Introduction
Cadmium is a highly toxic heavy metal that disrupts physiological processes in plants; this heavy metal induces oxidative stress and cause cellular damage to plants. Protonation of radical superoxide (O2•-) can produce the hydroxyl radical (•OH) and hydrogen peroxide (H2O2) that converts fatty acids into toxic lipid peroxides and degrade biological membranes (Weckx and Clijsters, 1996; Lu et al., 2010). Cadmium (Cd) is one of the most common heavy metal pollutants for humans, animals and plants (Wang et al., 2009). It enters the environment mainly from anthropogenic processes and agricultural soils (Januškaitienė, 2014), including sources as pesticides, mining waste and chemical fertilizers (Daud et al., 2013; Daud et al., 2015). In plants, Cd affects photosynthesis, while also damaging the light-harvesting complex and photosystems, reducing chlorophyll biosynthesis (Hu et al., 2009) and water status, decreasing the transpiration rate due to stomatal closure (Deng et al., 2014). Although Cd is not involved in cellular redox reactions, that is to say cannot produce reactive oxygen species (ROS) directly, it might impair the respiratory chain, inhibit antioxidant enzymes, and displace other ions in metalloproteins, which will eventually generate Fenton reactions (Romero-Puertas et al., 2019).Fenton reaction consist in the decomposition of hydrogen peroxide (H2O2) to highly reactive hydroxyl radical (•OH) in the presence of iron (Fe): Fe2+ + H2O2 → Fe 3+ •OH + OH- (Bhaduri and Fulekar, 2012). Thus, it induces the production of ROS as superoxide radicals (O2 •-), •OH, H2O2 and singlet oxygen (1O2) (Sytar et al., 2013). ROS can cause oxidative damage to several cellular constituents, including lipids, proteins and nucleic acids (Lu et al., 2010). In addition, ROS are the most common initiators of lipid peroxidation in living cells (Shahid et al., 2014). Some research has shown that there is an increased production of H2O2 in plants exposed to different concentrations of Cd, such as in Solanum lycopersicum (Nogueirol et al., 2016), Pteria vittata (Balestri et al., 2014), Oriza sativa (Roychoudhury et al., 2012) and in Hygrophila schulla (Mandal et al., 2015). These radicals affect the permeability of cell membranes and induce lipid peroxidation, due to the increased accumulation of ROS (Rellán-Álvarez et al., 2006; Chamseddine et al., 2009).
However, plants possess defense systems; this defense systems in plants include both enzymatic and non-enzymatic antioxidant defense systems, that work in concert to control cascades of uncontrolled oxidation and protect plant cells from oxidative damage (Gill and Tuteja, 2010; Hasanuzzaman et al., 2012). Antioxidant enzymes are fundamental, they catalyze or participate directly in generation of ROS (Gill and Tuteja, 2010; Gill et al., 2013). Antioxidant enzymes in plants include superoxide dismutase (SOD, EC 1.15.1.9), catalase (CAT, EC 1.11.1.6), ascorbate peroxidase (APX, EC 1.11.1.11), and guaiacol peroxidase (GPX, EC 1.11.1.9). Plants use this defense systems to counteract the effects of oxidative stress caused by heavy metals (Sharma and Chakraverty, 2013). Because the O2•- radical is usually the first to be generated (Gill and Tuteja, 2010), the SOD enzyme is the first line of defense as it converts and eliminate radical O2 •- to H2O2 (Muradoglu et al., 1015). However, H2O2 is also toxic to cells, so it is necessary to remove it from cells. The enzymes involved in this process (CAT, APX and GPX) convert H2O2 into water and oxygen. These biological processes entail maintaining a constant balance between the antioxidant systems and ROS content so that the radicals remain at levels compatible with cellular metabolism (Halliwell, 2006). In this sense, the equilibrium between a plant’s oxidative and anti-oxidative capacities determines its fate.
