Introduction
Lipases (E.C. 3.1.1.3) catalyze the hydrolysis of ester bonds in long-chain triglyceride molecules by releasing fatty acids, diglycerides, monoglycerides, and glycerol (Godoy et al., 2022). Lipases are hydrolytic enzymes that catalyze the breakdown of ester bonds of water-insoluble substrates. Lipases are versatile enzymes that undergo chemical reactions such as esterification, transesterification, acidolysis, and aminolysis (Mahfoudhi et al., 2022). The active site of these enzymes generally has a catalytic triad (Lim et al., 2022). There is currently great interest in lipases because of their potential use in numerous industrial applications, including biodiesel production, food flavoring, the detergent industry, cosmetics production, the paper industry, the pharmaceutical industry, and biosensors, among others (Pohanka 2019; Vivek et al., 2022). They are currently the third largest enzyme group in terms of total enzyme market value, after protease enzymes and carbohydrases (Godoy et al., 2022). Many approaches have been used to get lipases with biotechnological applications, from computational analysis (Haman et al., 2021) to recombinant enzymes (Oliart-Ros et al., 2021) and synthetic biology (Gamboa-Melendez et al., 2018).
Lipases are produced by all organisms; however, the most studied and used in industry and biotechnology are lipases obtained from microbial sources (Adetunji & Olaniran, 2021). The biotechnological importance of microbial lipases derives from their greater range of operating conditions (pH, temperature, etc.), and they can be synthesized in less time. Furthermore, their use can be a good alternative for reducing costs in the production of lipases. Optimal growth conditions, however, must first be examined to obtain the maximum yield of microbial lipases (Barik et al., 2022; Godoy et al., 2022). In microorganisms, the production of lipases is subject to the composition of the culture medium and the fermentation conditions, such as the culture temperature, the culture pH, and the speed of agitation, among other factors (Behera et al., 2019). To maximize the production of lipases, statistical tools must be used to find the best culture conditions. Then, statistically based optimization methods, such as response surface design, allow determining which are the most important factors in enzyme production and simultaneously determine optimal response values (Behera et al., 2019; Rmili et al., 2022).
Lipases from extremophilic organisms (called extremozymes) are more useful in some biotechnological applications because of their greater stability under conditions of high temperatures, high salt concentrations, and extreme pH values (Adrio & Demain, 2014). Examples of these enzymes are thermos-alkaline (TA) lipase from Bacillus thermoleovorans ID-1 with optimal activity at 70 °C and TA lipase from Bacillus sp.; LBN2 with optimal activity at 60 °C and pH 10; TA lipase from Geotrichum candidum with enzymatic activity in a pH ranging from 5 to 12; and TA lipases from organisms such as Microbacterium sp., Bacillus sp. RSJ-1, and Acinetobacter sp. with enzyme activity at 50 °C and alkaline pH (Lajis, 2018).
Geobacillus species are metabolically diverse extremophiles, and they exhibit a wide range of commercially useful extracellular enzymes, such as α-amylases, xylanases, catalases, DNA polymerases, nucleases, β-galactosidases, hemicellulases, proteases, and lipases, principally. Recently, two bacterial strains that could produce lipases were isolated from an extreme environment, such as the geothermal waters of the “El Chichón” volcano’s crater lake (Mexico). These strains were molecularly identified as belonging to the bacterial genus Geobacillus (Ovando-Chacon et al., 2020). It is therefore necessary to optimize the production of these enzymes and to know their biochemical characteristics so that they can be used in specific biotechnological processes.
The objective of the present work was to perform an optimization of culture conditions of the strain G. stearothermophilus CHI1 and the biochemical characterization of the activity of its lipases based on temperature, pH, the presence of solvents, the presence of metal ions, and the affinity for substrate type.
Materials and methods
Microorganisms
The strain G. stearothermophilus CHI1 has been isolated, characterized, and molecularly identified as belonging to the bacterial genus Geobacillus (Ovando-Chacon et al., 2020). The strain was maintained in Luria-Bertani modified medium (LBm) (Sodium Chloride, 0.5% m/v; peptone, 0.5% m/v; yeast extract, 0.3% m/v; pH 6.5) at 60 °C. The strain was designated as CHI1 and incorporated into ITTG culture collection.
