Introduction
Grasses present high requirement of iron (Fe) in relation to other cultures, such as sugar cane (Saccharum officinarum L.), which accumulates around 9000 g ha-1 (Prado, 2008). Iron is essential for chlorophyll formation, it acts as catalyst in respiration and photosynthesis, as a factor in a few enzyme systems (Madhuri et al., 2013); besides, it increases productivity and is connected to the nutritional quality of vegetal products (Briat et al., 2007).
The absorption of Fe by sugar cane is performed by a mechanism that releases phytosiderophorus of the roots, forming chelates with the nutrient (strategy II), resulting in the absorption of Fe3+. When the nutrient is deficient in the cultivation media there is an increment in the release of phytosiderophorus, promoting an auto regulation of the responses to this stress in strategy II plants, which is less studied than in strategy I plants (Morrissey and Guerinot, 2009).
Chelates are used in order to supply Fe, such as Fe-EDDHA, which is used due to its elevated stability constant (Ks=35.4) compared to other commercialized chelates as the Fe-EDTA (Ks=26.5) and Fe-EDDS (Ks=20.1); besides, it is stable even in elevated pH (Ylivainio et al., 2004). Despite its high stability, some cultures may decrease the efficiency of Fe absorption due to the difficulty in absorbing Fe ions from the compound.
Adequate Fe concentrations in the nutrient solution associated with higher dry matter (DM) production vary according to the species: corn BSC 6661 it is 120 μM of Fe-EDDHA (Çelik et al., 2010); soybean it is 50 μM EDTA-Fe-3 (Li et al., 2011); and durum wheat it is 80 μM EDTA-Fe-3 (Zuchi et al., 2012). These Fe concentrations differ from Hoagland and Arnon (1950) standard nutrient solution (184 umol of Fe-EDDHA) for hydroponic growth of different species. For sugar cane there is a limited number of studies using nutrient solution; thus, Vale et al. (2011) and Subasinghe (2006) utilized 184 μmol L-1 of Fe-EDDHA and 0.18 mM of Fe2+, respectively, but without scientific support.
We hypothesized that sugarcane is a demanding crop Fe and requires a higher concentration of this micronutrient in the nutrient solution established for optimal crop development, as pointed out by Hoagland and Arnon (1950). And the objective was to evaluate the effect of the concentrations of Fe in the nutrient solution on growth, green color index and DM production of sugar cane.
Material and methods
The experiment was conducted in a greenhouse at the University of Agrarian and Veterinary Sciences, Jaboticabal - SP, with sugar cane (variety IAC SP 93 3046), 21° 15’ 22’’ S and 48° 18’ 58’’ W. During the experiments, day and night average temperatures were 28 °C±1.0 and 25 °C±1.0, respectively, and average humidity was 40 %.
The experimental design was completely randomized, with four treatments (concentrations) of Fe-EDDHA [chelates ethylenediamine-N,N’-bis (2-hydroxyphenylacetic acid)]: 0 (control), 184, 368 and 551.4 μmol L-1, which are 0, 1, 2 and 3 times the reference concentration (184 μmol L-1; Hoagland and Arnon, 1950); and five replicates per treatment.
Billets of 5 cm were placed in 500 mL plastic recipients containing was hedsand. Fifteen day safter the emergence of tillers, seedlings were transplanted into polypropylene pots (7.5 L) with nutrient solution diluted to 50 %, and maintained for 7 d (adaptation period). Then, the solution was weekly renewed and continuously aerated, with pH adjusted (6.0±0.1) using either HCl 0.1 N or NaOH 1 N solutions.
Visual symptoms of nutritional deficiency were observed and described at the end of the experiment. A growth evaluation was performed for the following variables: plants height (from the basis to first leaf sheath when totally expanded); stem diameter measured with a digital caliper (Starrett 727-2001 ® , Brazil); and leaf area, measured by an image analysis system (Delta T Image Analysis System, Canada). Green color index was determined in the median part of leaf +1 using the chlorophyll-meter CCM200 (Opti Sciences, Canada).
Thirty days after the experiment was set up, seedlings were harvested and roots were separated from aerial parts, and dried in a forced ventilation heating oven at 65 °C until reaching constant weight. After drying, DM was weighed and milled in a Willey type mill, for chemical analysis of total Fe content in aerial parts and roots (Bataglia et al., 1983). With the results, total Fe accumulation was calculated.
The results were analyzed using ANOVA (p≤0.05) and a polynomial regression was carried out along with the greatest values of R2.
Results and discussion
The Fe concentrations in nutrient solution caused an increase with quadratic and cubic adjustment on the micronutrient accumulation both in shoots and roots, respectively (Figure 1A and 1B). The Fe-EDDHA concentration eliciting higher foliar accumulation of the nutrient was 250 μmol L-1 with a buildup of 0.30 g of Fe per pot. However, with the Fe-EDDHA concentration of 184 μmol L-1, there was a leaf accumulation of 0.28 g of Fe per pot. On roots, the greater Fe accumulation was 2 g per pot, when using 184 μmol L-1 of Fe-EDDHA. This concentration provides greater Fe accumulation on plants, since higher concentrations provide small increases on the nutrient accumulation on leaves, and on roots.
