Introduction
Electrospun (ES) fibers have recently been used as bioactive supporting matrices which allow them to be functionalized for their use in various health sector applications (Kriegel et al., 2008; Thenmozhi et al., 2017; Balakrishnan et al., 2018). Electrospinning is a versatile process that allows the production of fibers with a wide variety of applications in function of the polymer used. Biocompatible ES fibers combined with drugs, antibacterial or antioxidants engage the attention of biomedical and bioengineering researchers as they offer synergic effect between the fibers (large specific surface, excellent mechanical properties) and the properties of the additive molecule (Thenmozhi et al., 2017). Perumal et al. (2017) obtained a material for wound dressings applications with 25% of antioxidant release during a period of up to 72 hours. Zhang et al. (2017) showed the analysis of mechanical, physicochemical and biodegradation properties of a variety of natural and synthetic polymers. Food and Drug Administration (FDA) approved a diversity of synthetic polymers like Poly(lactic acid) (PLA) as biocompatible and biodegradable materials (Liu et al., 2012).
These features are critical if the composite materials will be used as scaffold involved in cellular adhesion and proliferation (Sridhar et al., 2011). A material useful as a wound dressings should provide a component that helps wounds to heal quickly in addition to offer protection against infections. Bioactive molecules are crucial to facilitate the acceleration of wound healing process (Haider et al., 2018; Rogers et al., 2014). Antioxidants extracted from plants have recently emerged as a useful tool for treating a broad range of diseases, in addition to having the advantage of being naturally available and can be combined in different ways to be used as therapeutic agents.
The extracts of different plants of the Polygonaceae family, including the Rumex genus, are used for the preparation of bioactive materials, amid which Rumex dentatus L. (El-Sherbiny et al. 2016) and R. vesicarius L. (Umoren et al. 2020) stand out. Several extracts of the Rumex genus have been successfully tested for diseases treatment, with activities such as bactericide, anti-inflammatory, astringent, antitumor, antidermatitic, amid other (Hawas et al., 2011; Mostafa et al., 2011; Sedaghat et al., 2011). Rodríguez et al. (2012) reported the use of R. hymenosepalus for nanoparticle preparation; the plant is known as a sour cane and can be found within the flora of northern Mexico, reported to have a high content of polyphenolic compounds and stilbenes.
The use of nanofibers as part of release systems has increased, since it allows to focus the release of bioactive compounds in a specific area improving effectiveness over conventional oral medication methods where the release does not occur in a specific area (Zhu et al., 2012). Castillo-Ortega et al. (2015) reported on the preparation of ES polymers added with (-) epicatechin and performed release studies that have been useful to our work, since epicatechin is a bioactive compound found in R. hymenosepalus. Fibers mats loaded with therapeutic agents have been studied for bio-medical applications, as wound dressings with antibacterial properties with reduced healing time (Ranjith et al., 2019).
Although the great potential of R. hymenosepalus has been published, there is no report of its integration to biomaterials, which represents an opportunity for the elaboration of useful biomaterials for wound dressings. Taking advantage of the biocompatibility and biodegradability characteristics of PLA and its ease of handling by electrospinning, in this work, PLA ES fibers loaded with R. hymenosepalus extract have been prepared and characterized, both morphological (using SEM), and thermally (by TGA); also the R. hymenosepalus extract release studies were performed. The addition of R. hymenosepalus extract to PLA fibers allow to obtain a biomaterial with antioxidant delivery properties that will be useful and novelty for tissue regeneration.
Materials and methods
Materials
Poly (lactic acid) (PLA 4060D, Mn=119,000 g mol-1) was supplied by Nature Works (USA), acetone 99.7% by Aldrich, and Sodium Hydroxide (NaOH pellets) by Meyer.
Preparation of PLA solution
For the ES fibers preparation process, PLA solutions were prepared adding one gram of PLA (poly(lactic acid), 4060D NatureWorks) to 10 mL of acetone and maintained under constant magnetic stirring at room temperature (25°C) for 4 h, in order to obtain a 10% (w/v) polymer concentration.
Electrospinning process
PLA fibers were obtained from the polymer solution using a horizontal electrospinning setup. Disposable 10 mL syringes with a needle of an internal diameter of 0.8 mm were used to hold the solution. Using a syringe pump KD Scientific, the flow velocity was varied in the range of 0.8-2.0 mL/h. Square aluminum plates of 100 cm2 were used as collectors, the distance between the collector plate and the tip of the needle was 15 cm. A high voltage power supply, Spellman, model CZE 1000R was used for the application of an electric field to the polymer solution in a range of 15-20 kV. To get unidirectional aligned fibers, the electrospinning proceeding was similarly performed with a change in the collector, which in this case a square aluminum plate of 100 cm2 over a rotating drum was used. The distance between the needle and the drum varied in the range of 15-18 cm. The experiments were made at room temperature and relative humidity between 10-30%.