Lipase activity
Lipolytic activity was determined by the quantification of p-nitrophenol (pNP) released by enzymatic hydrolysis from the substrate p-nitrophenyl palmitate (pNPP). The pNPP production was determined from a calibration curve and the absorbance was determined at 402 nm. For this, the cell-free extract (supernatant of the bacterial growth medium centrifuged at 4000 rpm for 10 min) was used, and an emulsion was prepared from one volume of 0.79 mM pNPP dissolved in 2-propanol, with nine volumes of 0.1% w/v gum arabic dissolved in 50 mM phosphate buffer (pH 8). The enzymatic reaction was started by adding 300 µL of the cell-free extract to 2700 µL of the described emulsion and was incubated for 10 min at 60 °C. Finally, the absorbance was determined at 402 nm. A unit of enzyme activity (U) was defined as the amount of enzyme releasing 1 nmol of pNPP per minute under the assay conditions (Vorderwülbecke et al., 1992). The pNPP esters were purchased from Sigma-Aldrich Mexico. All other reagents used in this work were of analytical grade.
Optimization of physicochemical cultivation parameters
To maximize lipase production in G. stearothermophilus CHI1 cultures, an optimization design was established for the physicochemical parameters’ temperature, pH, and agitation of the culture. The design employed was a central composite response surface design, with three variables and two levels for each variable. The design matrix consisted of 16 different treatments (eight factorial points, two axial points, and six central points), which were conducted in triplicate (Table 1).
Table 1 Treatments in the central composite experimental design for optimizing lipase production from Geobacillus stearothermophilus CHI1.
| Treatment | Temperature (°C) | pH | Agitation (rpm) |
| 1 | 50 | 3 | 50 |
| 2 | 70 | 3 | 50 |
| 3 | 50 | 5 | 50 |
| 4 | 70 | 5 | 50 |
| 5 | 50 | 3 | 150 |
| 6 | 70 | 3 | 150 |
| 7 | 50 | 5 | 150 |
| 8 | 70 | 5 | 150 |
| 9 | 43.2 | 4 | 100 |
| 10 | 76.8 | 4 | 100 |
| 11 | 60 | 2.3 | 100 |
| 12 | 60 | 5.7 | 100 |
| 13 | 60 | 4 | 16 |
| 14 | 60 | 4 | 184 |
| 16 | 60 | 4 | 100 |
Source: Autor’s own elaboration.
The experimental unit was considered to be a culture of G. stearothermophilus CHI1 with 24 h of incubation under the conditions described. The response variable was lipolytic activity (U/mL), which was calculated as the average of three individual measurements for each treatment.
Biochemical characterization of lipolytic activity
Biochemical characterization evaluated the effect of different temperature ranges, pH, presence of ions and solvents, and substrate specificity on enzyme activity. Extracellular lipolytic activity of cultures with 24 h of incubation was also determined. The results of the biochemical characterization were expressed in relative enzymatic activity, which was defined as the quotient of the enzymatic activity obtained in the presence of each of the factors evaluated concerning the enzymatic activity obtained in the control sample.
Effect of temperature on lipolytic activity
Enzyme activity was determined by varying only the temperature of the enzyme assay, which ranged from 30 °C to 90 °C in 10 °C intervals. The results were expressed as relative enzyme activity (Ekinci et al., 2016).
Effect of pH on lipolytic activity
The enzyme activity was determined by varying the pH values, which ranged from pH 3 to pH 11 at intervals of 1. Different buffer solutions at a concentration of 50 mM were used for this assay: a citrate solution (pH 3-6), a phosphate solution (pH 7-8), and a carbonate solution (pH 9-11). These solutions replaced the 50 mM phosphate buffer solution of the enzymatic assay. The results were expressed as relative enzyme activity (Ekinci et al., 2016).
Effect of metallic ions on lipolytic activity
The effect of the metal ions Ba2+, Ca2+, Co2+, Cu2+, Fe3+, K+, Mn2+, and Na+, as well as the chelating agent EDTA on enzyme activity was determined. To do this, the cell-free extract was incubated at 37 °C for 15 min with each of the ions at a concentration of 10 mM; the enzyme assay was then performed for each condition. The results were expressed as relative enzyme activity (Ekinci et al., 2016).