Likewise, Cd affects the morpho-physiological and biochemical processes of plants, such as germination, growth and the root/shoot ratio (Ashraf et al., 2015) and can causes a decrease in biomass production (Ammar et al., 2008). This can result in a decrease in the production of some crops to economic importance such as bean cultivation (Phaseolus vulgaris L.). Because of this, the aim of the present study was to assess the response of Phaseolus vulgaris plants to oxidative stress caused by cadmium, and its effects on protein content, lipid peroxidation and antioxidant enzymes in leaves and roots.
Materials and methods
Plant material, growth conditions and experimental design
Plants produced by seeds were grown in semi-hydroponic conditions in plastic pots with perlite and peat moss (3:1) as substrate. The pots were kept in a greenhouse under natural light conditions. The composition of the nutrient solution applied it was done according to Chaoui et al. (1997) at pH 5.5; pots were provided daily with 200 mL of the nutrient solution. Once the first trifoliate leaf appeared, Cd was added to the nutrient solution as Cd(NO 3)2. The treatments were: 0 (control), 0.25 µM, 0.50 µM, and 1 µM of Cd. After 15 days of adding Cd, leaves were cut and roots were carefully separated from the substrate.
Cadmium content determination
Roots and leaves were washed with deionized water and dried in an oven at 80°C. Then, samples were ground and stored in polyethylene bags until used in acid digestion. Approximately 0.5 g (DW) of sample was taken and digested in 15 mL of concentrated HNO3 in a microwave digestor (MARS 5 CEM). Potency was 1,200 W, pressure 195 psi, and temperature 210°C. The ramp and hold times were set at 15 and 10 min, respectively. The solution was then filtered through 42 µm filters, and deionized water was added to a final volume of 100 mL. Cd content was determined using Microwave Plasma Atomic Emission Spectroscopy (MP-AES) (Model 4200, Agilent Technologies). All concentrations of Cd in leaves and roots were reported in mg kg-1.
H2O2 content, lipid peroxidation and enzyme assays
H2O2 was estimated following the method of Jana and Choudhuri (1981) and lipid peroxidation was determined by measuring malondialdehyde (MDA) using the method of Gérard-Monnier et al. (1998). The MDA concentration was determined at 1,1,3,3-Tetramethoxypropane (0, 0.5, 1, 2, 3 and 4 µM) as a standard curve. For the determination of the enzymatic activity 0.5 g of fresh tissue of leaves and roots were taken and was homogenized in 1 mL of 50 mM Tris-HCl (pH 7.8) containing 1 mM of EDTA and 2% (w/v) polyvinyl pyrrolidone (PVP), using a chilled mortar and pestle, and then stored in an ice bath. The homogenate was centrifuged at 15,000 g at 4°C for 30 min (HERMELE Labnet, Z233 MK-2). The supernatant was used to determine SOD, CAT, APX and GPX activity, and soluble protein content. To measure APX (ascorbate peroxidase) activity, the tissue was ground separately in a homogenizing medium containing 2.0 mM of ascorbate and the other ingredients (Balestri et al., 2014) all assays were performed at 25°C. Superoxide dismutase (SOD) activity was determined following Beyer and Fridovich (1987); catalase (CAT) activity was measured following Aebi (1981); ascorbate peroxidase (APX) activity was assayed according to Nakano and Asada (1981) and guaiacol peroxidase (GPX) activity was determined according to Kato and Shimizu (1987). Protein content was determined using Direct Detect® Infrared Spectrometer equipment with Bovine Serum Albumin (BSA) as the standard.
Statistical analysis
Data were based from ten independent samples of each treatment (control, 0.25 µM, 0.50 µM and 1 µM); data was expressed as mean ± standard deviation. Normality was verified by the Shapiro-Wilk test, and the homogeneity of variance was tested using Levene’s test. Statistical analyses were carried out by a one-way analysis of variance (ANOVA) followed by Games-Howell test for multiple comparisons using SPSS 20.0 software. A significant difference was considered at level p < 0.05.