PLA ES fibers hydrolysis
PLA is a polyester with structure in which the carbonyl groups predominate and is characterized by its hydrophobic nature. Sadeghi-Avalshahr et al. (2017) reported an alkaline hydrolysis treatment using NaOH in order to activate the surface of the fibers. The treatment of hydrolysis should be performed smoothly and in short periods, since high NaOH concentrations and prolonged periods can fully degraded PLA fibers, conditions that allow treatment without significantly affecting the integrity of the fibers were determined experimentally. Following this approach, a soft alkaline hydrolysis treatment of the ES PLA fibers was carried out, an excess of 0.025 M NaOH solution was added to 2.25 cm2 of fiber mats in a glass bottle for 1.5 hours. Then the pieces of fibers were sonicated in deionized water during two cycles of 10 minutes.
Addition of Rh solution to PLA ES fibers
One hundred microliters of 0.2 µM (2x10-5µM) R. hymenosepalus extract in ethanol solution were added to the center of the fiber mats (squares of 2.25 cm2) and dried at room temperature for 24 h. The R. hymenosepalus extract was obtained according to the procedure described by Rodríguez-León et al. (2012).
Scanning electron microscopy
The morphology of the ES nanofibers was examined with a scanning electron microscope (SEM) using JEOL JSM-5410LV equipped with an INCA system and an Energy Dispersive X-Ray (EDS) microanalysis detector, operated at 20 kV. The samples were carefully cut with a scalpel to an adequate size and placed on a copper sample holder; the samples were coated with gold to allow electric conduction and to prevent the accumulation of charge under electron bombardment. For the analysis a homogeneous area is selected at the center of the sample. High-resolution scanning electron microscopy analysis was performed with electron microprobe JEOL 7610F. The diameter of the PLA nanofibers was measured using image analysis software ImageJ (National Institute of health (NIH), USA); 50 fiber diameters were measured and standard deviation analysis was made using STATGRAPHICS Plus 5.1 software to identify the effect of parameter modification on fibers morphology.
Thermogravimetric analysis (TGA)
The percentage of weight loss of the samples as a function of the temperature was measured by a thermogravimetric analysis using Pyris 1 TGA analyzer Perkin Elmer by heating 3 mg of the sample under nitrogen atmosphere in the temperature range of 25-600 °C with a heating rate of 10 °C/min. The first derivative of the TGA curve was calculated and differential thermogram (DTG, derived from the mass with respect to time) was obtained, allowing the differences between samples to be clearly visualized.
Fourier Transform Infrared Spectroscopy (FTIR)
The FTIR was performed to analyze the ES PLA, PLA hydrolyzed (PLA/H) and the PLA- R. hymenosepalus fibers by FTIR ATR with a Perkin Elmer FTIR Frontier spectrometer. The measurements were carried out at room temperature. Spectra were obtained in the 4000-400 cm-1 region.
Contact angle measurements
The water contact angle of the fiber was estimated using goniometer VSA optima, AST Products, Inc. by placing 0.1 µL of deionized water on fiber mats. The readings were measured at three different locations on the ES PLA mat.
UV-vis spectroscopy
UV-vis calibration curve of R. hymenosepalus was made by preparing dilutions of 0.2 µM of R. hymenosepalus extract to obtain experimental points and use linear regression with r2 value of 0.9962. The antioxidant release study of the PLA and PLA-hydrolysis mats obtained of the addition of R. hymenosepalus was subsequently carried out using 50 mL of water and constant stirring using a Lambda 20 Perkin Elmer UV-vis spectrometer, an aliquot of 3 mL was taken of the release solution at a determinate time period. The lecture was measured in the wavelength range of 190 to 1100 nm and wavelength of 278 nm is selected to study the release of R. hymenosapalus.
Results and discussion
Morphological characterization of the PLA ES fibers
Although the production of ES fibers has been reported on countless occasions in recent years, the tendency when acetone is used is to combine it with a higher dielectric constant solvent, a condition that favors the uniformity of the fibers. Unfortunately, the residues retention of these solvents in fibers restricts their use in health sector applications, since most of them are toxic. For this reason, it was decided to produce PLA fibers using acetone as a single solvent, which is completely volatilized during the production process and fibers free of solvent residues are obtained. The disadvantage of the single use of acetone will be the presence of irregularities in the morphology of the fibers. Therefore, a sweep of the flow rate from 0.8 to 2.0 mL/h was carried out in order to establish the effect of the flow rate on the diameters and homogeneity of the fibers obtained.