Effect of solvents on the lipolytic activity
The effect of the acetone, chloroform, ethanol, isopropanol, and methanol on the lipolytic activity was determined. To do this, the cell-free extract was incubated at 37 °C for 15 min with each of the solvents at a final concentration of 30% (v/v), then the enzymatic assay was performed for each of the conditions. The results were expressed as relative enzyme activity (Ekinci et al., 2016).
Substrate specificity
The specificity towards pNPP esters of different acyl chain lengths (pNP octanoate, C8; pNP dodecanoate, C12; and pNP palmitate, C16) was evaluated using each of these substrates at a 0.79 mM concentration in the enzymatic assay methodology. The results were expressed as relative enzyme activity (Ekinci et al., 2016).
Statistical analysis
An analysis of variance was performed. A 95% confidence interval was used for the analysis of variance, and a p-value of less than 0.05 was considered statistically significant in the analyses. All tests were performed in triplicate. The response variable was the enzyme activity obtained as the average of three individual measurements of the treatments. Design-Expert version 11 and Statgraphics version 19 programs were used for data analysis and the generation of surface plots.
Results
Optimization of the lipase production
To maximize lipase production, a mathematical model must first be established to explain the relationship between the factors evaluated (temperature, pH, and agitation) and the response variable (enzyme activity) using a central composite experimental design. Four models were evaluated: a linear model, a linear model with two-factor interaction (2FI), a quadratic model, and a cubic model (Table 2).
Table 2 Models evaluated in the optimization of the physicochemical parameters of culture to maximize the lipase production of Geobacillus stearothermophilus CHI1.
| Model | p-value of the model | R2 | R2 adjusted |
| Linear | 0.4242 | -0.0032 | -0.2416 |
| 2FI | 0.6975 | -0.0418 | -0.2549 |
| Quadratic | < 0.0001 | 0.6563 | 0.5088 |
| Cubic | 0.1655 | 0.6824 | 0.4753 |
Source: Autor’s own elaboration.
To choose the model with the best fit for the data, the following parameters were considered: the p-value of the model, the coefficient of determination R2, and the adjusted coefficient of determination R2, which quantifies the percentage of the variability present in the data explained by the model. In this case, the quadratic model is the model that best fits the data since it shows the statistical significance and the highest determination coefficient. The following is the equation resulting from the quadratic model (equation 1):
where EA is the enzymatic activity, T is the temperature, A is the agitation, and pH of the culture medium.
The optimal conditions to maximize the enzymatic activity were 53.7 °C, pH of 5.7, and agitation of 80 rpm. The estimated response surface graph generated from the quadratic model shows the interaction between the pH and temperature factors (Figure 1). The graph shows two zones with higher enzymatic activity: the first corresponding to the interaction point between 65 °C and a pH of 2.3, where the enzymatic activity was approximately 45 U/mL; and the second corresponding to the interaction between 50 °C and a pH of 6.3, with the enzymatic activity of approximately 95 U/mL. In this work, in addition to the results obtained from the optimization of culture conditions, we found that the composition of the culture medium is not critical in the production of lipolytic enzymes in G. stearothermophilus CHI1 (data not shown).
