29.5) | 294(67.6) | 292(67.0) | |||||
| TXP1c | 0.250(21.0) | 0.274(14.7) | 7.00(0.20) | < D.L | 1.58×104(38.9) | 1.55×104(38.4) | 420(21.4) | 384(31.3) | 1.50×104(25.9) | 1.51×104(26.1) | 372(68.5) | 377(69.3) | |||||
| TXP2a | 0.100(7.25) | 0.106(8.91) | 6.00(0.30) | 2.00(0.10) | 9.05×103(44.4) | 8.57×103(42.0) | 415(33.7) | 385(28.9) | 4.43×104(53.5) | 4.20×104(50.7) | 506(78.7) | 511(79.4) | |||||
| TXP2b | 0.170(12.1) | 0.179(13.0) | 5.00(0.20) | < D.L | 1.25×104(43.9) | 1.21×104(42.3) | 495(31.7) | 451(28.9) | 1.70×104(22.5) | 1.74×104(23.1) | 593(74.8) | 605(76.3) | |||||
| TXP2c | 0.220(15.6) | 0.165(11.7) | 6.00(0.30) | < D.L | 1.24×104(43.7) | 1.24×104(43.7) | 463(34.8) | 439(33.0) | 1.74×104(24.1) | 1.78×104(24.7) | 536(96.4) | 561(101) | |||||
| TXP3a | 0.080(20.0) | 0.090(22.5) | 13.0(10.8) | 8.00(6.40) | 21.0(14.3) | 12.0(8.16) | 12.0(20.3) | 13.0(22.0) | 170(80.4) | 79.0(37.2) | < D.L | < D.L | |||||
| TXP3b | 0.030(2.23) | 0.028(2.09) | 85.0(4.20) | 54.0(2.70) | 17.0(0.49) | 12.0(0.35) | 3.00(1.88) | 7.00(4.38) | 46.0(5.20) | 42.0(4.70) | < D.L | < D.L | |||||
| TXP3c | 0.090(12.0) | 0.079(10.5) | 92.0(10.0) | 78.0(8.50) | 857(44.4) | 451(23.4) | 410(41.5) | 378(38.2) | 154(3.30) | 131(2.80) | < D.L | < D.L | |||||
| TXP4a | 0.070(14.3) | 0.061(12.5) | 18.0(9.20) | 17.0(8.80) | 34.0(8.76) | 31.0(8.00) | 19.0(18.8) | 16.0(15.8) | 314(50.3) | 267(42.8) | < D.L | < D.L | |||||
| TXP4b | 0.180(21.4) | 0.189(22.5) | 3.00(0.20) | < D.L | 67.0(3.22) | 51.0(2.45) | 5.00(5.00) | 4.00(4.00) | 79.0(10.6) | 63.0(8.50) | < D.L | < D.L | |||||
| TXP4c | 0.320(46.4) | 0.351(50.9) | 95.0(11.0) | 89.0(5.90) | 1.10×103(57.9) | 1.07×103(56.3) | 141(44.5) | 138(43.5) | 7.34×103(46.4) | 7.33×103(46.3) | 85.0(64.3) | 84.0(61.9) | |||||
| TXP5a | 0.200(18.2) | 0.204(18.6) | 33.0(3.90) | 13.0(1.50) | 216(36.7) | 103(17.5) | 75.0(67.0) | 63.0(56.3) | 2.98×103(63.4) | 2.57×103(54.5) | 40.0(88.3) | 36.0(80.0) | |||||
| TXP5b | 0.200(20.0) | 0.216(21.6) | 54.0(8.00) | 19.0(2.10) | 236(45.0) | 144(27.5) | 72.0(52.9) | 64.0(47.1) | 2.57×103(61.1) | 2.50×103(69.3) | 35.0(61.7) | 35.0(61.7) | |||||
| TXP5c | 0.220(17.1) | 0.225(17.4) | 50.0(6.20) | 13.0(1.60) | 260(74.3) | 176(50.3) | 37.0(22.0) | 24.0(14.3) | 1.07×103(13.9) | 751(9.80) | 14.0(14.2) | 10.0(9.80) | |||||
| TXP6a | 0.140(11.7) | 0.134(11.2) | 23.0(2.70) | 15.0(1.80) | 102(32.6) | 56(17.9) | 41.0(47.1) | 46.0(52.9) | 1.69×103(60.4) | 1.29×103(46.1) | 21.0(58.3) | 16.0(44.4) | |||||
| TXP6b | 0.340(32.1) | 0.324(23.3) | 30.0(3.10) | 25.0(2.60) | 209(66.6) | 203(64.7) | 46.0(39.0) | 45.0(38.1) | 1.56×103(50.6) | 1.44×103(46.7) | 23.0(60.5) | 21.0(55.3) | |||||
| TXP6c | 0.360(27.7) | 0.335(22.4) | 157(23.8) | 97.0(14.6) | 238(61.8) | 238(61.8) | 26.0(21.0) | 24.0(19.4) | 630(24.6) | 524(20.8) | 8.00(18.6) | 7.00(16.3) | |||||