The surface morphology of PLA ES fibers produced from 10% w/v solution in acetone using 15 kV of voltage and 15 cm from the tip of the needle to the collector aluminum plate with flow variation from 0.8 mL/h to 2.0 mL/h was analyzed by SEM (Fig. 1).
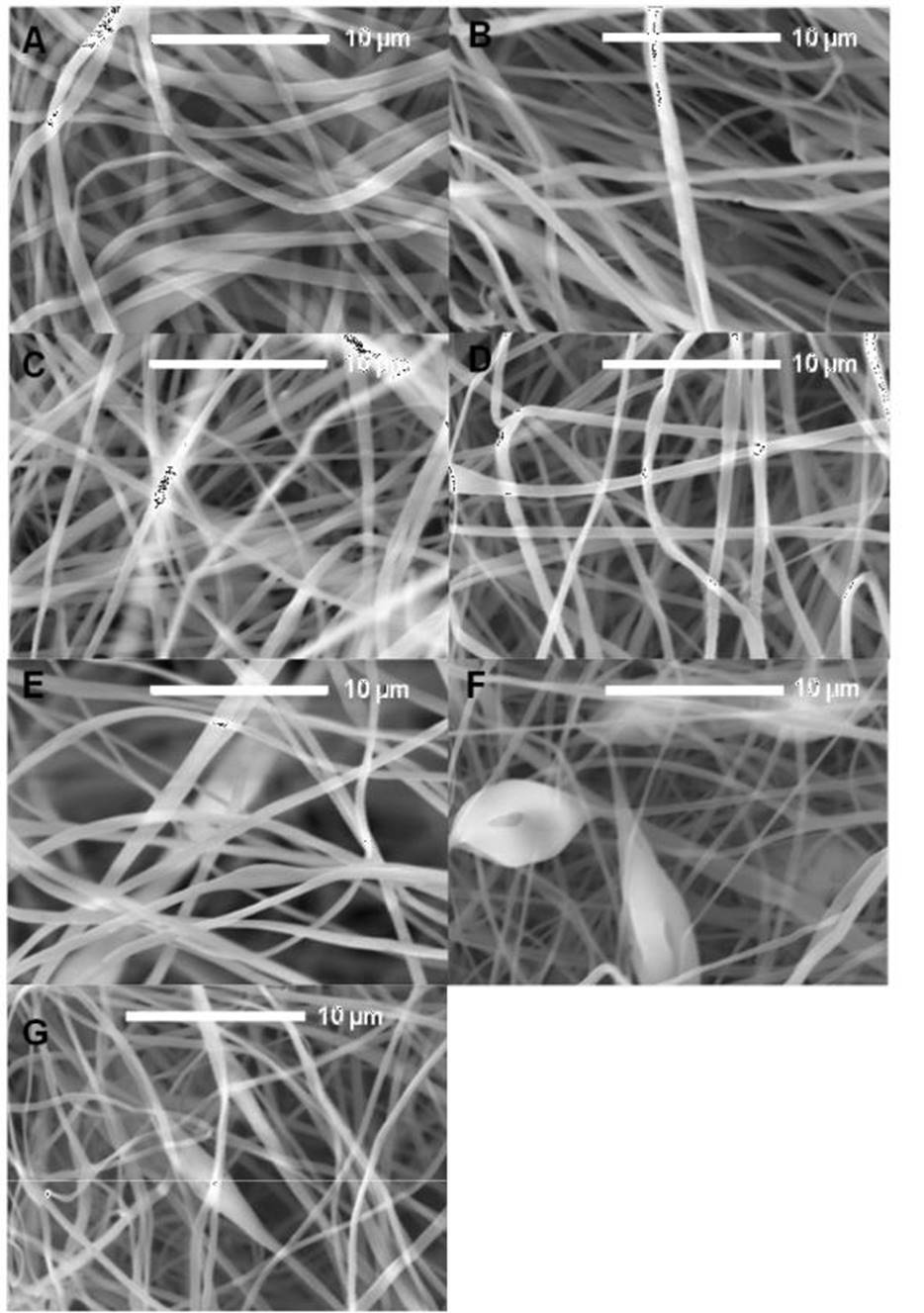
Figure 1 SEM micrographs of the PLA electrospun fibers with flow variation from (A) 0.8 mL/h, (B) 1.0 mL/h, (C) 1.2 mL/h, (D) 1.4 mL/h, (E) 1.6 mL/h, (F) 1.8 mL/h, (G)2.0 mL/h.
Figura 1.