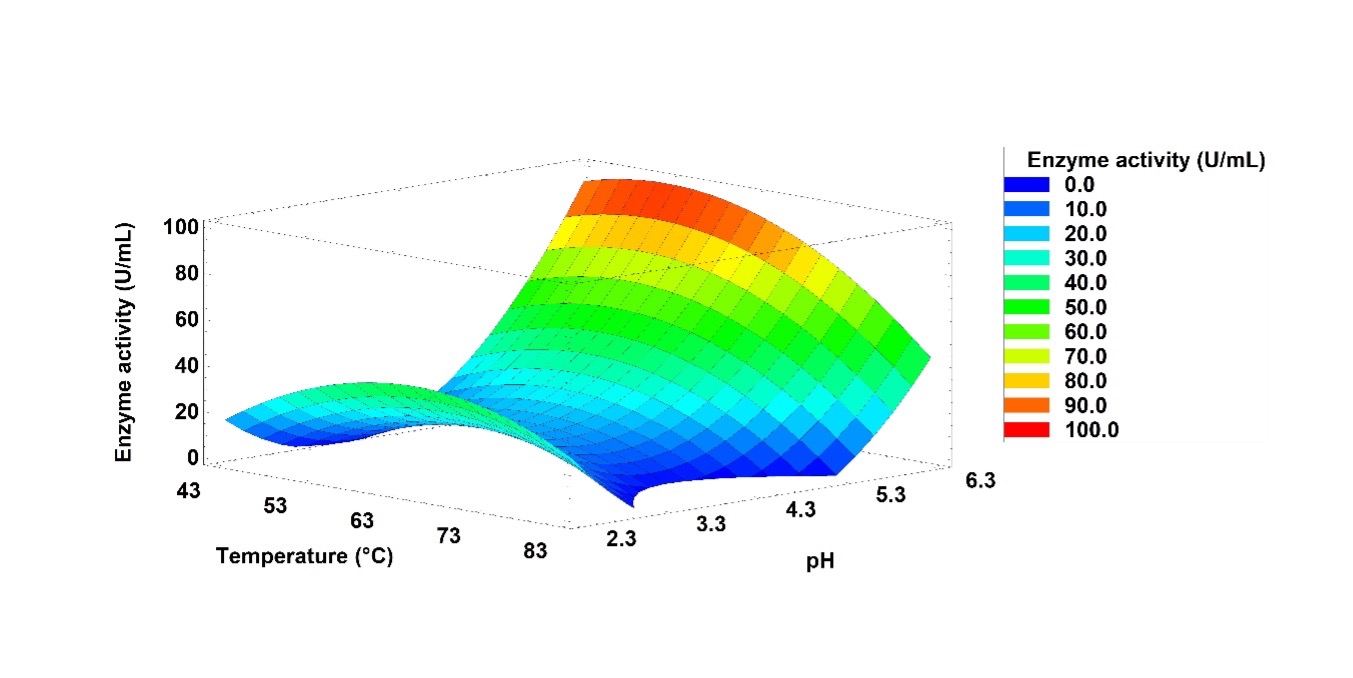
Source: Autor’s own elaboration.
Figure 1 Estimated response surface plot of the Geobacillus stearothermophilus CHI1 extracellular enzyme activity versus the temperature and pH factors. Two zones with greater activity are shown: the first zone in the interaction of 65 °C and pH 2.3 (45 U/mL), and the second zone in the interaction between 50 °C and pH 6.3 (95 U/mL).
The effect of different factors on the biochemical characteristics of the extracellular lipase activity present in G. stearothermophilus CHI1 were analized. Figure 2 shows the effect of temperature on relative enzyme activity for G. stearothermophilus CHI1. Higher relative lipolytic activity was observed at higher temperatures, with 2 points of maximum activity at 60 °C (84 U/mL) and 80 °C (85 U/mL). These results indicate the presence of thermophilic lipolytic enzymes in G. stearothermophilus CHI1. The enzymatic activity was evaluated.
Effect of pH on lipolytic activity
The effect of pH on the lipolytic activity of G. stearothermophilus CHI1 is shown in Figure 3, with higher lipolytic activity at pH values of 5 (310 U/mL), pH 9 (149 U/mL), and pH 11 (161 U/mL). These results indicate the presence of acid and alkaline lipases produced by G. stearothermophilus CHI1.
Effect of metallic ions on lipolytic activity
The effect of different metal ions at a concentration of 10 mM on the relative lipolytic activity of G. stearothermophilus CHI1 is shown in Table 3. This table shows that there is a significant decrease in enzyme activity in the presence of Na+, Mn2+, and Fe3+ ions, as well as the chelating agent EDTA and the control (absence of metallic ions). However, no improvement in enzymatic activity was present with the remaining ions: Ca2+, K+, Co2+, Cu2+, and Ba2+. The ions that most affected the activity were Mn2+ and Fe3+ ions with a 28% and 35% reduction in activity, respectively. These results suggest that the lipolytic enzymes produced by G. stearothermophilus CHI1 do not require metal cofactors for catalysis.