Results and discussion
Cadmium content
Cd concentrations in roots increased significantly (p>0.001) at higher concentrations of this contaminant in the nutrient solution; when adding 0.25 μM of Cd, the content in roots was 2.96 ± 0.54 mg kg-1; at 0.50 μM, it was 6.52 ± 0.66 mg kg-1; and at 1 μM, 21.08 ± 2.84 mg kg-1. However, no Cd was detected in leaves at any of the Cd concentrations after two weeks of contamination (Table 1). On the other hand, the length of root was reduced with the addition of 0.25 and 0.50 µM of Cd in the nutrient solution, but at 1 µM, the length was greater (Fig. 1). In leaves symptoms of phytotoxicity were observed caused by Cd although Cd was not detected, also leaves showed symptoms like chlorosis and necrosis, so the possibility of a very low concentration of Cd in leaves is not discarded (Fig. 2).

Fig. 1 Phytotoxic effects caused by the accumulation of cadmium in Phaseolus
vulgaris leaves. Control (A), 0.25 µM (A) 0.50 µM (B)
and 1 µM (C).
Fig. 1. Efectos fitotóxicos causados por la acumulación
de cadmio en las hojas de Phaseolus vulgaris. Control (A), 0.25 μM
(B) 0.50 μM (C) y 1 μM (D).
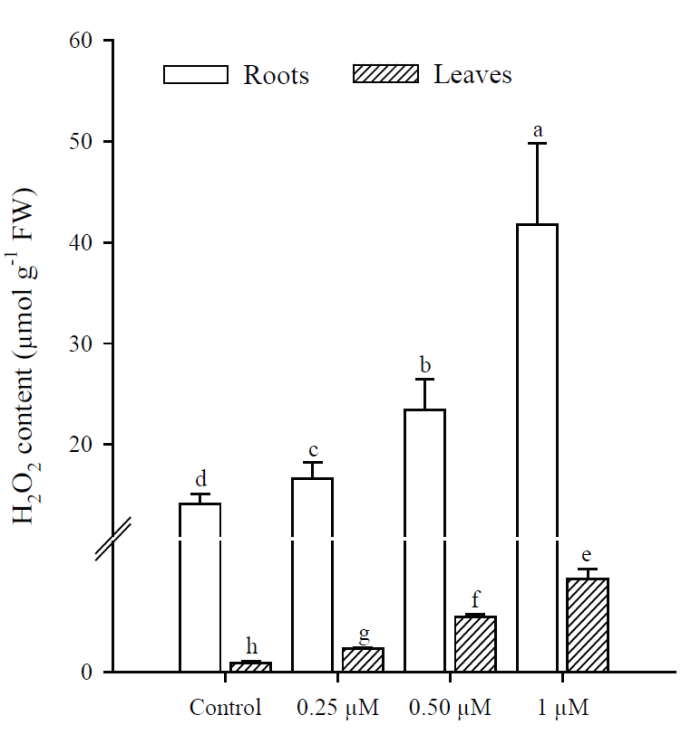
Fig. 2 Phytotoxic effects caused by the accumulation of cadmium in Phaseolus
vulgaris roots. Control (A), 0.25 µM (B) 0.50 µM (C)
and 1 µM (D).
Fig. 2. Efectos fitotóxicos causados por la acumulación
de cadmio en las raíces de Phaseolus vulgaris. Control (A), 0.25 μM
(B) 0.50 μM (C) y 1 μM (D).
Nevertheless, it has been reported that Cd influences the absorption and transport of nutrients and compete with their transport. This is because nutrient transporters show a wide specificity with divalent metals, including Fe+2, Zn+2, Mn+2 and Cd+2 (Liu et al., 2017). The mechanism by which Cd inhibits the uptake of essential nutrients is not completely clear (Hédiji et al., 2015). It is assumed that Cd may interfere with nutrient uptake by affecting the permeability of plasma membrane and modify the activity of nutrient transporters, leads to changes in its concentration and composition (Boulila-Zoghlami et al., 2006; López-Millán et al., 2009; Hédiji et al., 2015). These interactions can cause serious nutritional deficiencies and the symptoms of chlorosis and necrosis, it can not only be due to the phytotoxicity produced by Cd, being as the nutritional balance in P. vulgaris leaves and roots could be altered.
Table 1 Cadmium content (mg kg-1) in roots and leaves of Phaseolus
vulgaris plants.
