Introduction
There is still an incomplete understanding of how plant species control their own nutrition, as well as how management practices modify plant nutrition across the tropics (Wieder et al. 2015, Jakovac et al. 2021). Foliar nutrient contents are a widely used tool for understanding plant nutrition; however, comparative studies among are complex because of the heterogeneity of plant communities, soils, climate (Chakkour et al. 2023) and management practices. Foliar nutrient contents in plants vary according to nutrient availability in soil, plant functional traits (growth, habits, phenology, etcetera), and climate conditions (Bai et al. 2019).
In homegardens, the owners select the species and implement management practices to achieve plant establishment and/or increase their productivity; on the other hand, in the forest sites the management is barely null, being limited mainly to extractive practices (Toledo et al. 2008, DiGiano et al. 2013). Some management practices affect foliar nutrient content, the number of leaves, and decay rates (García-Palacios et al. 2013). By promoting conditions for crop growth, carbon and nutrients cycles are affected to a greater extent (Schmidt et al. 2019). Harvesting results in a negative nutrients balance if extractions are not compensated (Vitousek et al. 2009, Briat et al. 2020). Management practices, such as the elimination of litter (by removal or burning) have also a negative effect on the physical, chemical, and biological properties of soil (Miao et al. 2019, Mayer et al. 2020, Ahlawat et al. 2023); thus, altering nutrient availability and plant uptake. Regional environmental gradients, driven by multiple factors, including precipitation which contributes to soil moisture also affects nutrient dynamics and plant nutrient uptake (Tian et al. 2018). Soil moisture is an important factor for nutrients dynamics in soils because it promotes the decomposition of organic matter and nutrient mineralization (Sierra et al. 2015, Kuchenbuch et al. 1986).
Tree phenological adaptations have also been showed to be related to plant nutrient uptake. Deciduous species generally have higher growth rates, larger specific foliar area, and greater photosynthesis rates, suggesting increased nutritional requirements and nutrient accumulation compared to evergreen species (Huang et al. 2018). However, other authors have proposed that evergreen species deliver more nutrients to soil because the foliage is continuously falling (Aerts and Chapin 1999, Givnish 2002). The aim of this study was to investigate the foliar nutrient contents of three tropical tree species growing under two management systems (forests and homegardens) at two climate regions of the state of Yucatan (Northeast and Southwest). One of the species is evergreen, Brosimum alicastrum, while the other two are deciduous, Cordia dodecandra and Spondias purpurea. The assessment included: i) physicochemical conditions of the soil in which the species grow, ii) variations in foliar contents (C, N, P, K, Na, and Ca) for the three species, and iii) analyses of the soil properties and the foliar nutrient contents per region, management system and species.
Materials and methods
Study sites
The study was conducted at two municipalities of the state of Yucatan: Tizimin (Northeast region) with a predominant Aw1 (X’) climate (tropical sub-humid with summer rains and medium humidity) and an annual precipitation of 1 500 mm (INEGI 2010a) and Tzucacab (Southwest region), with predominant Aw0 climate (sub-humid with summer rains and low humidity), with an annual precipitation of 1 200 mm (INEGI 2010b) (Figure 1). Both regions are undulated karstic plains associated to limestone from the Neogene (Tizimin) and Paleogene (Tzucacab) (INEGI 2010a, 2010b). Leptosols (41.29%) dominate in Tizimin, associated to Luvisols (27.47%) and Phaeozems (20.82%) (INEGI 2010a). Luvisols (64.11%) are dominant in the Tzucacab, associated to Vertisols (20.18%) and Phaeozems (12.69%) (INEGI 2010b). The type of natural vegetation in both regions is medium height semi-deciduous secondary forest.
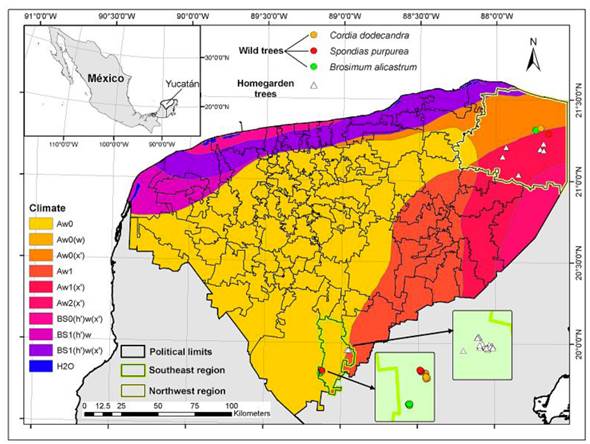
Figure 1 Management systems distribution at study sites. Forests (dots) and Homegardens (triangles) in two climate regions of Yucatan: Northeast (yellow border) in the municipality of Tizimin and Southwest (green border) in the municipality of Tzucacab. Sites where Brosimum alicastrum (green dots), Cordia dodecandra (yellow dots) and Spondias pupurea (red dots) grow in forests had different locations. Due to the availability of the tree individuals in homegardens, in Tzucacab all homegardens were in a single village whereas in Tizimin homegardens were more dispersed.