Table 3 Effect of metal ions on the relative lipolytic activity of Geobacillus stearothermophilus CHI1.
| Treatment | Relative lipolytic activity (%) |
| Control | 97.76 ± 2.05 |
| Na+ | 78.08 ± 4.36 |
| K+ | 92.39 ± 5.70 |
| Ca2+ | 87.25 ± 0.67 |
| Mn2+ | 70.02 ± 11.51 |
| Fe3+ | 63.76 ± 10.80 |
| Co2+ | 79.19 ± 0.68 |
| Cu2+ | 80.54 ± 1.78 |
| Ba2+ | 83.22 ± 11.47 |
| EDTA | 77.85 ± 3.08 |
Source: Autor’s own elaboration.
Effect of solvents on the lipolytic activity
The effect of the presence of solvents at a concentration of 30% (v/v) on the enzymatic activity of G. stearothermophilus CHI1 is shown in Table 4. Table 4 shows a decrease in lipolytic activity in the chloroform and methanol treatments, although it was not significant concerning the control. There was also a decrease in lipolytic activity in the presence of solvents, such as acetone, ethanol, and isopropanol of 40%, 25%, and 20%, respectively.
Table 4 Effect of different solvents on the lipolytic activity of Geobacillus stearothermophilus CHI1.
| Solvents | Relative enzyme activity (%) |
| Control | 97.49 ± 2.18 |
| Acetone | 57.86 ± 3.81 |
| Chloroform | 99.53 ± 2.36 |
| Ethanol | 73.27 ± 6.63 |
| Isopropanol | 77.36 ± 9.00 |
| Methanol | 87.74 ± 2.49 |
Source: Autor’s own elaboration.
Substrate specificity
Finally, the substrate affinity of the enzymes present in G. stearothermophilus CHI1 was evaluated using p-nitrophenol esters of different acyl chain lengths (Table 5). The highest enzymatic activity was obtained with pNP octanoate (C8), a medium-chain substrate with eight carbon atoms; the activity towards pNP dodecanoate (C12) and pNP palmitate (C16) represented 50% and 25% of the maximum activity obtained with the substrate pNP octanoate.
Table 5 Substrate affinity for the lipolytic enzymes from Geobacillus stearothermophilus CHI1.
| Substrates* | Relative enzyme activity (%) |
| pNPO-C8 | 93.67 ± 5.50 |
| pNPD-C12 | 45.80 ± 1.63 |
| pNPP-C16 | 23.23 ± 0.49 |
* pNPO-C8: p-nitrofenil-octanato; pNPD-C12: p-nitrofenil-dodecanato; p-nitrofenil hexadecanoato.
Source: Autor’s own elaboration.
The results obtained indicate the clear preference of the enzymes present in G. stearothermophilus CHI1 towards medium-chain substrates, which is characteristic of lipase enzymes and allows them to be differentiated from other enzyme groups such as esterases.
Discussion
Lipases are hydrolytic enzymes that are in high demand in industrial processes, especially those enzymes from extremophilic organisms (Adrio & Demain, 2014). The G. stearothermophilus strain CHI1, isolated from a volcanic crater lake, produce lipolytic enzymes (Ovando-Chacón et al., 2020). One of the objectives of the current work has been to optimize the culture conditions using statistical models to generate a higher production of these lipolytic enzymes. The mathematical model obtained allowed us to explain the relationship between the factors evaluated (temperature, pH, and agitation) and the response variable (enzyme activity), and to find the optimal conditions to maximize enzyme production. According to Figure 1 generated from the model, there are two zones with higher enzyme activity: one showing activity at a low pH and the other at high pH. If we also consider the results obtained in the biochemical characterization, specifically the effect of temperature and pH on enzyme activity (Figure 2 and 3), we see how this observation is presented again: concerning temperature, there are two points where the maximum enzyme activity occurs (at 60 °C and 80° C), and for pH, something similar occurs at low pH (pH 3) and alkaline pH (pH 9-11). Taken together, the results of the optimization and characterization allow us to suggest the presence of at least two enzymes with lipolytic activity produced by the G. stearothermophilus strain CHI1, one of which shows higher catalysis at acid pH (acid lipase) and one more that shows higher catalysis at alkaline pH (alkaline lipase). In both cases catalysis at high temperatures is also shown, thus, considering both enzymes as thermophilic lipases.
