1. Introduction
Alkali-aggregate reactions (AAR) are chemical reactions that occur between reactive mineralogical constituents of aggregates, alkaline ions and hydroxyl present in the interstitial solution of cement paste. The reaction product is an expansive gel that in the presence of water expands, causing an increase in the internal structural forces, generating deformations and cracks in the concrete surface. These can have a highly detrimental effect on the cement, compromising even the lifespan of the structure (Thomas, 2011).
Currently, three types of deleterious reaction are considered, depending on the mineralogical composition of the aggregates and the mechanisms involved: alkali-silica reaction, alkali-silicate reaction and alkali-carbonate reaction. The alkali-carbonate reaction is not discussed in greater detail in this paper because it was not part of our research. The term alkali-aggregate reaction (AAR) when mentioned in the text refers to the alkali-silica or alkali-silicate reactions.
The first stage of the alkali-silica reaction (ASR) is the reaction between the hydroxyl ions (OH-) present in the pore solution, and the reactive silica of the aggregate. Initially the alkalis increase the concentration of hydroxyl ions in the solution, and the expansive gel production (Thomas, 2011; Beyene et al., 2013). ASR can be represented in a simplified form by Equations (1) and (2) (West, 1996 apud Campos, 2015).
For AAR to occur, three conditions are necessary: i) presence of reactive phases in the aggregate (ii) enough moisture, and iii) high alkali hydroxides concentration in the concrete pore’s solution, enough to react with the reactive phases of the aggregates (Giordano, 2007).
The raw materials used in the manufacture of Portland cement are generally responsible for the alkalis present in the cement, ranging from 0.2% to 1.5% Na2O equivalent (Na2O + 0.658K2O). Because of cement hydration, there is an interstitial solution in the concrete containing essentially sodium, calcium and potassium hydroxide. Normally, depending on the amount of alkalis, the pH in the pore solution ranges from 12.5 to 13.5. This pH represents a strongly alkaline liquid in which some acid rocks (aggregates composed of silica and siliceous minerals) do not remain stable (Giordano, 2007). In other words, the presence of alkalis influences the reactivity of the aggregate because the more alkalis available, the higher the concentration of OH- in the solution of the pores and, consequently, the more silica will dissolve (Beyene et al., 2013).
If the expansive gel is formed, there is no way to reverse the process, only minimizing its damage. Recent studies (Thomas, 2011; Beyene et al., 2013) showed that AAR expansion is reduced when a pozzolanic cement or pozzolanic mineral additions are used. Therefore the use of pozzolanic mineral additions, such as silica fume and metakaolin, in the concrete mixture is recommended. These studies evaluate the effects of mineral additions on the alkali-aggregate reaction and note that the use of a sufficient amount of a suitable mineral addition is one of the most efficient measures for the AAR prevention, by controlling expansion when a reactive aggregate is used in the production of concrete (Thomas, 2011).
2. Materials and methods
2.1. Materials
In the present work, a special cement produced by the Brazilian Association of Portland Cement (ABCP), containing high alkali contents and indicated for accelerated AAR tests was used. Additionally, an ordinary Portland cement CP V ARI-RS (equivalent to type V cement, high sulfate resistance, according to ASTM C150), composed of clinker and calcium sulphate, non-mitigating of the alkali-aggregate reaction, was used so as to evaluate the effect of the additions (metakaolin and silica fume).
Portland cement CP V ARI, metakaolin, silica fume, sand and gravel commercialized in the city of Salvador, BA, Brazil, water from the public supply system and a water-based superplasticizer chemical additive were used.
2.2.1 Materials characterization
Characterization of the materials included the physical parameters, such as the specific surface area (estimated by BET, using a Micromeritics Gemini 2370V1.02 surface area analyzer) and specific gravity (Micromeritics Accupyc 1330V2.01 helium pycnometer) for the cement, metakaolin and silica fume. Sieving was done according to Brazilian standard NBR 9776 ("Aggregates - Determination of the specific mass of small aggregates by Chapman flask"), for the sand. The chemical composition of the cement was determined by X-ray diffraction (XRD).