| TXSC1 | 0.170(23.0) | 0.164(22.2) | 94.0(4.30) | 36.0(1.16) | 4.41×103(43.7) | 3.46×103(34.3) | 166(31.0) | 158(29.4) | 2.06×104(53.5) | 1.05×104(27.3) | 234(71.2) | 213(64.0) | |||||
| TXSC2 | 0.165(20.4) | 0.166(20.5) | 27.0(14.5) | 21.0(11.1) | 1.34×103(104) | 1.16×103(90.0) | 328(28.8) | 35.0(31.5) | 3.09×103(80.7) | 2.68×103(70.0) | 32.0(107) | 32.0(107) | |||||
| TXSF1 | 0.020(4.00) | 0.014(2.80) | 3.00(0.20) | < D.L | 581(13.8) | 478(11.4) | 75.0(21.8) | 68.0(19.8) | 413(28.2) | 351(23.9) | 12.0(60.0) | 12.0(60.0) | |||||
| TXSF2 | 0.050(10.4) | 0.038(7.92) | 21.0(2.30) | 23.0(2.60) | 165(3.00) | 239(4.30) | 12.0(13.0) | 14.0(15.2) | 578(42.9) | 551(40.9) | < D.L | < D.L | |||||
*In samples TXP1 to TXP6, a, b, c refer to the progressively higher depths of the vertical profiles sampled (cf. Table SI).
SLP samples showed the highest contents of extractable PTEs. This is because the metallurgical treatment usually consists of aggressive oxidation processes, which makes PTEs concentrated in the residues more mobile and soluble (Cruz-Hernández et al. 2018).
In the TX mining area the tailings contain PTEs in various stages of oxidation, from more reduced to more oxidized forms. However, a high degree of oxidation is expected in the soils of this zone, but a lower percentage of BA is observed for As (Table II) when comparing the results of both zones (SLP and TX).
BA contents of As were lower in the samples of oxidized tailings (brown, TXP1 and TXP2) than in the samples of reduced tailings (gray, TXP5 and TXP6) (Table II). This is due to the presence of secondary minerals, such as jarosites that can retain As in their structures, and which were detected by XRD (Table SII). This is the reason why the % BA of As in the gastric phase is low compared to the other PTEs (Table II). Therefore, it would be worth investigating this speciation with more mineralogical detail in future studies. In contrast, in SLP soils the BA values reach up to over 60 %, which is indicative of an As mineralization very different from that of the tailings and soil samples from TX. Most samples of SLP contain arsenolite (As2O3), a very soluble mineral of As(III) (Koch et al. 2011).
Unlike As, the BA contents of Cd are higher in oxidized tailings and soils (67.6 to 96.4 %) than in reduced tailings (14.2 to 88.3 %). The high BA values reflect that Cd is not strongly bound to minerals or that it forms highly soluble minerals. The acidic conditions of the gastric phase produce the release of As, Cd, Cu, Zn, Tl and Pb (Juhasz et al. 2010) due to the dissolution of the mineral phases present in addition to the formation of stable complexes. The chlorides from the HCl extractant play an important role in the formation of cadmium chloride complexes, which make it more soluble and mobile (EEA 2014). In reduced tailings where sulfides prevail, Cd mobility from CdS formation is expected to be much lower than in their oxidized (sulfated) counterparts.