Sampling design
In both study regions, we selected 20 adult individuals of each species with a diameter at breast height greater than 15 cm from the forest and 20 individuals from homegardens, resulting in a total of 120 individuals per region (N = 240). For the forest, we selected a wild population of each species. However, due to variability in the total number of individuals of each species in the homegardens, we selected a different number of individuals from each species in each homegarden. Specifically, in the Northeast region, we selected 9 homegardens with B. alicastrum individuals, 5 with C. dodecandra individuals, and 9 with S. purpurea individuals. In the Southwest region, we selected 7 homegardens with B. alicastrum individuals, 5 with C. dodecandra individuals, and 9 with S. purpurea individuals. We randomly selected five soil sub-samples from below each treetop at a depth of 0-30 cm or until the R horizon was reached. These sub-samples were combined into one sub-sample for each tree of each species, resulting in a total of 20 sub-samples per species. To determine foliar nutrient content, we randomly collected 20 healthy mature leaves from the middle part of the canopy of each of the 240 tree individuals. We ensured that the leaves were free of foliar damage caused by pests or diseases.
Sample processing and laboratory analyses
The laboratory analyzes were carried out in the Soils, Plants and Water Analysis Laboratory of the Autonomous University of Yucatan. Soil samples were air-dried, and sieved at 2mm, leaves samples were oven dry at 105 °C to constant weight and ground in a mechanical mill. The analyzes that were carried out were: particle size analyses (Bouyoucos’s densimeter method) (Gee and Bauder 1986), pH (water ratio 1:2, potentiometric) (Thomas 1996), electric conductivity (ratio 1:5, potentiometric) (Rhoades 1996), total organic C content (Walkey-Black method) (Nelson and Sommers 1987), Total N content (Kjeldahl’s method) (Bremner 1996), and K, Na, and Ca contents (flamometry) (Helmke y Sparks 1996, Suarez 1996). Leaves were dried (at 60 °C until constant weight) to analyze total organic C (Walkey-Black method) (Nelson and Sommers 1987), total N (Kjeldahl’s method) (Bremner 1996), and K, Na, and Ca contents (flamometry) (Helmke y Sparks 1996, Suarez 1996).
Statistical analysis
For the heuristic exploration of possible data pooling, a principal component analysis (PCA) was conducted. To homogenize the difference in the magnitude of the measurements of the different variables (Manly and Navarro 2016), data transformation through standardization [(Xi -
Subsequently, soil physicochemical properties and nutrient contents and foliar nutrients content were analyzed for the combination between region (Northeast and Southwest) and type of system (forest and homegarden) for each of the species. A discriminant analysis was performed (Supplementary file 3) (Manly and Navarro 2016) to see whether the variations among the three species were associated with variation in soil physicochemical properties, soil nutrients content or with a pattern in foliar nutrients contents. Discriminant analyses were performed for each of the species to assess the variations in soil physicochemical properties, soil and foliar nutrient contents between management systems at the two regions for each species. In addition, a hierarchical analysis of variance was carried out separately for each species to study the variance of soil physicochemical properties, soil nutrient contents, and foliar nutrient contents (Manly and Navarro 2016). Region and type of system nested within the region were considered fixed effects factors, while soil physicochemical properties and soil and leaves nutrient contents were response variables. Analyses were conducted with the original variables after verifying that the assumptions of homoscedasticity and normal distribution of residuals were met. Tukey’s test was conducted to assess the differences between the average of each variable and the combinations of region and system for unbalanced samples. All the analyses were performed with JMP v. 12.01 (SAS Institute, NC, USA).
Results
Variation among species
The MANOVA results highlight differences within the physicochemical properties and nutrient contents of the soil among the three species (λ Wilks = 0.411, F20,346 = 9.68; P < 0.0001) and between effects for the species (F2,182 = 19.18; P < 0.0001). Similarly, significant differences within the nutrient contents of the leaves among the three species (λ Wilks = 0.061, F10,442 = 134.63; P < 0.0001) and between effects for the species (F2,182 = 19.18; P < 0.0001) were found.