Tabla 1. Contenido de cadmio (mg kg-1) en raíces y
hojas de plantas de Phaseolus vulgaris.
| Organ | Control | 0.25 µM | 0.50 µM | 1 µM |
|---|---|---|---|---|
| Roots | ND | 2.96 ± 0.54c | 6.52 ± 0.66b | 21.08 ± 2.84a |
| Leaves | ND | ND | ND | ND |
ND = Not detectable
Data presented as means ± SD (n = 10); different letters indicate significant differences in each treatment.
Oxidative stress
Exposure to Cd resulted in a significant increase (p<0.001) in H2O2 content, which was greater at higher Cd concentrations. The highest production of H2O2 in roots and leaves was in plants treated with 1 μM Cd; however, H2O2 concentrations were always higher in roots than in leaves under all treatments (Fig. 3). The results obtained show that Cd significantly increased (p < 0.001) MDA content in roots and leaves of P. vulgaris (Fig. 4A). In roots, MDA content increased 2.0, 3.1 and 5.38 times more than in control (0.25, 0.50 and 1 µM Cd, respectively), while the increases in leaves were 1.6, 4.1 and 7.7, compared to control. Contrary to H2O2 content, lipid peroxidation was more notable in leaves under all treatments. Finally, protein content decreased significantly (p < 0.001) in leaves when adding 0.50 and 1 µM of Cd, with respect to the control (Fig. 4B). However, no significant diferences were observed between treatments with 0.50 and 1 µM of Cd. In roots, as in leaves, protein content decreased with respect to the control upon adding 0.25, 0.50 and 1 µM of Cd (p = 0.036; p = 0.035; p = 0.041, respectively).
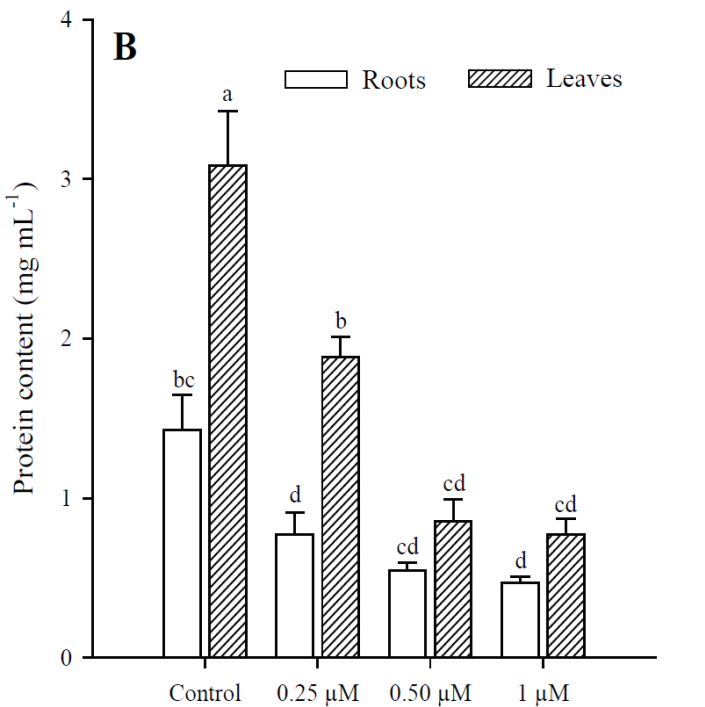
Fig. 3 Hydrogen peroxide content in leaves and roots of Phaseolus vulgaris
plants exposed to cadmium. Data presented as means ± SD
(n = 10); different letters indicate
significant differences in each treatment.
Fig. 3. Contenido de peróxido de hidrógeno en las hojas
y raíces de las plantas Phaseolus vulgaris expuestas al cadmio. Los
datos son presentados como medias ± DE (n = 10); letras diferentes
indican diferencias significativas en cada tratamiento.