Micrografías MEB de fibras electrohiladas de PLA con variación
deflujo de (A) 0.8 mL/h, (B) 1.0 mL/h, (C) 1.2
mL/h, (D) 1.4 mL/h, (E) 1.6 mL/h, (F) 1.8 mL/h, (G)2.0 mL/h.
The SEM analysis showed randomly aligned, where smooth fibers with a heterogeneous diameter distribution can be observed, from 418 nm to almost 2 µm, with an average diameter of 800 nm and uniform fibers with diameter from 412 nm to 1.1 µm, and an average diameter of 688 nm (Fig. 1 A and B). We can see that the diameter of the fibers decreases to 221 nm showing an average diameter of 596 nm (Fig. 1 C). Micrograph show diameter averages between 671 nm and 680 nm when the flow rate of 1.4 and 1.8 mL/h was used. The wide distribution of diameters observed in the fibers obtained by using flow rate between 0.8 mL/h and 1.4 mL/h evidences the effect of the acetone low electrical conductivity, where it is difficult for the polymer solution to be attracted towards the collector plate with uniformity. In the case of fibers obtained at 1.6 mL/h although there is variety in the diameters distribution, homogeneous, smooth, and uniform surface was observed, indicating the better condition for the fiber production. The Fig. 1 F correspond to fibers obtained with flow rate of 1.8 mL/h; the diameters vary between 100 nm and 1 µm, with bead formation. The formation of bubbles in the fiber is observed, when the maximum flow rate was used in this study (Fig. 1 G). The presence of bead and bubbles when a flow rates higher than 1.8 mL/h are used, is due to the fact that the solvent does not evaporate completely on the path between the tip of the needle and the collector plate. The average diameters of the PLA ES fibers with flow variation are listed in Table 1.
Table 1 Average diameters calculated from SEM micrographs of the PLA electrospun
fibers with flow variation.
Tabla 1. Diámetros promedio calculados utilizando micrografías MEB de fibras electrohiladas de PLA con variación de flujo.
| Flow rate (mL/h)N=50 | Average (um) | ±SD | Min | Max |
|---|---|---|---|---|
| 0.8 | 0.666 | 0.181 | 0.376 | 1.254 |
| 1.0 | 0.572 | 0.222 | 0.258 | 1.232 |
| 1.2 | 0.548 | 0.189 | 0.221 | 1.110 |
| 1.4 | 0.592 | 0.197 | 0.264 | 1.112 |
| 1.6 | 0.591 | 0.155 | 0.278 | 1.004 |
| 1.8 | 0.482 | 0.209 | 0.176 | 1.034 |
| 2.0 | 0.507 | 0.144 | 0.283 | 0.939 |
To evaluate the voltage effect on the morphology of the fibers, the same conditions mentioned above were used, maintaining a flow of 1.6 mL/h, which was better, as was seen before. Fig. 2 show the micrographs of PLA ES fibers obtained at different voltages, from 15 to 20 kV. It can observed that when the lowest voltage used in this study was applied, the fibers obtained were more homogeneous and uniform. The fiber surface becomes rougher and more irregular, with some microfractures in different patterns in the surface observed when the voltage increases.
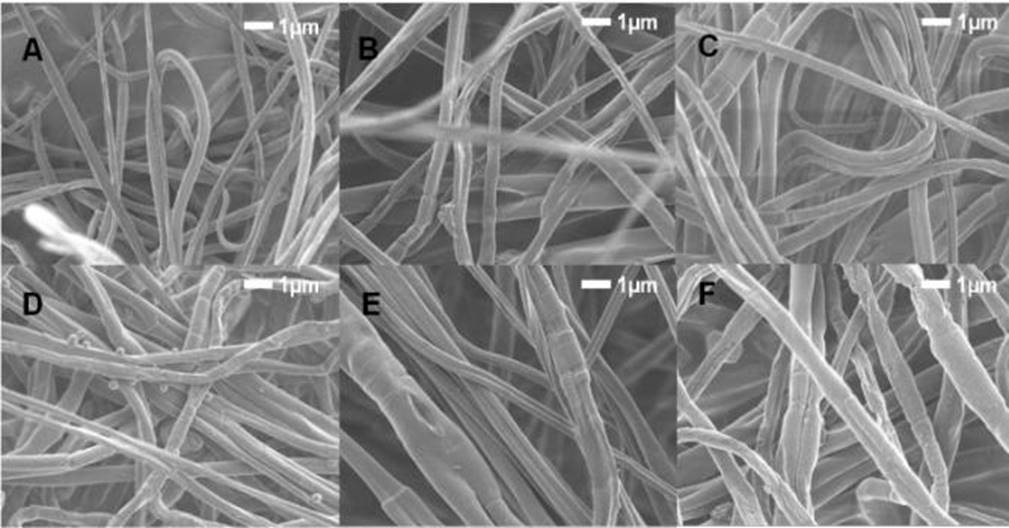
Figure 2 SEM micrographs of the PLA 10% electrospun fibers with voltage variation from (A) 15 kV, (B) 16 kV, (C) 17 kV (D) 18 kV, (E) 19 kV, (F) 20 kV.