There are few reports of biochemical characterization where more than one enzyme with lipolytic activity is presented. This is because most of these reports are made using different enzymatic purification methods, where the main objective is the isolation of a single protein (Dako et al., 2012). During the purification process, there can be a loss of enzymatic activity and consequently a loss of protein material in each of the steps (e.g., with precipitation methods, an affinity to specific matrices, an exclusion by molecular size or charge, among others), which seriously hinders identifying more than one enzyme with lipolytic activity, as reported in the present work (Dako et al., 2012). Moreover, two studies reported the presence of two different lipase enzymes produced by the Bacillus subtilis strain 168. These enzymes, named LipA and LipB, are regulated differently, have different inducers, and possess different biochemical characteristics (Eggert et al., 2001, 2003). Within the genus Geobacillus, the presence of more than one enzyme with lipolytic activity has also been reported: a lipase enzyme and an esterase enzyme in G. thermoleovorans, with different structural and biochemical characteristics (Soliman et al., 2007).
The results presented in this paper suggest that the enzymes with lipolytic activity produced by G. stearothermophilus CHI1 could also be regulated differently and could have different inducers. This is probably the main reason why we could not establish a fixed nutrient composition in the culture medium of G. stearothermophilus CHI1, even after using different statistical models.
The second objective of the present investigation was to biochemically characterize the lipolytic enzymes produced by G. stearothermophilus CHI1. Regarding the catalysis temperature, the lipolytic enzymes produced by G. stearothermophilus CHI1 showed activity at elevated temperatures (60 °C-80 °C). For the genus Geobacillus, lipolytic enzymes have been reported with an optimum catalysis temperature, ranging from 50 °C to 70 °C, depending on the organism: for the lipase enzymes of G. stearothermophilus strain 5 (Sifour et al., 2010), G. thermoleovorans CCR11 (Castro-Ochoa et al., 2005; Quintana-Castro et al., 2009), G. thermodenitrificans AV-5 (Christopher et al., 2015), and G. thermoleovorans YN (Soliman et al., 2007), optimum temperatures between 60 °C and 65 °C were reported. For the lipases of G. stearothermophilus AH22 (Ekinci et al., 2016) and Geobacillus sp. TW1 (Li & Zhang, 2005), optimum temperatures of 50 °C were reported. And for lipase from the Geobacillus sp. strain T1 (Leow et al., 2007), an optimum catalysis temperature of 70 °C was reported. Regarding the pH of catalysis, the lipolytic enzymes produced by G. stearothermophilus CHI1 showed higher enzymatic activity at pH values of 5, pH 9, and pH 11. According to what has been reported in the literature for the genus Geobacillus, most of the studied lipases show an optimum pH of catalysis under alkaline conditions. For example, with lipase of G. stearothermophilus strain-5 (Berekaa et al., 2009), the optimum pH was 7. Furthermore, the lipase enzymes of G. stearothermophilus AH22 (Ekinci et al., 2016) and Geobacillus sp. TW1 (Li & Zhang, 2005), the optimum pH was 8. For the lipase enzymes of G. thermoleovorans CCR11 (Castro-Ochoa et al., 2005; Quintana-Castro et al., 2009), the Geobacillus sp. strain T1 (Leow et al., 2007), and G. thermodenitrificans AV-5 (Christopher et al., 2015), the optimum pH was 9. Finally, for the lipase enzyme of G. thermoleovorans YN (Soliman et al., 2007), the optimum pH was 10. Since the vast majority of lipolytic enzymes present in Geobacillus showed activity exclusively under alkaline conditions, this study of G. stearothermophilus CHI1 may be the first report of these enzymes with enzymatic activity at acidic pH.