The distribution of the studied species is associated with the percentage of silt and clays, pH, and electrical conductivity (λ Wilks = 0.75, F8,468 = 9.23; P < 0.0001) (Table 1). B. alicastrum individuals tended to be in soil with higher pH and electrical conductivity, while C. dodecandra and S. purpurea tended to be in soils with higher content of silt (Figure 2a). The nutrient contents of soils associated with each species were significantly different. (λ Wilks = 0.61, F12,372 = 8.70; P < 0.0001). Soils associated with B. alicastrum had greater contents of C, P, Ca, K, and N. C. dodecandra and S. purpurea individuals were found in soils with lower contents of P, C, and K. (Figure 2b, Table 1). Foliar nutrient contents of the three species were significantly different (λ Wilks = 0.11, F12,458 = 77.71; P < 0.0001). B. alicastrum had greater foliar contents of Na and K; C. dodecandra had more Ca and C, and S. purpurea had higher levels of N and P (Figure 2c, Table 2). Foliar N content was lower in the deciduous species C. dodecandra (1.89%, SD = 0.2), and similar between the evergreen tree B. alicastrum (2.37%, SD = 0.3) and the deciduous species S. purpurea (2.77%, SD = 0.3). Foliar Na content was more than double in B. alicastrum (0.69 cmol kg-1, SD = 0.3) than in C. dodecandra (0.32 cmol kg-1, SD = 0.1), and 5 times higher than in S. purpurea (0.12 cmol kg-1, SD = 0.2). Foliar K content was almost two times higher in B. alicastrum (4.6 cmol kg-1, SD = 1.3) compared to the levels of C. dodecandra (2.7 cmol kg-1 SD = 0.3) and S. purpurea (2.43 cmol kg-1, SD = 0.2). Foliar Ca content was double in C. dodecandra compared to B. alicastrum (2.43 cmol kg-1, SD = 0.5) and almost 4 times higher compared to S. purpurea (1.22 cmol kg-1, SD = 0.4) (Table 2).
Table 1 Physicochemical properties and nutritional contents of soils associated with three tree species (Brosimum alicastrum, Cordia dodecandra, and Spondias purpurea) growing in homegardens and forests of two regions (Northeast and Southwest ) of Yucatan, Mexico.
| R | S | pH | EC | Clay | Silt | Sand | C | N | P | K | Na | Ca |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| μS cm-1 | % | mg kg-1 | cmol kg-1 | |||||||||
| Brosimum alicastrum | ||||||||||||
| NE | H | 6.81b | 0.35ab | 29.60b | 17.20a | 53.20a | 8.84b | 0.58c | 160.61a | 0.47a | 0.11ab | 1.92a |
| (0.06) | (0.04) | (3.08) | (1.59) | (3.77) | (1.41) | (0.08) | (15.56) | (0.02) | (0.02) | (0.10) | ||
| F | 7.18a | 0.29ab | 45.40a | 13.89ab | 40.71a | 19.08a | 0.91b | 9.64b | 0.23c | 0.03b | 1.49b | |
| (0.06) | (0.04) | (3.08) | (1.59) | (3.77) | (1.41) | (0.08) | (15.56) | (0.02) | (0.02) | (0.10) | ||
| SW | H | 7.33a | 0.25b | 35.50ab | 11.60ab | 52.90a | 9.29b | 0.72bc | 138.93a | 0.31b | 0.15a | 1.61ab |
| (0.06) | (0.04) | (3.08) | (1.59) | (3.77) | (1.41) | (0.08) | (15.56) | (0.02) | (0.02) | (0.10) | ||
| F | 7.19a | 0.43a | 39.22ab | 10.00b | 50.78a | 12.70b | 1.26a | 20.31b | 0.25bc | 0.03b | 1.54b | |