2.2.2 Mortar molding
The mortar mix proportions were 1.0 : 3.0 (Portland cement : sand) and the water/binder ratio was 0.6 (by weight), with the mineral additives and superplasticizer additive present in varying contents according to Table 1.
Table 1 Material consumption needed to produce 1m3 of mortar with silica fume or metakaolin.
| Content | Cement (kg) | Sand (kg) | Water (kg) | Additive (kg) | Silica Fume or Metakaolin (kg) | Water/ binder ratio | Water/ cement ratio |
|---|---|---|---|---|---|---|---|
| REF | 485.45 | 1456.35 | 291.27 | 1.37 | 0.00 | 0.60 | 0.60 |
| 10% | 436.90 | 1456.35 | 291.27 | 3.55 | 48.55 | 0.60 | 0.67 |
| 15% | 412.63 | 1456.35 | 291.27 | 7.10 | 72.82 | 0.60 | 0.71 |
| 20% | 388.36 | 1456.35 | 291.27 | 14.20 | 97.09 | 0.60 | 0.75 |
In order to relate the physical properties of the mortars to their behavior according to the alkali-aggregate reaction and to analyze the influence of mineral additions, some tests were performed on the mortars used. The experimental study involved mechanical strength tests (ABNT, 2005), water capillarity and apparent density tests using the Archimedes principle.
The AAR expansion was measured using prismatic specimens (25×25×285 mm3) into which steel bars were inserted during molding, according to NBR 15577/2008 Parts 4 and 5 (ABNT 2004a; ABNT 2004b). These tests were carried out to verify the aggregates reactivity in relation to the alkali-aggregate reaction and the capacity of the mineral additions to mitigate this reaction.
A minimum of 4 samples were tested for each mixture.
2.2.3 Mortar performance analysis
a) Mechanical resistance
For mechanical strength performance analysis, axial compression and flexural tensile strength tests were performed at 3, 7 and 28 days, according to NBR 13279 (ABNT, 2005).
The axial compression strength (RC) is given by the ratio between the maximum load (P) supported by the specimens and the specimens original section area (A), according to Equation (3).
The flexural tensile strength (RTF) is determined by Equation (4).
Where P = maximum load applied, in N; L = distance between support blades, in mm; B = specimen width in the section of rupture, in mm; D = specimen height in the breaking section, in mm.
b) Apparent Density and Porosity
For the determination of the apparent density and porosity of the concretes, the Archimedes principle was used. The technique consists of comparing the masses of the test specimens, 28 days during, before and after immersion in water. The samples are weighed still dry (Ms) and then immersed in water for 24 hours. After this period, the immersed mass (Mi) and the wet mass (Mu) are determined. With these values, the apparent porosity (Pa) and the apparent density (Da) are calculated using Equations 5 and 6.
In which ρ is the density of the liquid used (in this case, the water, ρ equals 1.0 g/cm³).
c) Sorptivity
The sorptivity is defined as a measure of the capacity of the matrices to absorb or desorb liquid by capillarity. High sorptivity is an indicator of a greater diffusion of elements and solutions in the cementitious matrix, increasing the chances of AAR occurrence.
Capillary water absorption (sorptivity) was determined from specimens measuring 40 mm x 40 mm x 160 mm. The specimens were demolded 24 hours after being cast and were cured for 28 days in a humid chamber (>95% RH). During the test, the saturated mass of the specimens with time intervals standardized by the NBR 9779:2012 ("Mortar and hardened concrete - Determination of water absorption by capillarity") is determined. The capillary absorption coefficient (or absorptivity, S), which represents the mass of water absorbed per square meter of the mortar in contact with water, can be calculated as a function of the square root of the time elapsed until reaching this point of absorption.