Considering now the effect of various metal ions on enzymatic activity, G. stearothermophilus CHI1 produces lipolytic enzymes that do not require metal ions as catalytic cofactors (Table 3). Most of the Geobacillus lipolytic enzymes described in the literature are calcium-dependent as they utilize these ions as cofactors for enzymatic activity, significantly enhancing catalysis (Ekinci et al., 2016). Some examples of calcium-dependent lipolytic enzymes are the lipases of G. thermoleovorans CCR11 (Castro-Ochoa et al., 2005), which increases its activity by 60% in the presence of calcium. The lipase present in Geobacillus sp. TW1 (Li & Zhang, 2005) increases its activity by 40%, and the esterase present in Geobacillus thermoleovorans YN (Soliman et al., 2007) increases its activity by 50% in the presence of this ion. Some other divalent ions such as Co2+, Cu2+, and Ba2+ have been shown to enhance enzyme activity in lipases such as those present in the G. stearothermophilus strain AH22 (Ekinci et al., 2016). Since there was no significant improvement in catalytic activity in the presence of metal ions and there was little decrease of the enzymatic activity in the presence of the chelating agent EDTA, it is respectively suggested that the lipolytic enzymes present in G. stearothermophilus CHI1 may be metal ion-independent enzymes, also referred to as non-metalloenzymes.
Finally, regarding the effect of different organic solvents on enzymatic activity and regarding substrate specificity, the lipolytic enzymes produced by G. stearothermophilus CHI1 maintained more than 75% of their activity in the presence of chloroform, isopropanol, and methanol, and they showed greater preference towards medium-chain substrates (C8). Generally, the enzymes that are resistant to high temperatures are also resistant in the presence of organic solvents; this also applies to the lipolytic enzymes present in G. stearothermophilus CHI1, which, in addition to showing the highest activity at high temperatures, also maintains at least 60% of its enzymatic activity in the presence of the organic solvents evaluated. The stability of these enzymes in organic solvents (denoted by the presence of enzymatic activity in the conditions evaluated) suggests that they can be used in biocatalysis processes in the presence of nonaqueous solvents, such as in transesterification processes, in the synthesis of chiral compounds, and in the production of biofuels, among others (Lajis, 2018). The results of the specificity by substrate indicate a clear preference of the lipolytic enzymes produced by G. stearothermophilus CHI1 towards medium-chain substrates; these results agree with those reported for G. thermoleovorans CCR11 (Castro-Ochoa et al., 2005), G. thermoleovorans YN (Soliman et al., 2007), and Geobacillus sp. T1 (Leow et al., 2007), with a higher affinity towards medium-chain substrates (C8, C10, and C12) compared with short-chain substrates (C6, C4, and C2).
According to the biochemical characteristics present in the lipolytic enzymes produced by G. stearothermophilus CHI1, they could be used in different biotechnological processes: 1) in the detergent industry, given their activity at alkaline pH; 2) in bioconversion reactions (fatty acid hydrolysis, esterification, alcoholysis, among others) and in the cosmetic industry, given their tolerance to organic solvents; 3) in the industrial generation of polyunsaturated fatty acids due to their high activity at elevated temperatures; 4) in the industrial production of biodiesel due to tolerance to organic solvents such as methanol; and 5) in wastewater treatment due to their activity at acidic pH (Salihu & Alam, 2015; Sharma & Kanwar, 2014).
Conclusions
The enzymes evaluated in this work are characterized by having catalytic activity at elevated and acidic and alkaline pH, in the presence of solvents, independently of the presence of metal ions, and the enzymes have an affinity towards medium-chain substrates. The combination of the biochemical characteristics of these enzymes shows their potential for being used in different biotechnological processes that require high temperatures (e.g., the paper industry), the presence of solvents (e.g., biofuel production), and activity in alkaline conditions (e.g., detergent production). The results obtained in the optimization and characterization of the enzyme activity suggest the presence of more than one enzyme with lipolytic activity in G. stearothermophilus CHI1. Further studies are needed to fully elucidate the nature of the enzyme group (lipases or esterases) to which these proteins belong.
Conflict of interest
The authors declare they have no financial interests.
Author Contributions
Conceptualization, methodology, validation, formal analysis: C.O.G.H. and P.E.A.G.; software and data curation: M.A.A. and C.O.G.H.; investigation and methodology: C.O.G.H., M.A.A., L.M.C.V.C., and P.E.A.G.; resources: S.L.O.C.; writing-original draft preparation: C.O.G.H., M.A.A., L.M.C.V.C., and P.E.A.G.; supervision, project administration, funding acquisition: P.E.A.G. All authors have read and agreed to the published version of the manuscript.











 nueva página del texto (beta)
nueva página del texto (beta)