| (0.06) | (0.04) | (3.08) | (1.59) | (3.77) | (1.41) | (0.08) | (15.56) | (0.02) | (0.02) | (0.10) | ||
| Cordia dodecandra | ||||||||||||
| NE | H | 6.67b | 0.17b | 19.10c | 19.80ab | 61.10a | 10.55a | 0.84a | 32.76b | 0.30a | 0.04b | 2.13a |
| (0.06) | (0.02) | (2.13) | (1.31) | (2.31) | (0.91) | (0.06) | (9.87) | (0.02) | (0.01) | (0.11) | ||
| F | 7.20a | 0.27a | 31.42b | 15.10b | 53.48a | 8.40ab | 0.63ab | 1.89b | 0.08b | 0.02b | 1.15b | |
| (0.06) | (0.02) | (2.13) | (1.31) | (2.31) | (0.91) | (0.06) | (10.76) | (0.02) | (0.01) | (0.11) | ||
| SW | H | 7.39a | 0.24ab | 51.62a | 24.20a | 24.18c | 6.45b | 0.60b | 80.02a | 0.26a | 0.03b | 1.56b |
| (0.06) | (0.02) | (2.13) | (1.31) | (2.31) | (0.91) | (0.06) | (9.87) | (0.02) | (0.01) | (0.11) | ||
| F | 6.46b | 0.21ab | 57.00a | 9.90c | 33.10b | 9.42ab | 0.77ab | 8.48b | 0.25a | 0.17a | 1.28b | |
| (0.06) | (0.02) | (2.13) | (1.31) | (2.31) | (0.91) | (0.06) | (19.25) | (0.02) | (0.01) | (0.11) | ||
| Spondias purpurea | ||||||||||||
| NE | H | 7.12a | 0.15c | 36.50b | 19.50a | 44.00b | 6.86b | 0.59b | 7.62b | 0.09b | 0.01b | 0.85b |
| (0.08) | (0.02) | (2.38) | (1.41) | (2.57) | (1.04) | (0.10) | (13.61) | (0.01) | (0.01) | (0.15) | ||
| F | 6.77b | 0.34a | 27.30c | 17.06a | 55.64a | 14.39a | 1.37a | 1.39b | 0.21 a | 0.03b | 2.08a | |
| (0.08) | (0.02) | (2.38) | (1.41) | (2.57) | (1.04) | (0.10) | (19.77) | (0.01) | (0.01) | (0.15) | ||
| SW | H | 7.11a | 0.23bc | 47.72a | 21.60a | 30.68c | 5.81b | 0.47b | 69.84a | 0.19a | 0.11a | 0.90b |
| (0.08) | (0.02) | (2.38) | (1.41) | (2.57) | (1.04) | (0.10) | (13.26) | (0.01) | (0.01) | (0.15) | ||
| F | 7.10a | 0.29ab | 23.52c | 19.50a | 56.98a | 13.74a | 1.22a | 10.73ab | 0.21 a | 0.12a | 1.35b | |
| (0.08) | (0.02) | (2.38) | (1.41) | (2.57) | (1.04) | (0.10) | (22.42) | (0.01) | (0.01) | (0.15) | ||
Note: EC= Electrical Conductivity; R = Region (NE = Northeast, SW = Southwest ; S = System (H = Homegarden, F = Forest). Data averages: figures in parenthesis indicate standard deviation (n = 20). Different superscripts in each column indicate significant differences between systems for each variable by species.
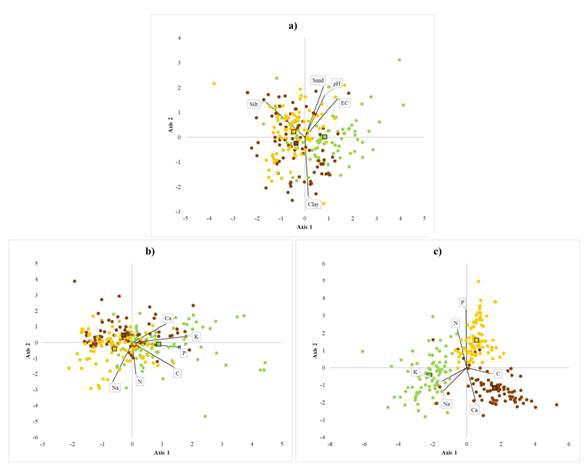
Figure 2 Canonical discriminant groups of the three study species. Note: Brosimum alicastrum (green), Cordia dodecandra, (brown) and Spondias purpurea (yellow), for two canonical axis considering: a) physicochemical conditions of soil: pH, electrical conductivity (uS cm-1), clay (%), silt (%) and sand (%); b) soil nutrient contents: C (%), N (%), (mg kg-1), K (cmol kg-1), Na (cmol kg-1), and Ca (cmol kg-1); and c) leave nutrient contents: C (%), N (%), (mg kg-1), K (cmol kg-1), Na (cmol kg-1), and Ca (cmol kg-1)
Table 2 Nutrients content in leaves of three multi-purpose species (Brosimum alicastrum, Cordia dodecandra, and Spondias purpurea) growing in homegardens and forests at two regions (Northeast and Southwest ) of Yucatan, Mexico.