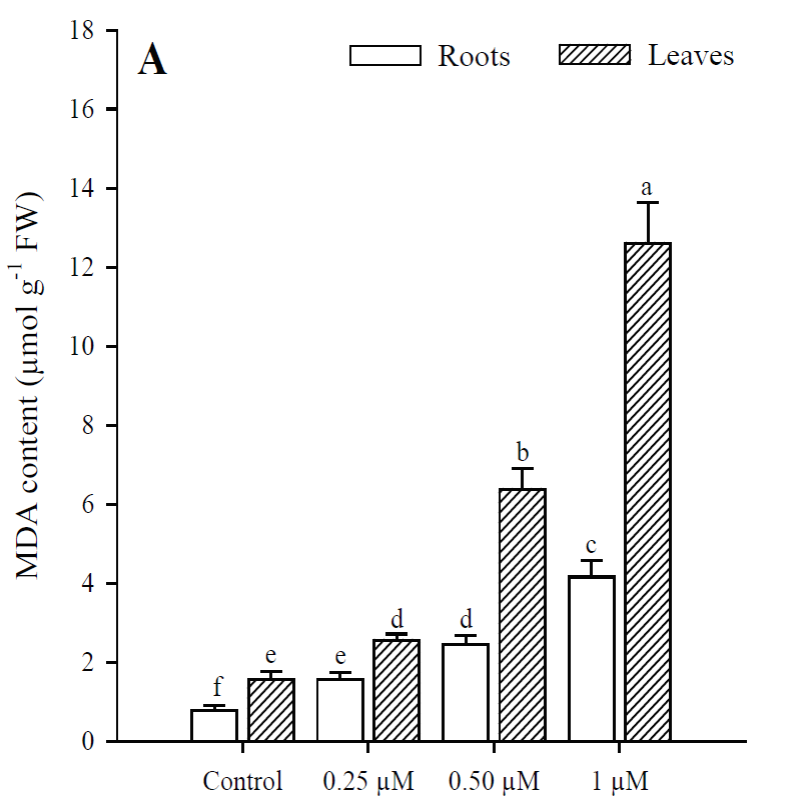
Fig. 4 Malondialdehyde (A) and protein (B) content in leaves and roots of Phaseolus
vulgaris plants exposed to cadmium. Data presented as
means SD (n = 10); different letters indicate
significant differences between treatments.
Fig. 4. Contenido de malondialdehído (A) y proteína (B)
en las hojas y raíces de plantas de Phaseolus vulgaris expuestas al
cadmio. Los datos son presentados como medias ± DE (n = 10); letras
diferentes indican diferencias significativas entre
tratamientos.
Both in leaves and roots of P. vulgaris, the increase in Cd concentrations significantly increased the generation of H2O2 and caused lipid peroxidation with the increase in MDA; in leaves a greater induction of MDA was observed and in roots, of H2O2. Evidence has been reported suggesting that Cd toxicity takes the form of oxidative stress caused by ROS production (Sanità di Toppi and Gabbrielli, 1999; Gao et al., 2010). Cd is not involved in cellular redox reactions and does not produce ROS directly. However, biochemical and transcriptomic studies show that oxidative stress is one of the first consequences of Cd toxicity in plants and other organisms (Romero-Puertas et al., 2019). Although Cd is not a transition metal, it commonly causes oxidative stress in plants, but the way to conducting cell damage is far to be elucidated (Gallego et al., 1996; Groppa et al., 2007; Lin et al., 2007; Iannone et al., 2010).
In plants, H2O2 is continuously produced as a product of various metabolic reactions. On the other hand, it is important to keep in mind that Cd in leaves was not detected in any of the treatments, but despite this there were increases in the content of MDA, H2O2 and enzymatic activity. This could be explained by the fact that H2O2 can act as a local and systemic signaling molecule against oxidative stress caused by exposure to heavy metals (Wang et al., 2006; Zayneb et al., 2015). ROS can also serve as signaling molecules, and a possible mitigating effect of H2O2 derived from Cd stressors has been proposed (Gill and Tuteja, 2010). In recent years, several studies have focused on the role of H2O2 in response to tolerance for a wide range of stress conditions, so it is interesting to see what happens in response to internal and external stimuli to improve stress tolerance (Cuypers et al., 2016). This seems to be used positively in plants to activate multiple stress-sensitive genes, so it is widely accepted that H2O2 is one of the signaling molecules due to its high stability and mobility (Christou et al., 2014). However, in high concentrations, H2O2 is harmful to plants and leads to programmed cell death (Gill and Tuteja, 2010).