Copper in soils usually is strongly but reversibly bound to organic matter (Violante et al. 2010). The samples analyzed have total Cu contents that range between 59.0 ppm and 4.87×104 ppm (Table I), with the soils of the metallurgical zone having the highest contents, followed by oxidized tailings > soils in the mining area > reduced tailings. Cu forms very stable complexes in the presence of organics, which promote high BA values. The calculated bioaccessible contents in the samples ranged between 5 % and 75 %, depending on the characteristics of the sample. For example, SLP09 shows that although it has a high total copper content, 4.87×104 mg/kg, its high BA value of 1.91×103 mg/kg represents only 3.90 %.
Gastric BA values of Pb in TX are greater in reduced tailings (gray - TXP5, TXP6) than in oxidized tailings (brown - TXP1, TXP2), in contrast to Cd, although the total content of Pb in gray tailings is lower than in oxidized tailings. It is note worthy that gray tailings contain much higher content of anglesite (PbSO4), where Pb is more BA than in other Pb minerals, including Pb arsenates like mimetite, which are found in similar proportions in both types of tailings (Palumbo-Roe et al. 2013) (Table SII).
In summary, the BA contents of Pb in the soil samples vary greatly from one zone to another, indicating a wide range of Pb minerals or Pb associations with variable extractability behavior at low pH. Pb carbonates and sulfates may be very soluble, while arsenates may not (Vaca-Escobar and Villalobos 2005).
Smithsonite (ZnCO3) is the main zinc mineral species in the tailings (Table SII), which is very soluble in the extraction conditions and contributes highly to Zn BA. Oxidized tailings have the highest total zinc content. The bioaccessible percentage varied from 5.00 % to 70.0 %, indicating again a wide range of BA in the different Zn minerals, however a trend is observed in that the surface samples have the highest BA contents. According to the observed results, in the TXP3 and TXP4 (varied tailings) vertical profiles, the BA contents in the b horizon are lower than in the a and c horizons. Whereas a higher BA content is found in the c horizon of the reduced tailing (a, b, and c indicating progressively deeper horizons - cf. Table SII).
Thallium (Tl), although found in much lower content than all the PTEs analyzed, is the most toxic inorganic pollutant regulated in soil and water (Peter and Viraraghavan 2005, Rodriguez-Mercado and Altamirano-Lozano 2013). The BA percentages of Tl vary between 4.00 and 86.7 %, the SLP zone showing the highest BA contents of Tl, evidently highly correlated to the Mn BA content (Fig. SI). Previous studies on this metallurgical zone (Cruz-Hernández et al. 2018) showed evidences of Tl bonding with poorly-crystalline manganese oxides. The considerably high BA Mn contents is surprising, considering Mn(III/IV) oxides cannot dissolve, except through a reductive dissolution mechanism. This suggests that HCl may be acting as a mild reducing agent (Table SIII), because using HNO3 instead yields much lower extracted Mn (Cruz-Hernández et al. 2018).
In the case of TX samples, the percentages of Tl BA may be attributed to carbonate binding (Table SII), where Tl(I) can be coprecipitated in the mineral calcite (Cruz-Hernández et al. 2018). Also, weakly sorbed Tl species may be present.
It is difficult to compare these Tl data with other studies, due to the different extraction protocols used, the different types of soils and the different particle sizes used, in addition to the non-existence in the literature of Tl BA data in contaminated soils from mining waste.
Finally, in general, the contents extracted with HCl alone (pH 1.5) are very close to those obtained using the glycine (SBRC) method. Although for some samples and elements the contents obtained with the glycine method are slightly higher, as will be described below.