| R | S | C | N | P | K | Na | Ca |
|---|---|---|---|---|---|---|---|
| (%) | (mg kg-1) | (cmol kg-1) | |||||
| Brosimum alicastrum | |||||||
| NE | H | 41.99a | 2.61a | 331.81a | 3.67b | 0.42b | 0.43c |
| (1.05) | (0.06) | (43.76) | (2.39) | (0.57) | (0.22) | ||
| F | 40.29a | 1.92b | 89.85b | 3.32b | 0.45b | 0.44c | |
| (1.05) | (0.06) | (43.76) | (2.39) | (0.57) | (0.22) | ||
| SW | H | 38.49a | 2.50a | 444.34a | 5.85a | 1.09a | 3.68b |
| (1.05) | (0.06) | (43.76) | (2.39) | (0.57) | (0.22) | ||
| F | 33.34b | 2.46a | 153.79b | 5.57a | 1.27a | 5.16a | |
| (1.05) | (0.06) | (43.76) | (2.39) | (0.57) | (0.22) | ||
| Cordia dodecandra | |||||||
| NE | H | 35.34c | 2.17a | 211.74a | 2.64a | 0.24b | 3.31b |
| (0.84) | (0.07) | (18.13) | (2.70) | (0.41) | (0.24) | ||
| F | 33.45c | 1.94ab | 122.70b | 2.41a | 0.18b | 4.01b | |
| (0.84) | (0.07) | (18.13) | (2.70) | (0.41) | (0.24) | ||
| SW | H | 52.25b | 1.70b | 227.53a | 2.75a | 0.72a | 5.06a |
| (0.84) | (0.07) | (18.13) | (2.70) | (0.41) | (0.24) | ||
| F | 57.49a | 1.73b | 134.25b | 3.08a | 0.63a | 4.22ab | |
| (0.84) | (0.07) | (18.13) | (2.70) | (0.41) | (0.24) | ||
| Spondias purpurea | |||||||
| NE | H | 40.73b | 3.23a | 809.01a | 2.64a | 0.07c | 0.78b |
| (0.78) | (0.06) | (28.54) | (1.36) | (0.02) | (0.13) | ||
| F | 40.71b | 2.54b | 319.48c | 2.12b | 0.17b | 1.08b | |
| (0.78) | (0.06) | (28.54) | (1.36) | (0.02) | (0.13) | ||
| SW | H | 44.17a | 2.70b | 494.50b | 2.98a | 0.38a | 1.19b |
| (0.78) | (0.06) | (28.54) | (1.36) | (0.02) | (0.13) | ||
| F | 41.51ab | 2.60b | 215.78c | 1.97b | 0.36a | 1.81 a | |
| (0.78) | (0.06) | (28.54) | (1.36) | (0.02) | (0.13) | ||
Note: R = Region (NE = Northeast, SW = Southwest, S = System (H = Homegarden, F = Forest). Data averages: figures in parenthesis indicate standard deviation (n = 20). Different superscripts indicate significant differences between systems and regions for each variable by species.