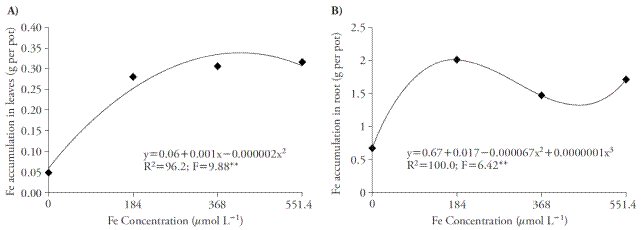
Figure 1 Effects of Fe concentration in leaves (A) and roots (B) of sugar cane cultivated in four concentrations of nutrient solution. ** p≤0.01; F test.
Elevated Fe content in roots relative to leaves may be explained by the use of phytosiderophorus by the grasses (Strategy II). These compounds are liberated on the rhizosphere from iron deficiency, facilitating the absorption of this nutrient (Nozoye et al., 2011). Therefore, Strategy II plants possess higher iron acquisition efficiency and resistance to its deficiency, as compared to Strategy I plants (Zuo and Zhang, 2011). In the control treatment (0 μmol L-1) it was possible to observe Fe accumulation, mainly on roots, since the spreading way of this plant occurs by vegetative parts; therefore, the billet was used, which had Fe concentrations that influenced on the plant’s maintenance. However, this source was exhausted in less than 15 d, which allowed the verification of deficiency symptoms.
Another possible explanation to high Fe accumulation in roots would be the likely nutrient adsorption in fixed negative charges in root apoplasm, or precipitation in Fe form (III) oxide-hydrate in rhyzoplane (Jin et al., 2007). The plants present the strategy of accumulating metallic elements on roots for alleviating possible risks of metal toxicity, diminishing its content in aerial part, which may affect their development (Sarma, 2011).
The increase of Fe concentrations improved green color index, reaching the maximum point in 551.4 μmol L-1 of Fe-EDDHA (Figure 2A). The rise in this index is due to the increase in Fe accumulation in plant, because there is a correlation between these two variables (Figure 2B).
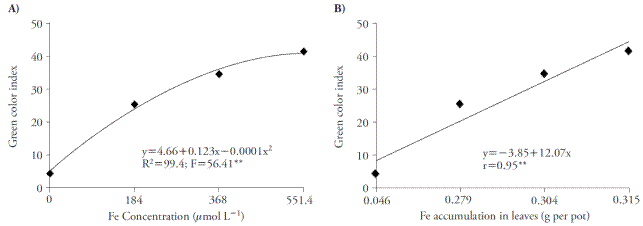
Figure 2 Effect of Fe concentrations in green color index (A) and the correlation between green color index and Fe accumulation in leaves (B) of sugar cane cultivated in four concentrations of nutrient. **p≤0.01; F test.
Plants in the control group showed chlorosis, compared to the other treatments (Figure 3A). Fe deficiency symptoms induce chlorosis leaf interveinal, which evolved into white stains (Figure 3B). In the control treatment roots showed lower density and darkened color (Figure 3C), but with higher Fe concentration greater root density was visualized, as well as lighter coloration (Figure 3D). This visual diagnosis probably reflects physiological changes in roots, due to a higher Fe accumulation as compared to aerial parts (Figure 1).
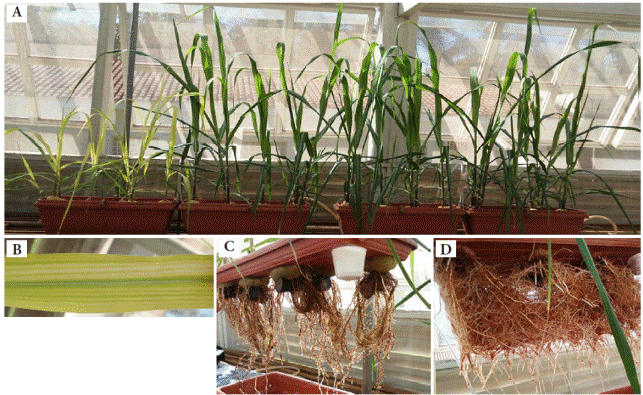
Figure 3 (A) All treatments; (B) Control treatment, indicating Fe deficiency in new leaves; (C) Control treatment, indicating Fe deficiency in roots; (D) Higher Fe concentration treatment.
The increased green color index was an effect of iron, as reported by Huang et al. (2012), who used nutrient solution containing Fe-EDDHA. Decreased leaf chlorophyll content in Fe-deficiency plants may be recovered using nutrient solution with greater Fe concentrations (Pestana et al., 2012). According to Fernández et al. (2008) and Pestana et al. (2005), chlorophyll concentrations and Fe accumulation may be used to diagnose ferric deficiency in plants.