Figura 2. Micrografías MEB de fibras electrohiladas de PLA al 10% con variación de voltaje de (A) 15 kV, (B) 16 kV, (C) 17 kV (D) 18 kV, (E) 19 kV, (F) 20 kV.
In the electrospinning technique the type of collector used is decisive for the characteristics of the fiber obtained. The fibers with unidirectional alignment stand out for their utility in applications of wound dressings, the orientation favors interactions with the tissue; in our work, a rotating drum was used as a collector. Based on the above, PLA ES fibers obtained using a rotating drum are shown in Fig. 3. The PLA solution 10 % w/v in acetone was ES using 15 kV of voltage at different flow rates, (A) 0.6 mL/h, (B) 0.8 mL/h, (C) 1.0 mL/h and (D) 1.2 mL/h; it was observed that the fibers were not aligned in the rotating direction of the drum, probably due to the rotating rate of the drum not being enough to contribute to the fiber alignment. From these fibers, the flow rate of 0.6 mL/h was selected to continue the study.
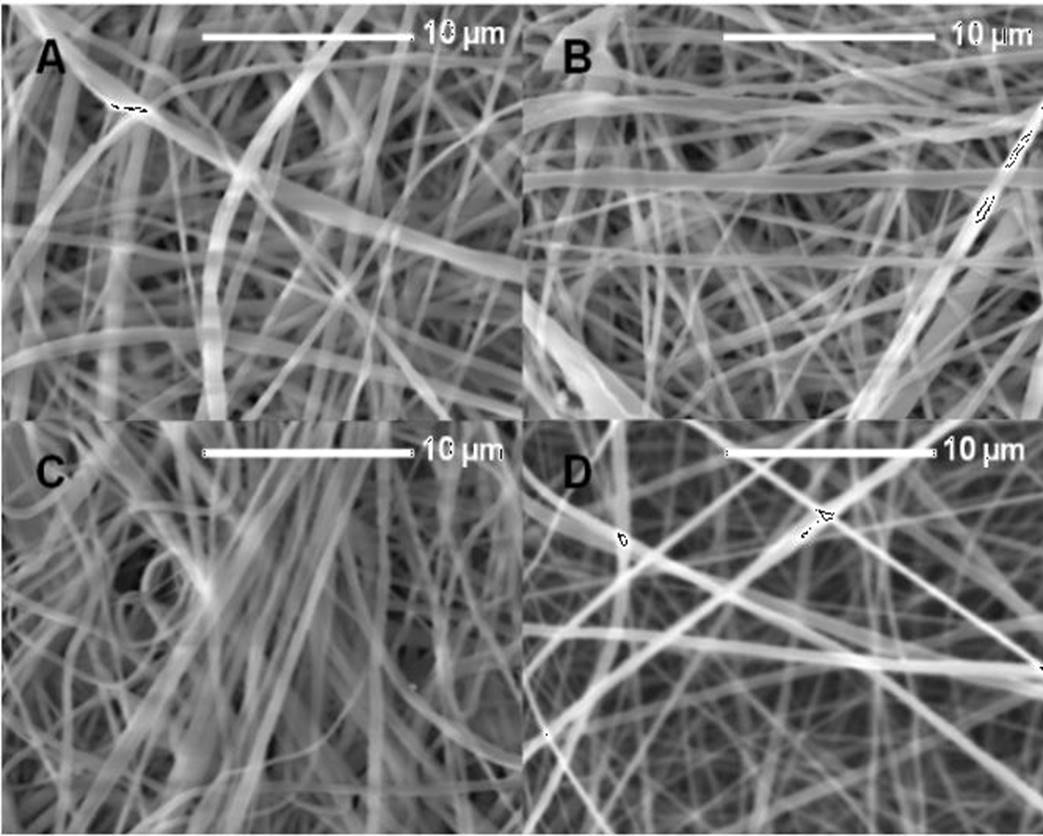
Figure 3 SEM micrographs of the PLA 10% electrospun fibers obtained using a rotating drum with flow variation from (A) 0.6 mL/h, (B) 0.8 mL/h, (C) 1.0 mL/h, (D) 1.2 mL/h.