2.2.4 Alkali-Aggregate Reactivity (AAR) determination
To evaluate the reactivity of the Salvador metropolitan region aggregates to AAR, the Brazilian standard NBR 15577-4 (ABNT, 2008a) was used. This method consists of evaluating the dimensional mortar bars expansion subjected to a sodium hydroxide alkaline solution at 80°C.
For the mortar preparation, the gravels must be obtained with minimum crushing in order to produce a product classified according to the standard. Bars (25 mm x 25 mm x 285 mm) were molded with weight ratios of cement: aggregate 1: 2.25 and with ratio water/cement fixed and equal to 0.47, using a special cement produced by ABCP, suitable for conducting this type test and meeting the ABNT NBR 5732 (ABNT, 1991) requirements.
2.2.5 Efficiency evaluation of the active additions in mitigating the occurrence of AAR
The method defined by NBR 15577-5 (ABNT, 2008b), using the same principle as the ABNT method NBR 15577-4 (ABNT, 2008a) is recommended to evaluate the efficiency of pozzolanic materials to mitigate expansion due to AAR.
In this method mixtures are made with additions and without additions. Three mortar bars with dimensions 25 mm x 25 mm x 285 mm were made for each. Portland cement CPV ARI-RS (equivalent to type V cement, according to ASTM C150) was used, which proved to be non-mitigating to the AAR and the reactive aggregate available in the region.
The expansion measures are done in a similar way to NBR 15577-4 (ABNT, 2008a), and after 30 days the comparative expansion analysis in the reference mortar bars (without additions) and the mortar bars with addition of metakaolin and silica fume, in the contents of 10%, 15% and 20% are carried out. As a result, it is possible to see whether or not the material contributed to the reduction of expansion caused by the AAR.
3. Results and discussions
3.1. Materials characterization
The chemical composition of the cement and the physical characteristics of the materials used in the study are shown in Tables 2 and 3.
Table 2 Cement chemical composition, determined by XRD.
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Alkali Content | ||
|---|---|---|---|---|---|---|---|
| Na2O | K2O | Na2Oeq* | |||||
| 19.10 | 4.84 | 3.19 | 61.12 | 2.73 | 0.24 | 0.70 | 0.70 |
* Na2Oeq = Na2O + 0,658K2O
3.2 Mortar performance analysis
Figures 1 and 2 present the compressive strength and flexural tensile strength results, at 3, 7 and 28 days, of the mortars with partial cement replacement with silica fume and metakaolin, respectively. The traces used were 1: 3, with water / binder ratio (cement + addition) equal to 0.6, according to Table 1. A super plasticizing additive was also used in order to ensure the workability of the mixture.
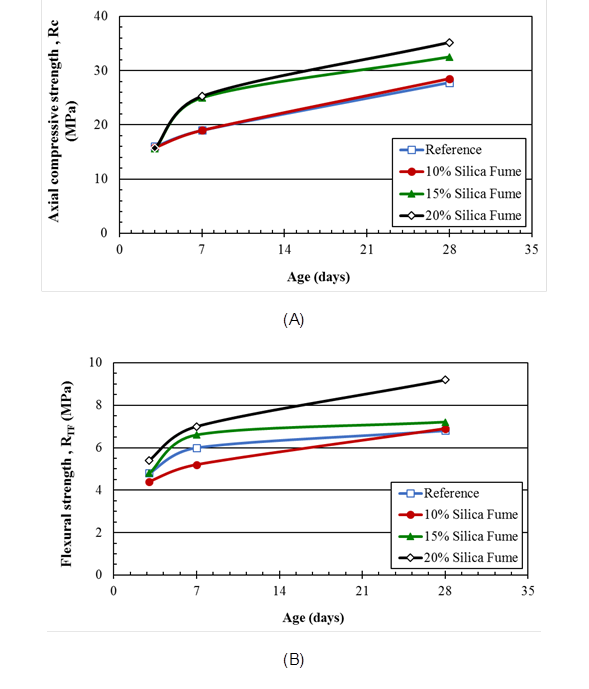
Figure 1 (A) Axial compressive and (B) flexural tensile strength of mortars containing silica fume, partially replacing Portland cement, as a function of days of curing.