Variation associated with systems management
Contents of sand, silt, and clay are essential variables at the level of species, region, and system. Both, soil physicochemical properties and nutrient contents helped are clearly distinct between homegardens from forests. The variation in soil properties suggests that the homegarden species develop in soils with higher pH and less electrical conductivity than those of the forest (Table 1, Figure 3). Homegardens variation in soil texture also exhibits different patterns per region in the case of B. alicastrum and C. dodecandra. S. purpurea grows in homegardens with more clay and less sand (Figure 3). The variance in physicochemical properties was significant between management systems at both regions, except for the percentages of silt in soils associated with C. dodecandra and sand in those associated with B. alicastrum. Averages and standard deviations of soil physicochemical properties are shown in Table 1. Soils pH in homegardens ranged from 6.67 - 7.39, and electrical conductivity oscillated between 0.17 - 0.35 µS cm-1. Soil pH in the forest oscillated between 6.46 - 7.20 and electrical conductivity from 0.21 to 0.43 µS cm-1. B. alicastrum and C. dodecandra pH averages were significantly higher in forest soils than in homegardens at the Northeast region; in contrast, S. purpurea and C. dodecandra soil pH averages were significantly higher in homegardens at the Northeast region and Southwest region, respectively. Electrical conductivity averages were 1.5 and 2 times lower in homegardens soils than in the forests for the species B. alicastrum at the Southwest and C. dodecandra and S. purpurea at the Northeast. B. alicastrum and C. dodecandra homegardens soils had twice less clay at the Northeast; soil of C. dodecandra at the Southwest region had 2.4 times more silt and 1.4 less sand. S. purpurea soils in both regions had 1.3 - 2 times more clay and 1.2 - 1.8 times more sand. Foliar C, Na, K, and Ca contents were higher at the Southwest region, whereas P and N contents were higher in leaves from the Northeast region; foliar P content was three times higher in trees from homegardens than in forest individuals (Table 2). At each region, it was possible to discriminate between individuals from homegardens or forests based on the variation of nutrient contents in soils and leaves for each of the species (Figure 4a). Soils at homegardens associated to B. alicastrum had higher contents of P, K, and Ca, while forest soils at the Northeast region had higher levels of C; forest soils in Southwest region had greater contents of N and Na (Figure 4a). Foliar Na and Ca contents were higher in the Northeast forests; at the Southwest, C levels were higher in both homegardens and forests. P, N, and Ca contents were more elevated in Southwest homegardens (Figure 4b). Northeast homegardens where C. dodecandra grows have higher contents of Ca, K, and N, while Southwest homegardens have higher levels of P and Na (Figure 4c). Foliar P, K, and Na content were higher in Southwest homegardens; in the Northeast, homegardens and forest leaves had higher levels of N; Southwest forest leaves were more abundant in C (Figure 4d). Homegarden soils of the Northeast region where S. purpurea grows had higher contents of P in both regions; P and K levels were higher in Southwest forests compared to Northeast forest. In contrast, C, N, and Ca levels were higher at the Northeast (Figure 4e). Foliar K and Na contents were greater in Southwest homegardens, and C in Southwest forest. Northeast homegardens had higher levels of N and P (Figure 4f). Soil nutrient contents exhibited differences between systems at both regions, except for P values in the case of S. purpurea. Foliar nutrient contents also differed, except for B. alicastrum K and N, Na, and K in C. dodecandra and S. purpurea (Table 2). C and N contents in homegardens soils were approximately two times lower than in the forests. However, only C averages were significantly different for B. alicastrum at the Northeast, and for S. purpurea in both regions. N averages also had significant variations in B. alicastrum and S. purpurea at both regions. At the Southwest, foliar C content was slightly greater in homegarden Brosimum alicastrum and C. dodecandra species than in forests. The same was true for N in C. dodecandra at the Northeast. Soil P content in was 5 to 18 times larger in homegardens, with significant differences in both regions for B. alicastrum and C. dodecandra at the Southwest. The three species P foliar contents were between 1.7 and 3.7 greater in homegardens at both regions. Soil K and Ca contents at the Northeast region were between 1.2 and three times more abundant in homegardens than in the forest’s soils where B. alicastrum and C. dodecandra grow, and approximately two times lower in S. purpurea soils at both regions. Foliar K content was 1.2 - 1.5 times higher in S. purpurea, and Ca was 1.5 times lower in Southwest B. alicastrum trees, and S. purpurea leaves. At the Southwest region, B. alicastrum Na content in homegarden soils was three times higher, while C. dodecandra levels were three times lower. However, foliar Na content in both species was similar in homegardens and forests at both regions. It was 2.4 times lower at homegardens than in forest leaves at the Southwest region.
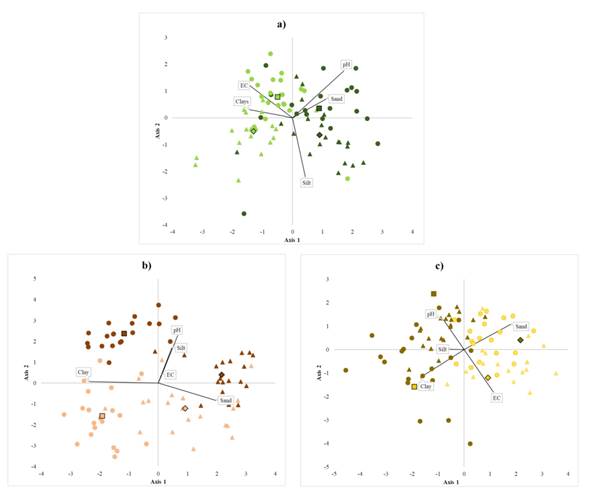
Figure 3 Canonical discriminant groups of study species. Note: a) Brosimum alicastrum, b) Cordia dodecandra and c) Spondias purpurea for two management systems: forest (light colors) and homegardens (dark colors) in two regions: Northeast (triangles) and southweast (dots). Canonical axis considering the physicochemical conditions of soil: pH, electrical conductivity, clay (%), silt (%) and sand (%).