Antioxidant enzymes
The effect on SOD activity (Fig. 5A) in roots were significant with the application of 0.25 μM of Cd (p < 0.001), as it increased 9.1 times more than in the control; whereas at 0.50 and 1 μM Cd, activity was 8.5 and 5.9 times greater than in the control, respectively. In leaves, the activity of this enzyme increased at higher concentrations, and significant differences were found compared to the control in all Cd treatments (p < 0.001). CAT activity (Fig. 5B) in roots was significantly higher (p < 0.001) in the treatment with 0.25 μM of Cd (6.9 times more than in the control) but decreased with the application of 0.50 and 1 μM Cd. No significant differences were observed between these two treatments (p = 0.340). In contrast, the greatest activity of this enzyme in leaves occurred in treatments with 0.50 and 1 μM of Cd (11.6 and 12.1 times more, respectively), compared to the control (p < 0.001). However, no significant differences were found (p = 0.996) between these two treatments (0.50 and 1 µM). Regarding GPX activity (Fig. 5C), the study found significant differences in roots among all treatments (p < 0.001). The highest activity was in the treatment with 0.25 μM of Cd (2.6 times compared to the control) but was inhibited in treatments with 0.50 and 1 μM of Cd. In leaves, this enzyme’s activity was lower than in roots under all treatments. However, in the treatment with 1 μM of Cd it was 8.1 times higher than in the control (p < 0.001). Finally, APX activity (Fig. 5D) was significantly higher in roots (p < 0.01) at the concentration of 0.25 μM of Cd, compared to all other treatments. In treatments with 0.50 and 1 μM of Cd, this enzyme’s activity was inhibited; as shown by the finding that concentrations increased. In terms of leaves, APX activity increased at concentrations. The highest activity was with 1 μM of Cd (11 times more than the control).