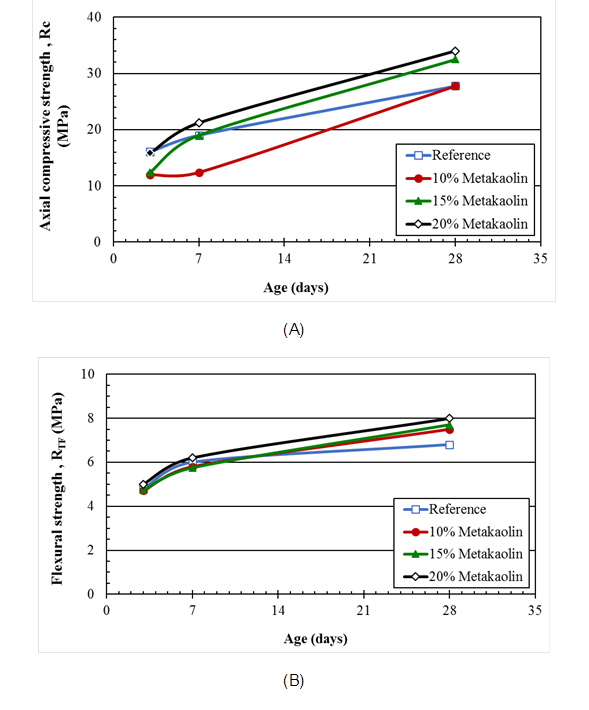
Figure 2 (A) Axial compressive and (B) flexural tensile strength of mortars containing metakaolin, partially replacing Portland cement, as a function of days of curing.
It can be observed that as the silica fume content increases, there is an increase in the mechanical strength of the mortar, reaching axial compression values of 35.2 MPa with 20% silica fume, while the reference mortar presented a resistance of 27.8 MPa (a 27% increase). Similar behavior is observed for the flexural tensile results, however, for silica fume contents of less than 20%, at 28 days, the specimens showed a resistance close to the reference for the mortar specimens.
The increase in strength is also observed with 15% metakaolin (Figure 2), where the axial compression reached 32.5 MPa, while the reference mortar presented a resistance of 27.8 MPa (a 17% increase). Although the mortars of 10% showed less resistance than the reference mortars, the results are in very close error ranges, and it was concluded that there was no considerable resistance variation with a 10% addition of material.
In the flexural tensile results, no significant resistance increase was observed in the mortar with the addition. This behavior was also observed by Beltrão (2010) when testing concrete specimens with added metakaolin contents of 6%, 10% and 14%.
According to Hassan et al. (2012) the compressive strength results at 28 days show that the addition of any metakaolin content increases the compressive strength of the concrete. However, there is no "linearity" in the relationship between the content used and the increase in resistance because concrete with 8% metakaolin showed better results than with 11%. Munhoz (2007) also observed a decrease in compressive strength when comparing the results of specimens with active additions of 5% and 10%, the result was 10% lower when compared with contents of 5% up to 20%.
In addition, an improvement of only 7% in the compressive strength was observed with the 8% of metakaolin addition, when using contents up to 25% (Munhoz, 2007). It is possible that "mortar saturation" occurred because the available addition amount was much greater than the calcium hydroxide amount (cement hydration product). With this "disproportional" relationship, the pozzolanic reaction will occur more slowly, delaying the formation of C-S-H and resulting in a smaller increase in the resistance with high addition contents (Beltrão, 2010) as a result.
The increase in the mortar resistance with the addition of silica fume and metakaolin occurs because pozzolana, together with calcium hydroxide, generates binding products, like the phases resulting from the direct hydration of the clinker grains. Consequently, the structure is more compact, chemically and mechanically stronger than that of ordinary Portland cement. Thus, the presence of pozzolanic cements contributes to greater compactness and compressive strength after 28 days curing and greater impermeability to water (Giordano, 2007).