Figura 3. Micrografías MEB de fibras electrohiladas de PLA al 10% usando un cilindro giratorio con variación de flujo de (A) 0.6 mL/h, (B) 0.8 mL/h, (C) 1.0 mL/h, (D) 1.2 mL/h.
For the next stage, ES fibers obtained with the rotating drum using a flow rate of 0.6 mL/h were subjected to alkaline hydrolysis with the objective to improve hydrophilicity, PLA/H fibers were obtained (Fig. 4). It can be observed that the treated fibers have a diameter that varies between 200 to 563 nm, thus we assume that the alkaline treatment reduces the diameter of the fibers, and increases the roughness of the surface; this morphological change is the result of hydrolysis process. Since the fibers may be considered as a promising material to be used as the matrix or scaffold for tissue engineering, the effect of the observed rugosity could influence the behavior of the cellular adhesion.
Thermogravimetric analysis (TGA)
Thermogravimetric analysis of PLA and PLA/H fibers was performed to evaluate the effect of hydrolysis treatment on the structure of PLA. The thermogram of the samples are shown in Fig. 5, where the TGA curve resulted as expected and shows a small difference between the PLA and PLA/H curves. The insert of Figure 5 shows the DTG curve and it is observed that the PLA/H fibers were degraded at a temperature of 335 °C while the fibers without hydrolysis degraded at 350 °C, the small variation between the samples proves that the alkaline hydrolysis was slight as desired and did not significantly compromise the thermic stability of PLA fibers.
FTIR characterization of PLA, PLA/H and PLA/R. hymenosepalus fibers
The infrared spectrum study was realized to evaluate the effect of alkaline hydrolysis and the addition of R. hymenosepalus, for this PLA, PLA/H, PLA/R. hymenosepalus and PLA/H/R. hymenosepalus samples were analyzed. In this way, the differences observed in the FTIR spectrum will show modifications to fibers, as shown in Fig. 6. Spectrum 6 (A) and 6 (B) show the same signal pattern and agrees with the sample composition, sample PLA/H was slightly hydrolyzed, so their chemical structure is not affected by the process.
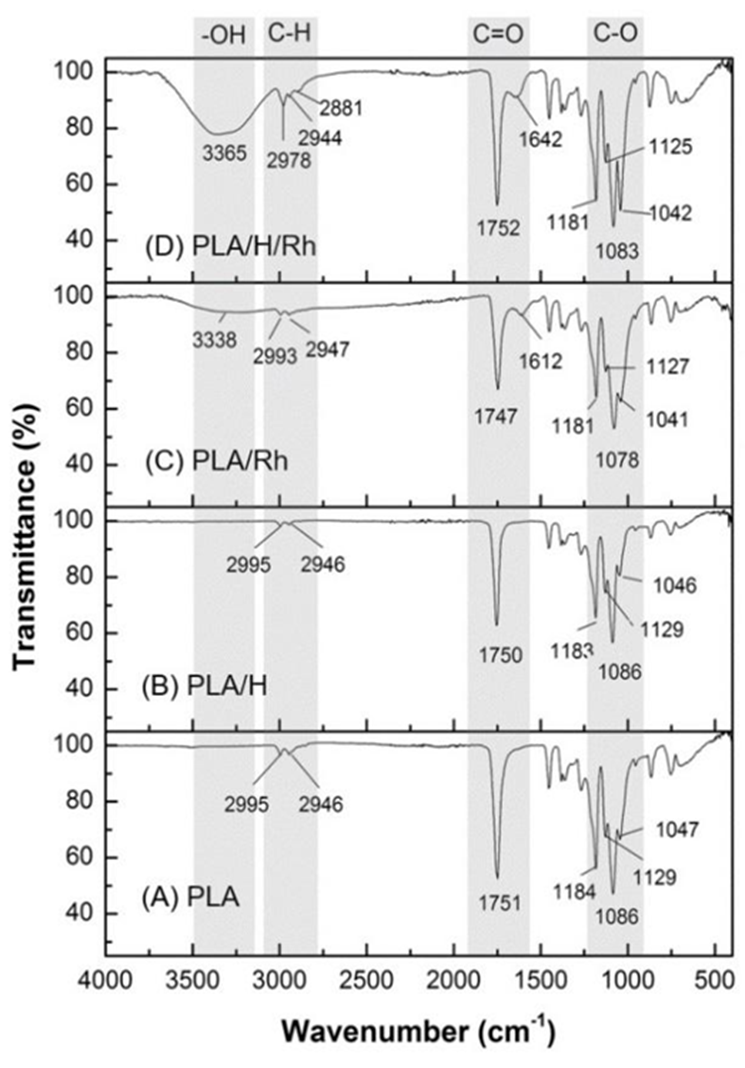
Figure 6 FTIR spectra of electrospun fibers A) PLA, B) PLA/H, C) PLA/R. hymenosepalus and D) PLA/H/R. hymenosepalus.