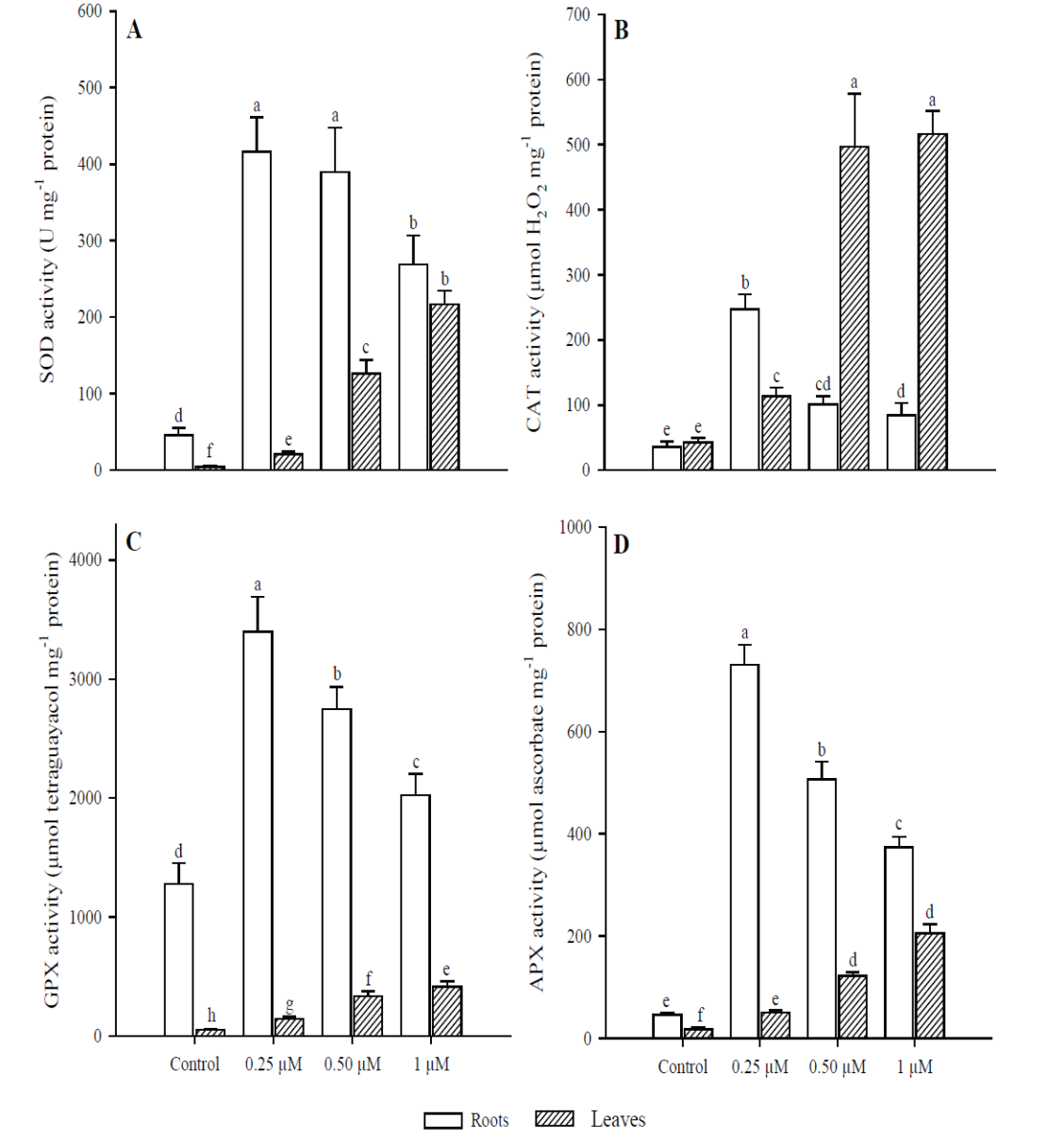
Fig. 5 Antioxidant response in leaves and roots of Phaseolus vulgaris plants
exposed to Cd. Superoxide dismutase (A); catalase (B); guaiacol
peroxidase (C); and ascorbate peroxidase (D). Data presented as
means ± SD (n = 10); different letters indicate
significant differences between treatments
Fig. 5. Respuesta antioxidante en las hojas y raíces de
plantas Phaseolus vulgaris expuestas a Cd. Superóxido dismutasa (A);
catalasa (B); guaiacol peroxidasa (C); y ascorbato peroxidasa (D).
Los datos son presentados como medias ± DE (n = 10); letras
diferentes indican diferencias significativas entre
tratamientos.
The enzymes considered most important to eliminate intracellular H2O2 levels in plants are CAT, APX and GPX (Al-fadul and Al-Fredan, 2013). Like SOD, these enzymes showed a different behavior between leaves and roots of P. vulgaris. The APX and GPX activity was higher in roots when adding 0.25 μM of Cd, since at 0.50 μM this activity decreased, although it was always greater than in the control. Regarding CAT, it was inhibited in roots with the addition of 0.50 μM of Cd, in contrast to leaves where the highest activity was at 0.50 and 1 μM. The decrease in CAT activity in roots of P. vulgaris can be compensated with a higher APX and GPX activity (0.25 μM Cd), indicating that these two enzymes can act simultaneously. Therefore, the decrease in CAT with the increase in H2O2 in roots suggests the sensitivity of this enzyme to Cd (Roychoudhury et al., 2012). A higher H2O2 concentration was observed in roots than in leaves of P. vulgaris. In addition, the sensitivity of CAT to O2•- radicals and Cd levels can cause its inactivation (Nouairi et al., 2009).