The Fe concentration that promoted greater diameter (12.2 mm) and height (25 cm) was 184 μmol L-1 of Fe-EDDHA (Figures 4A and 4B). However, considering leaf area, the concentration that promoted greater increase was 551.4 μmol L-1 of Fe-EDDHA for 906 cm2, whereas 184 μmol L-1 promoted 750 cm2 (Figure 4C).
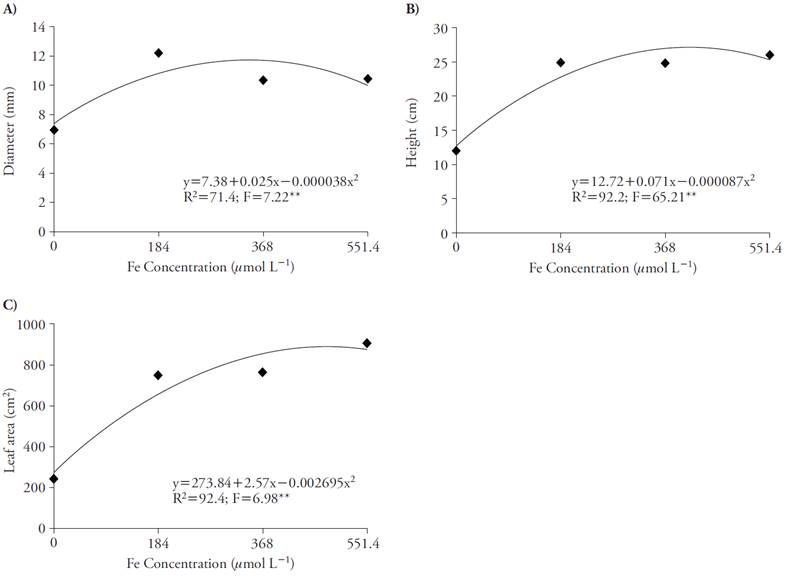
Figure 4 Effect of Fe concentrations in stem diameter (A), plant height (B) and leaf area (C) of sugar cane in four nutrient concentrations. **p≤0.01; F test.
The beneficial effect of the use of Fe on growth, green color index and leaf area in tillers cultivated in nutrient solution may be explained due to the participation of the micronutrient in several stages of the chlorophyll biosynthesis metabolism and chloroplasts ultrastructure (Jeong and Guerinot, 2009). In addition, this nutrient composes the chloroplast proteome, playing an important role in reducing the quantity of proteins involved in electron transfer complexes, while raises the proteins involved in carbon fixation (Briat et al., 2007).
The Fe concentration that promoted greater DM production on both shoots and whole plant was 551,4 μmol L-1, with which was obtained 5.20 g and 6.78 g of DM per pot, respectively (Figure 5). However, there wasn’t a considerable increase over the concentration of 184 μmol L-1, which provided 4.10 g of DM for shoots and 6.28 g for whole plant. Considering the roots, 184 μmol L-1 of Fe-EDDHA promoted greater DM production (2.18 g per pot).
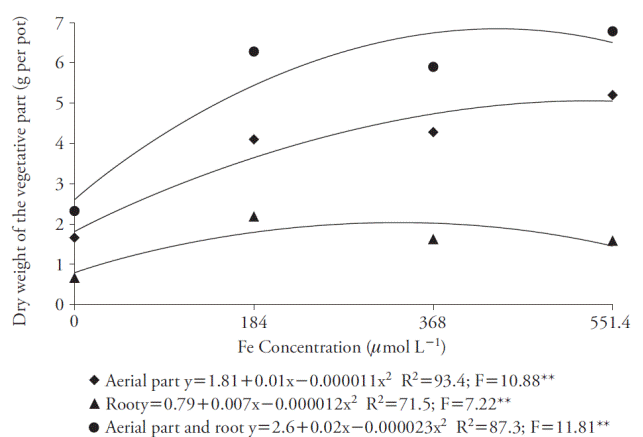
Figure 5 Effect of Fe concentrations in dry matter production in aerial parts, roots and whole plant (aerial part and roots) of sugar cane cultivated in four concentrations of nutrient. **p≤0.01; F test.
The effect of Fe on DM, using Fe-EDDHA, was observed in other species by Moro et al. (2013), who point out that with high Fe doses, the oxidative stress is induced, raising the production of free radicals and reducing plants’ dry matter accumulation.
The adequate iron concentration for the variables was 184 μmol L-1 of Fe-EDDHA, and the law of diminishing increments prevailed since other concentrations showed no significant larger increments. The Fe-EDDHA concentration of 184 μmol L-1 promoted greater DM production on the whole plant for hydroponic cultivation in nutrient solution; thus, it would be the adequate concentration as indicated by Hoagland and Arnon (1950). Furthermore, these results showed that the Fe-EDDHA can be used as an iron source for sugarcane.











 texto en
texto en 