Correlations between extraction methods
To evaluate whether the simple extraction method with HCl is an adequate method to predict gastric-BA as dictated by the SBRC test, the degree of linear correlation between both extraction results was determined for each element investigated, and the values of the slopes obtained were compared to a desirable value of 1. These correlations are presented in figure 3, but separately for SLP (Fig. 3A) and TX (Fig. 3B) since the PTEs contents of SLP are much higher and overwhelm those of TX when plotted together.
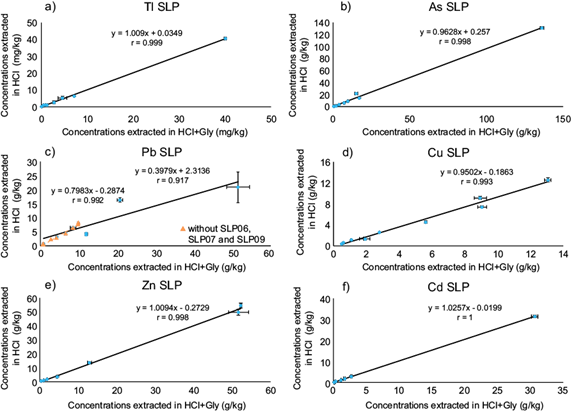
Fig. 3A Correlations obtained between extracted potential toxic elements (PTEs) for the two bioaccessibility (BA) methods evaluated in San Luis Potosí (SLP) samples, at pH 1.5: with hydrochloric acid (HCl) alone and with hydrochloric acid + Glycine (HCl+Gly) (solubility and bioavailability research consortium, SBRC test). Errors bars indicate standard error
It is clear that all elements in both settings show perfect correlations, with r values above 0.99, and some even of 1, and with slopes from 0.92 to 1.03, which indicates that the SBRC test using additionally glycine can be replaced with full confidence by the simpler HCl method. With two notable exceptions: Pb in SLP samples (Fig. 3Ac), and As in TX mine tailings (Fig. 3Bh).
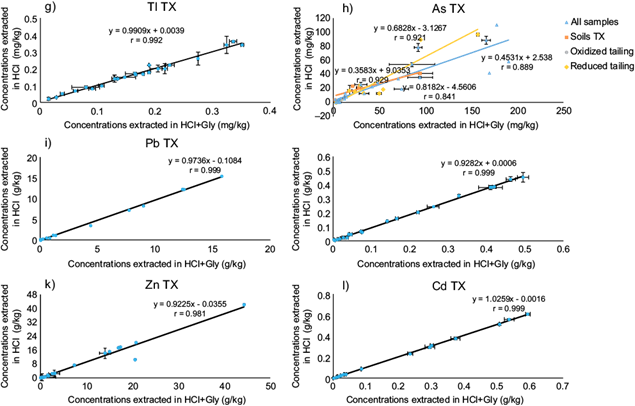
Fig. 3B Correlations obtained between extracted potential toxic elements (PTEs) for the two bioaccessibility (BA) methods evaluated in Taxco (TX) samples, at pH 1.5: with hydrochloric acid alone and with hydrochloric acid + Glycine (HCl+Gly) (solubility and bioavailability research consortium, SBRC test). Errors bars indicate standard error
In contrast, Pb in TX samples yielded an equally perfect correlation as the other PTEs with an r = 1.0, p ˂ 0.01 (Fig. 3Bi), as did As in SLP (Fig. 3Ab). In the Pb case in SLP, samples were plotted separately, excluding SLP 06, 07 and 09 (shown in triangles Fig. 3Ac), because of their higher EC values and total Fe contents as compared to the other samples (Table I). The remaining samples showed a perfect correlation between BA extraction methods (r = 0.99, p ˂ 0.01), although their slope was lower than 1 (0.8), which denotes a higher extractability of the combination of glycine and HCl (SBCR test) as compared to the HCl alone, and suggests Pb minerals have a higher solubility when glycine is present. Nevertheless, in those cases, when the correlation is so high, it is still unnecessary to include glycine in the BA test, and the correlation found may be used to convert to actual (SBCR) BA values.