On the other hand, APX plays an important role in the elimination of H2O2, but its activity depends on the concentration of metal, and its main function is to quickly eliminate H2O2 at the site where it is generated (Asada, 1992; Gill et al., 2013). GPX can be induced by toxicity generated by heavy metals and is more efficient than CAT in the elimination of H2O2 (Wang et al., 2010; Nadgórska-Socha et al., 2013). However, it was observed that the CAT enzyme proved to be more efficient in removing H2O2 in leaves than in roots of P. vulgaris. Depending on Cd concentration and plant species, Cd may inhibit or stimulate the activity of several antioxidative enzymes before any visible symptoms of toxicity appear (Anjum et al., 2011; Gill et al., 2012; Xu et al., 2014). Excessive levels of H2O2 could be minimized through the activities of APX and GPX; it may be due to the damage to enzymatic proteins due to the excess of radicals (Lou et al., 2011). This could explain the fact that in roots after the addition of 0.50 µM Cd, the excess of free radicals inhibited the enzymatic activity of APX and GPX.
Other studies have also observed increases or decreases in enzymatic activities at different concentrations of metals. In the case of Brassica juncea, the activity of CAT and GPX progressively decreased in leaves exposed to 30 μM of Cd, but APX activity was induced (Markovska et al., 2009). Bankaji et al. (2015) showed that the excess of Cd or Cu mainly decreased the enzymatic activity of CAT, GPX and APX in the leaves of Suaeda fruticosa, suggesting that antioxidant systems are altered by the action of heavy metals. Likewise, the patterns produced in response to the stress caused by heavy metals vary in the different organs of the plants, as was seen in the case of P. vulgaris.
These differences in enzymatic activity can be explained by the fact that roots are the first organ to come into direct contact, so a quick and effective response is important in response to oxidative stress caused by this type of contaminants (Souza et al., 2015). Some studies have shown that SOD activity increases due to the stress produced by Cd, which was observed in P. vulgaris leaves; however, this enzyme was inhibited in roots by adding 0.50 and 1 μM of Cd. This reduction may be due to the inactivation of SOD by the action of H2O2 (Namdjoyan et al., 2011). Therefore, the H2O2 produced by SOD must be rapidly metabolized, since it is still toxic and needs to be converted into H2O and O2 by other enzymes (Rahoui et al., 2014).
In the other hand, it is also important to note that no Cd was detected in the leaves in any treatment, though increases in MDA and H2O2 content and enzymatic activity were observed. This could be explained by the fact that H 2O2 can act as a local and systemic signaling molecule against oxidative stress caused by exposure to heavy metals (Wang et al., 2006; Zayneb et al., 2015). ROS can also serve as signaling molecules, and a possible mitigating effect of H2O2 derived from the stress factors of Cd has been proposed (Gill and Tuteja, 2010). In recent years, several studies have focused on the role of H2O2 as a response to tolerance for a wide range of stress conditions, so it is interesting to see what is produced in response to internal and external stimuli in order to improve tolerance of stress (Cuypers et al., 2016). This seems to be used positively in plants to activate multiple stress-sensitive genes, which is why it is widely-accepted that H2O2 is one of the signaling molecules due to its high stability and mobility (Christou et al., 2014). However, at high concentrations, H2O2 is actually harmful to plants, and leads to programmed cell death (Gill and Tuteja, 2010).
The results of this study show that although cadmium was deposited in roots but not detected in leaves, an enzymatic antioxidant response was observed in both roots and leaves of P. vulgaris. This could be due to the induction of systematic signaling mechanisms due to oxidative stress that occurs at roots as a means to protect the leaves. However, it is possible that Cd accumulates at such low concentrations in the leaves, so it was not detected.
Conclusions
Our results suggest the generation of oxidative stress caused by Cd by the accumulation of MDA and H2O2 in leaves and roots. However, the antioxidant defense system in the roots of P. vulgaris was not effective enough in the elimination of free radicals generated with the addition of 0.50 and 1 µM of Cd.
Regarding the enzymatic activity in leaves, there was an increase in all concentrations with Cd, although this contaminant was not detected, could be due to the induction of systematic signaling mechanisms caused by oxidative stress as a means to protect the leaves. Therefore, it is necessary to carry out further studies on oxidative stress and the antioxidant response at low concentrations of Cd and gain a better understanding of the connection between ROS production, antioxidant defense mechanisms and signaling mechanisms in plants.











 nueva página del texto (beta)
nueva página del texto (beta)