Figura 6. Espectro FTIR de fibras electrohiladas A) PLA, B) PLA/H, C) PLA/R. hymenosepalus y D) PLA/H/R. hymenosepalus.
The samples added with R. hymenosepalus are expected to have more differences at FTIR analysis. Fig. 6 (C) show small differences over PLA/R. hymenosepalus and PLA or PLA/H samples, this result leads us to know that the PLA/R. hymenosepalus sample has minimum interaction between PLA fibers and R. hymenosepalus extract. Fig. 6 (D), on the other hand, show a significant difference over the other samples, this result shows that the hydrolysis process was effective in improving the interaction of extracts on PLA fibers. This can be explained by considering the functional groups exposed in PLA/H/R. hymenosepalus fibers. As is known, the R. hymenosepalus extract has high polyphenols content, being epicatechin and epicatechin gallate the main components, which are characterized as having several hydroxyl groups in their structures. Moreover, PLA/H fibers have functionalized carbonyl groups as a result of the alkaline hydrolysis to which they were submitted. The interaction between the functional groups PLA-R. hymenosepalus occurs through the formation of hydrogen bond which can be verified by the presence of a broadband characteristic of an associated alcohol in the region of 3365 cm-1, if we look at the FTIR spectrum of the R. hymenosepalus extract, Figure.6, we see the -OH band at 3232 cm-1.
Contact angle measurements of PLA and PLA/H/R. hyme-nosepalus samples
Although the determination of the contact angle is a technique that allows to study the surfaces of materials, the variations in the contact angle values will allow to observe the treatments effects on the fibers. Water contact angle was measured for the sample’s PLA, PLA/H, PLA/R. hymenosepalus, and PLA/H/R. hymenosepalus to evaluate the effect of alkaline hydrolysis and R. hymenosepalus addition. Figure 7A shows the characteristic behavior of PLA giving a high contact angle of 130° for water proper of PLA hydrophobicity. PLA added with R. hymenosepalus, Fig. 7B otherwise show contact angle of 0°, since FTIR of this sample had minimum differences with PLA sample, the difference at contact with water because the ethanolic extract increases the permeability of the PLA fiber. Fig. 7C show water contact angle measurement of PLA/H with the result of 126°, where the effect of hydrolysis reducing the hydrophobicity is evident. The sample PLA/H/R. hymenosepalus added with R. hymenosepalus, shows the effect of permeability of the fiber mat giving a water contact angle of 0°.
R. hymenosepalus release study by UV-vis spectroscopy
The antioxidant release studies were conducted using water to keep the hydroxyl groups of the polyphenols contained in the R. hymenosepalus extract protonated. Because the structure of the PLA is hydrolyzed at alkaline pH, the use of water is also appropriate to ensure that the observed changes in absorbance are due to PLA fibers releasing the antioxidant and not released for PLA degradation. The concentration of extract used for the release process was chosen taking into account the reports made by Rodríguez et al. (2018), in which antibacterial capacity of the R. hymenosepalus was observed using aliquots of 30 µL of extract.
A UV-vis calibration curve was performed with R. hymenosepalus extract (Fig. 8A). Once the maximum absorption wavelength of R. hymenosepalus has been obtained, the 278 nm maximum was selected as the wavelength by which the concentration variation of R. hymenosepalus was evaluated. A constant 50 mL volume of water was used to carry out the R. hymenosepalus release study, to compare the effect of alkaline hydrolysis on the release, the study was performed with the PLA/R. hymenosepalus and PLA/H/ R. hymenosepalus samples. At Fig. 8B the PLA/R. hymenosepalus fiber shows a fast release during the first 90 min and then follows a gradual release to reach a 15.5% release of the R. hymenosepalus extract. To interpret the rapid release of R. hymenosepalus extract during the first few minutes, it should be considered that ES fibers have large contact area and that Fibers-R. hymenosepalus interaction have low intensity since the extract was added superficially and not integrated into the polymer structure. While for the extract incorporated into the alkaline hydrolysis contract fibers PLA/H/ R. hymenosepalus the release has a slight delay, in the 90th minute it starts to be gradually released to reach a 13.5% release, this behavior indicates that there is greater interaction between the surface of the fibers and the polyphenols present in the extract. The R. hymenosepalus release is comparable to previously reported release systems for wound dressings applications (Perumal et al., 2017). After 8 h of release study, the ES PLA fibers were degraded in the media with constant magnetic stirring. Fig. 8 C and 8D shows the UV-Vis spectroscopy release study for PLA/R. hymenosepalus and PLA/H/R. hymenosepalus fibers, where a gradual release of R. hymenosepalus can be observed. The maximum release of antioxidants was reached at 6 h, since 2x10-5 µm of R. hymenosepalus was added to the fiber mats, the release percentage of PLA/R. hymenosepalus and PLA/H/R. hymenosepalus represents 3.1x10-6 µM and 2.7x10-6 µM of R. hymenosepalus respectively. The obtained results about antioxidant release, the high content of polyphenols of the R. hymenosepalus extract and their antibacterial properties reported by Rodriguez et al. (2018) makes PLA ES fibers loaded with R. hymenosepalus appropriate for wound dressings applications, giving a synergic effect between the contact surface of PLA structure and R. hymenosepalus extract properties.