In the SLP samples that did not yield correlation for Pb, we observed that they all contained anglesite (PbSO4), especially SLP09 and 06 (Table SII), in higher concentrations than all others, including those of TX. Therefore, we tested if indeed anglesite yielded a different extraction capacity in the presence of glycine by applying the same procedure as in the real samples, but using only pure anglesite. Indeed, glycine+HCl dissolved a considerably higher concentration of anglesite (6.20×104 mg/kg) than HCl alone (1.40×104 mg/kg) under the same extraction conditions.
In the case of As in TX mine tailings, the explanation for the lack of correlation between the two BA extraction methods was not that clear. The results have been separated according to the characteristics of the samples: in oxidized tailings, reduced tailings, and soils, in order to evaluate whether the correlations improved. Only slight improvements were obtained for the soil samples (r = 0.93, p ˂ 0.01), although their slope remained considerably lower than 1, while the tailings still showed high dispersions (r = 0.84 for oxidized tailings and r = 0.92 for reduced tailings) (Fig. 3Bh). Separating oxidized (brown) from reduced (gray) tailings, the latter expected to contain higher concentrations of arsenopyrite, did not yield much improvement in the correlation. This suggests that several arsenic minerals are responsible for the variability in As extractability with and without glycine, and with the data that we produced this is not easy to unravel. A more in depth study is required, with several different As minerals, as well as adsorbed As(V) (mainly to Fe oxides), which is expected to be high at low pH, and thus not very BA. In the case of SLP, As speciation is expected to be more homogeneous, mainly as arsenolite (As2O3) (cf. previous section), which shows a very good correlation between BA methods.
In general, Cu, Zn, and Pb (in TX), show a slightly higher extractability in glycine than in HCl alone (slopes between 0.92 and 0.95). But Pb in SLP samples (with low anglesite contents), shows a much higher extractability with this ligand (slope of 0.8).
Overall, the correlations obtained in this research are considerably better than those reported in the literature in general, with the exception of the results obtained for Pb present as anglesite in high proportions, and As in various mineral phases (Santana-Silva 2016) in TX.
CONCLUSIONS
Metallurgical and mining affected environments were selected to compare the BA SBCR method that uses glycine and HCl at pH 1.5, with a method using only HCl also at pH 1.5. Very strong to perfect correlations (r from 0.98 to 1) between both methods were found for Tl, Cd(II), Cu(II), and Zn(II). Also, slopes in almost all cases were close to 1, meaning glycine does not show an evident extraction activity. For Pb extractions perfect correlations were also found but only when the solid matrix does not contain anglesite, which shows a considerably higher solubility in glycine and HCl than in HCl alone. Of these elements, Cu, Zn and Pb showed slightly higher solubilities in glycine+HCl than in HCl alone, but with no effect on the strong correlation found between methods.
Arsenic was the only problematic species that only shows perfect correlations in the metallurgical zone, where its speciation is more homogeneous due to generalized oxidation to arsenolite (As2O3). A very large variation in the As BA data was obtained for mine tailings, which must be related to a suite of mineralogical associations, in addition to arsenopyrite, abundant in fresh, unoxidized tailings. The three experimental factors imposed that were crucial to obtain such high correlations, and higher than previously reported in the literature for the suite of elements analyzed here were: a rigorous pH of 1.5 ± 0.1 and stirring time of 1 h ± 1 min, as well as the use of filtering membranes of 0.05 µm pore size. We recommend that in BA work in general, these conditions, even if chosen at different values (except the pore size of filtering membranes), should be kept as rigorous as possible. Therefore a simple use of HCl at pH 1.5 is perfectly suitable to determine gastric bioaccessibility in most important PTEs, which can be used to assess risk situations in areas contaminated by the mining and metallurgical industry.
The advantages of excluding glycine from the extraction method can be summarized as the evident lowering of costs brought about not only from not having to acquire this reagent, but also in the total amounts of HCl added and time consumed in adjusting the pH to 1.5. Glycine required final HCl concentrations between 0.3 M and 0.4 M, while in its absence final HCl concentrations were reduced to between 0.1 M and 0.03 M.











 nueva página del texto (beta)
nueva página del texto (beta)