The use of silica and metakaolin in cement produces a less permeable mortar as expected and also observed in studies by Gomez-Zamorano et al. (2015). From the characterization results (Table 3), it is possible observe that silica and metakaolin are finer than cement and thus they have a larger surface area. The finer particles of these materials tend to decrease the relative amount of capillary pores, reflecting a reduction in water absorption by capillarity, as seen in Figure 3.
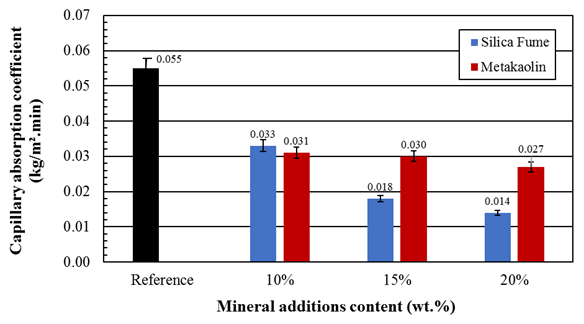
Figure 3 Capillary absorption coefficient of mortars containing silica fume and metakaolin partially replacing the Portland cement after curing for 28 days.
Figures 4 and 5 presents the apparent density and porosity results for the reference mortars (without addition) and those containing silica fume and metakaolin in partial replacement of the Portland cement. It can be observed that there is no significant variation in mortar density when partially replacing Portland cement with pozzolanic materials, however, a significant increase in porosity is observed when using silica and a reduction in porosity is observed when using metakaolin.
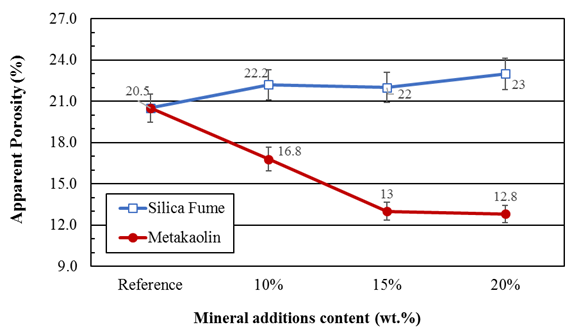
Figure 4 Apparent porosity of mortars containing silica fume and metakaolin partially replacing the Portland cement after curing for 28 days
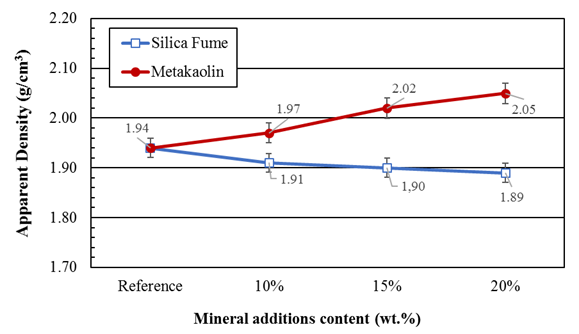
Figure 5 Density of mortars containing silica fume and metakaolin partially replacing the Portland cement after curing for 28 days
Since metakaolin and silica fume are finer than cement, it is expected that there will be pore filling, as well as its refinement and, consequently, porosity reduction.
The results reported by Siddique (2011) indicate that the use of silica fume reduces the porosity of the cement unlike what was observed in this work. It is believed that the use of the additive without partial water reduction influenced this behavior. This is because despite the water / binder ratio being constant, the w / c ratio varied, as shown in Table 1. For higher silica contents, a higher a/c ratio and, consequently, a higher water amount in the mixture is observed. The additional water, which is not consumed in the hydration of the cement, remains free in the system and, upon evaporation, gives rise to the increase in the porosity of the mortar.