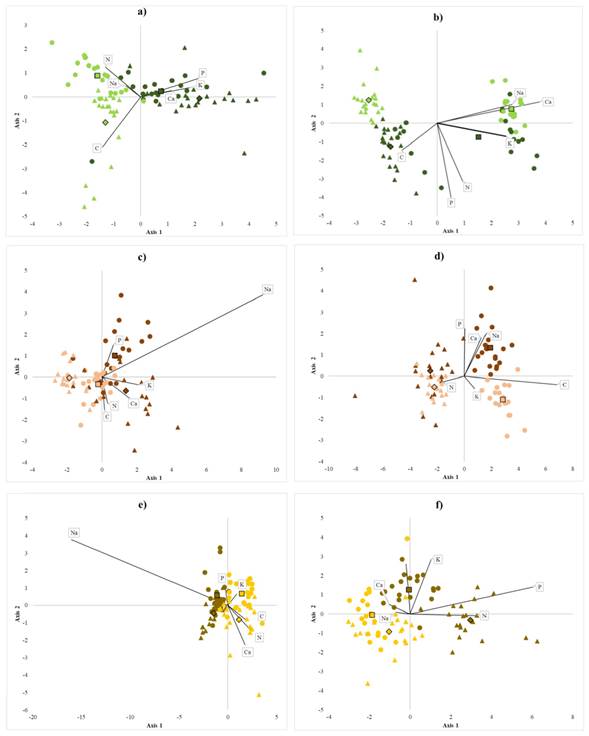
Figure 4 Canonical discriminant groups of study species Note: Brosimum alicastrum (green), Cordia dodecandra (brown), and Spondias purpurea (yellow) for two management systems: forest (light colors) and homegardens (dark colors) in two regions: Northeast (triangles) and Southwest (circles). Canonical axis considering: nutrient contents in soil (a, c, e): C (%), N (%), (mg kg-1), K (cmol kg-1), Na (cmol kg-1), and Ca (cmol kg-1); and nutrient contents in leaves variables (b, d, f): C (%), N (%), (mg kg-1), K (cmol kg-1), Na (cmol kg-1), and Ca (cmol kg-1).
Discussion
Nutrients additions to soil and plants uptake and reabsorption are highly dependent of several factors such as climate, species type and, management, the latter especially important in agroecosystems. Understanding the response of species to the different environmental factors poses a challenge because there always are multiple assemblages of coupled gradients (Muscarella et al. 2006), and not all can be studied or controlled at the same time. Climate (particularly soil humidity and precipitation) is one of the main factors leading ecosystems nutrients cycling (Engelhardt 2021, Grau-Andrés et al. 2021, Singh et al. 2021). This study compared soil and foliar nutrients contents associated with three tropical tree species growing in two climate regions, the Southwest region with an Aw0 climate and the Northeast region with a Aw1 with an average annual rainfall 15% higher. The forest soils in which the three studied species (B. alicastrum, C. dodecandra and S. purpurea) were established had different properties; niche differences can be associated not only to the heterogeneity of the habitats, but also to the specific traits of the species that foster its distribution, such as its radicular system, trunk, and foliage traits, etcetera (Mori et al. 2019).