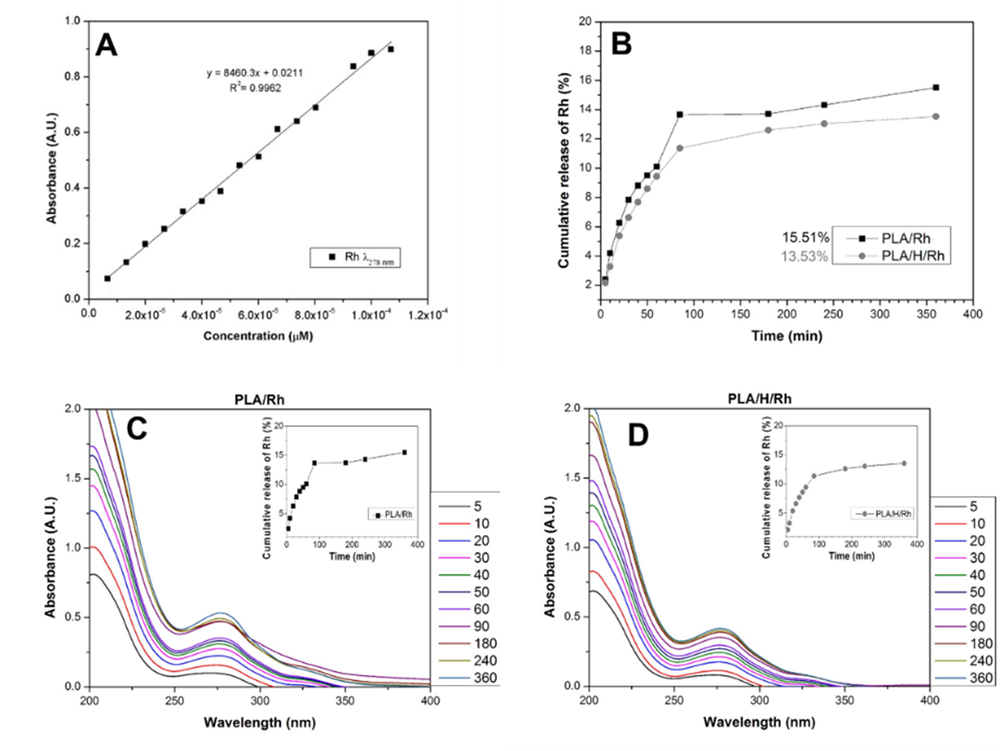
Figure 8 (A) UV-Vis calibration curve of R. hymenosepalus extract in water, (B) Rh release study of R. hymenosepalus in PLA/Rh and PLA/H/Rh samples, (C) UV-Vis release of PLA/R. hymenosepalus and (D) UV-Vis release of PLA/H/R. hymenosepalus.
Figura 8. (A) Curva de calibración UV-Vis del extracto R. hymenosepalus en agua, (B) Estudio de liberación de R. hymenosepalus en las muestras PLA/R. hymenosepalus y PLA/H/R. hymenosepalus, (C) liberación en UV-Vis de PLA/R. hymenosepalus y (D) liberación UV-Vis de PLA/H/R. hymenosepalus.
Conclusions
PLA ES fibers loaded with R. hymenosepalus extract were obtained and show release properties during the 6 h period, the comparation of release behavior between PLA ES fibers with and without hydrolysis process shows that the alkaline treatment contributes to a better interaction between the polymeric matrix and the R. hymenosepalus extract. The obtained results in the fiber treatment together with the R. hymenosepalus properties allow to consider PLA ES fibers loaded with R. hymenosepalus potentially useful for antioxidant release applications like wound dressings, giving a synergy effect between the PLA structure and R. hymenosepalus properties.











 nueva página del texto (beta)
nueva página del texto (beta)