3.3 Aggregates reactivity determination of the Salvador metropolitan region
Some sands and gravels from deposits located in the metropolitan region of Salvador (SMR) and Feira de Santana were analyzed. Figure 6 presents the results of the mortar bars expansion, submitted to the AAR test, as a test time function, for several gravels. According to the NBR 15577: 2008, for AAR tests for the evaluation of gravels, they must be ground in order to obtain a desired granulation similar to sand.
Figure 7 shows the results of the mortar bar expansions, submitted to the AAR test, as a test time function, for sands marketed in SMR and in Feira de Santana.
Analyzing the results obtained with the aggregates used, it can be observed that, in general, the gravels are reactive regarding AAR, whereas the sands are potentially innocuous. Therefore, there are restrictions on the use of gravels in SMR. Depending on the moisture conditions and the selected cement, it will be necessary to use active additions in the cement to mitigate the AAR.
3.4 Efficiency evaluation of the mineral additions in AAR mitigation
The results of the investigations of the expansions carried out using the accelerated method in the presence of silica fume and metakaolin are presented in Figures 8 and 9, respectively.
The behavior of the mortar specimens with the addition of silica fume (Figure 8) was similar to that observed by several authors (Thomaz, 2011; Hasparyk and Farias, 2013; Lindgard et al., 2012), who found that this contributes to the reaction mitigation due to the pozzolanic properties of the material.
The works cited used maximum levels of silica fume between 12% and 15%, however, in the present research, higher levels were added in order to verify if the indiscriminate use of this material interferes in the AAR. It was found that when the content reached 15%, there was a stagnation in the mitigation capacity of this additive. Thus, when using higher amounts than this no improvement in the material behavior is expected, and it may even result in an inverse behavior, that is, an AAR increase. This is because the silica fume is amorphous silica and therefore reactive, therefore it is thought that if used in excess, a "saturation" of this material can occur in the interstitial pores of the cementitious matrix. Part of the silica fume will react with Ca(OH)2 as a pozzolanic material, and the excess will be available to react with the alkalis which have not been incorporated into the C-S-H structure.
The behavior of the specimen with the addition of metakaolin (Figure 9) was similar to that observed by other authors (Munhoz, 2007; Hasparyk and Farias, 2013). The increase in the metakaolin content contributed to the mitigation of the reaction due to the pozzolanic properties of this material. An increasing reduction in reactivity was observed when higher contents of metakaolin was added. The Brazilian standard determines that the reaction mitigation proof is obtained when the accelerated test result in mortar bars is less than 0.10% at 16 days (França et al, 2016). From the results obtained, it is possible to predict that by using metakaolin contents higher than 15%, there will be a greater reduction in expansion and it will be possible to mitigate the AAR. However, these results were only effective when adding 20% of this material.
When comparing the performance of the mineral additions, it can be observed that the silica fume was more effective in mitigating the occurrence of AAR.
4. Conclusions
From the results of the present study, we conclude that:
All the gravels commercially available in the metropolitan region of Salvador and Feira de Santana, studied in the present research, can be classified as reactive aggregates regarding AAR
The small aggregates marketed in the metropolitan region of Salvador, from deposits in the Camaçari region, are classified as potentially innocuous aggregates in relation to the AAR
The mortar performance analysis confirmed that the silica fume and metakaolin improves the properties of the cementitious matrix, resulting in mortars that are more resistant to compression and tensile in flexion and have lower permeability
The silica fume proved to mitigate the expansions caused by AAR, reaching saturation point at 15% addition
When used in excess, occur a silica fume "saturation" in the interstitial solution of pores in the cementitious matrix leading to parts that will not react with the excess Ca(OH)2 to be available to react with the alkalis that were not incorporated into the CSH structure
The metakaolin showed an increased capacity to mitigate the expansions caused by the AAR when applied at levels of 10%, 15% and 20%
The silica fume was more effective in AAR mitigation than metakaolin.











 nueva página del texto (beta)
nueva página del texto (beta)