Comparison of foliar nutrient contents (C, N, P, K, Ca, and Na) between both humidity regions of the three studied species showed differences for all nutrients except P (all species) and K (C. dodecandra and S. purpurea). Campo (2016) studied a humidity gradient in sites of wild vegetation at Yucatan, finding low contents of N, NO3, and P in soils of the Northeast region, this study found that these relationships can be modified by the species and/or management, since only P contents were lower in Northeast forest soils in the three species. At the species level, foliar habit influences nutrients cycling dynamics in ecosystems (Reich 1995), whereas in perennial species leaf abscission occurs gradually along the year, in deciduous species occurs seasonally, determining their resistance/tolerance to the hydric stress, as well as nutrient reabsorption (Brodribb et al. 2021, Xu et al. 2021). However, drought can promote early leaf abscission in both evergreen and deciduous species, increasing litter phosphorus losses only in evergreens (Dallstream and Piper 2021). Regarding to soil properties, we find that B. alicastrum is found in soils with higher electrical conductivity and higher levels of clay. C. dodecandra and S. purpurea are associated with soils with lower pH and electrical conductivity and higher content of silt. Evergreen species, which demand great amounts of water, are less efficient in using water compared to deciduous species (Tomlinson et al. 2013). Hence, the association of B. alicastrum with soils with higher content of clay and better water retention is expected. C. dodecandra is associated with soils undergoing recurrent floods during the rainy season, while S. purpurea is associated with rolling hills where water drains easily. Thus, C. dodecandra seems to tolerate stress caused by water excess as it is a species relying on water of the upper 30 cm of soil (Querejeta et al. 2007), while S. purpurea endures stress caused by water deficit. It is generally accepted that evergreen species are more abundant than deciduous species in nutrientpoor soils (Goldberg 1982); on the contrary, our study found that B. alicastrum, an evergreen species, grew in soils with greater nutritional contents than the two deciduous species, suggesting there may be other physical factors determining its presence in such soils. These trees from forest soils are found in the best-preserved area of the ecosystems where nutrient cycles are better preserved. Absorption of C, N, P, K, Ca, and Na depended on the nutritional contents of the soils, suggesting that the three species have enough phenotypic plasticity to allow allocating nutrients to leaves depending on the different edaphic conditions. For instance, soils associated with S. purpurea were found to have on average, the lowest nutrient contents; however, its leaves had the highest concentration of the three species, suggesting that S. purpurea has a high nutrient extraction capacity and that it may cause low levels of nutrients in soils. Although we expected that deciduous species in the forest would have greater foliar concentrations of N and P than the evergreen species (Chabot and Hicks 1982), we found that S. purpurea had the highest values of N and P; however, B. alicastrum had higher values of foliar P than C. dodecandra. The latter is found in sites in which periodical flooding takes place each year, a factor that promotes an increase in P mobilization from the soil (Tsheboeng et al. 2014, Vivekananthan et al. 2023). B. alicastrum had the highest K and Na foliar contents and C. dodecandra, the highest Ca content, evidence of the different nutritional needs of each species. Nonetheless, the final balance is that the evergreen species B. alicastrum squanders nutrients, while the deciduous species C. dodecandra and S. purpurea transfer them because deciduous species release all their nutrients when their leaves fall off and decay (except for those that are reabsorbed), a process that is much slower in the evergreen species. Besides biotic and abiotic factors, management is a fundamental factor influencing nutrient soil availability in agroecosystems (Tuğrul 2019, Hartmann and Six 2023, Saliu et al. 2023). For instance, when new species are introduced in a homegarden, the edaphic conditions and water availability are often different from those from their place of origin due not only to the natural conditions of the new place but also to the management practices. People uses protective management practices (Vogl-Lukasser et al. 2010) to achieve the establishment and better performance of their species of interest. Nevertheless, only species with high phenotypic plasticity are suited to be cultivated under different conditions than those of their wild environments. Our results indicate that the three species have overlapped edaphic niches, with a small mismatch in the physicochemical properties of B. alicastrum individuals compared to the other two species, suggesting niche plasticity (Maia et al. 2020). Homegarden trees were distributed in soils with less variation compared to those in the forest, regardless of the species; in addition, most physicochemical properties and soil nutrients exhibited greater differences between management systems (homegardens vs forests) than between regions. The lower variation in soil properties among homegardens must be with their proximity because in the wild tree populations trees are often far from each other. Finally, phosphorous content was always greater in soils and tree leaves of homegardens (except by soils associated to S. purpurea in Northeast region); Estrada-Medina et al. (2018) found that the differences between homegarden and forest soils are likely caused by P enrichment in homegardens due to management practices, as well as contamination from detergents. Thus, it seems that tree species are taking advantage of this surplus of P in soils.
Conclusions
The study found that foliar nutrient contents varied according to the studied species, soil conditions, and management systems at each region. Our results suggest that foliar P content is determined primarily by management, whereas C and N depend mainly on the region and the species; K, Na, and Ca contents in leaves have more to do with the species and management. Management of species also affects nutrients availability because species have a differential response to soil conditions in homegardens and forests. In the forest, soils associated with each species have different properties, which influences foliar nutrient contents, while homegardens soils have more similarities. In homegardens, management facilitates the coexistence of species coming from different edaphic conditions. Nevertheless, further studies are necessary to determine the adaptation capacities of each species.











 nueva página del texto (beta)
nueva página del texto (beta)



