1 Introduction
The electron configurations of Zinc (Zn) and Zn2+ cation are
2 Experimental
2.1 Kinetic model in the chemical synthesis of ZnO nanocrystals
The Chemical Bath Deposition (CBD) technique, has been successfully applied at
chemical synthesis of nanocrystals because it is versatile and relatively
inexpensive, in which the chemical parameters of crystal growth are easily
experimentally controlled [17]. Previous report, we systematically
presented experimental details on the chemical synthesis to prepare rare earth
and oxide-hydroxide transition metal and semiconductor nanocrystals
[14,15,18]. The key chemical and physical
parameters in CBD technique are systematically investigated with the aim of
locating optimal conditions in small ranges of reaction temperature, dilute
concentrations of precursor reagents,
At the chemical synthesis, it is proposed the coordination complex
According to Hess’s law, we proceed to arrive at the final product
The intermediate complex of coordination
From (7) it is observed that
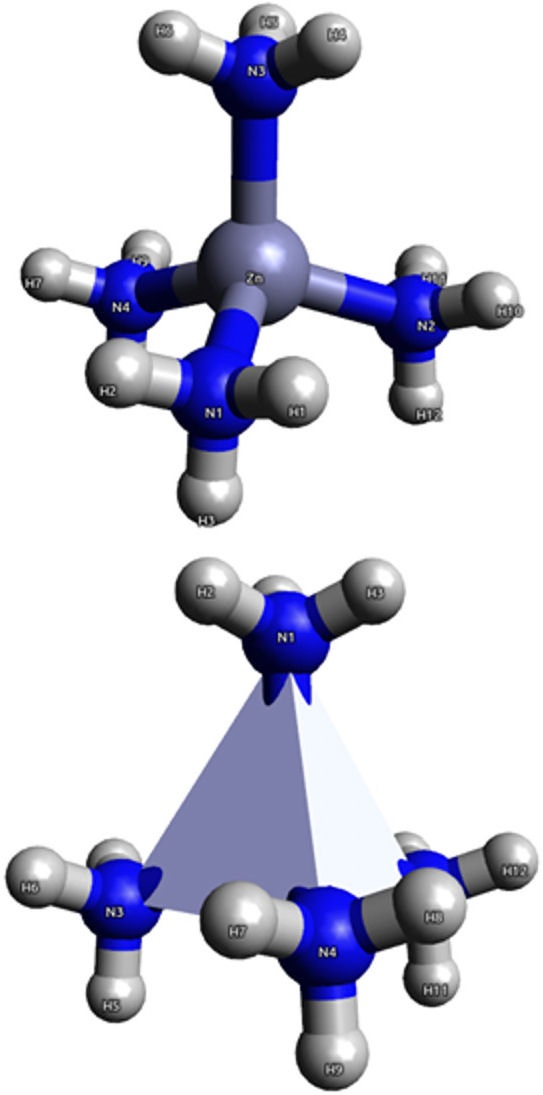
Figure 1 The spatial distribution of
On the other hand, the equilibrium constant
As mentioned above, in our conditions the following chemical equilibria are proposed:
Adding the chemical (1) and (5) equilibria
The precipitated white powder corresponds in a first stage, which synthesis
chemical was carried out with
Hydrolysis of thiourea at room temperature (RT), provides
On the other hand, according to the experimental results of XRD previously
reported in the structural investigation of ZnO product [14], the crystalline
The synthesis of the green chemistry CBD of inorganic materials has been
previously reported [14]. However, we will briefly point out some
of the strategic steps that we applied in the chemical synthesis of samples. The
CBD technique is reduced to preparing three solutions at MC of ~1.0 M, ~ 2.0 M
and ~3.0 M of
3 Discussion
In this manuscript, we continue to investigate the systematically obtained
experimental results of ZnO synthesized by the
The nanocrystals labeled by the
Optical propertie were investigated through the refractive index
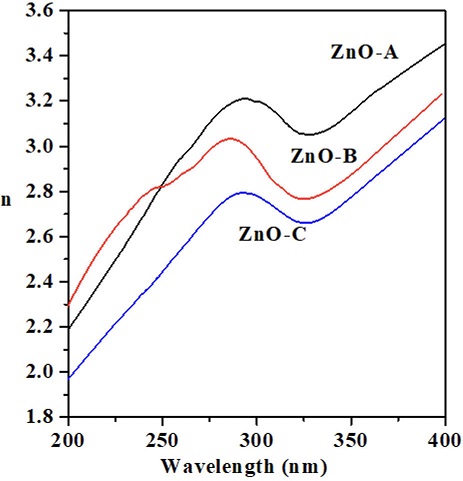
Figure 3 Real refractive index
To determinate the values of extinction coefficient (k) we used the following equation:
The data were recorded in the wavelength range
The electronic transitions of greater relative intensity are seen at ZnO-A and ZnO-B
nanocrystals. A plausible explanation is proposed;
Urbach’s energy
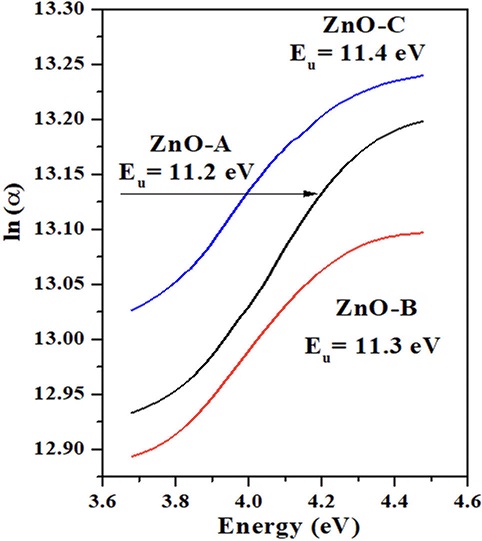
Figure 4 ln (α) as a function of energy eV spectrum of ZnO-A, ZnO-B and ZnO-C samples [Zn(NH3)4]2+ ion.
It has been recently reported that the
The photoluminescence (PL) spectroscopy technique, is applied in research of the band
edge electronic transition levels of a material have been performed by many research
groups [48]. We present a previous study applying the technique of PL located at
UV-Visregion with the aim of investigating the green (GE) and yellow (YE) emission
bands, respectively [14]. Generally, native defects located at UV-Vis
region by means of PL technique and recorded at range
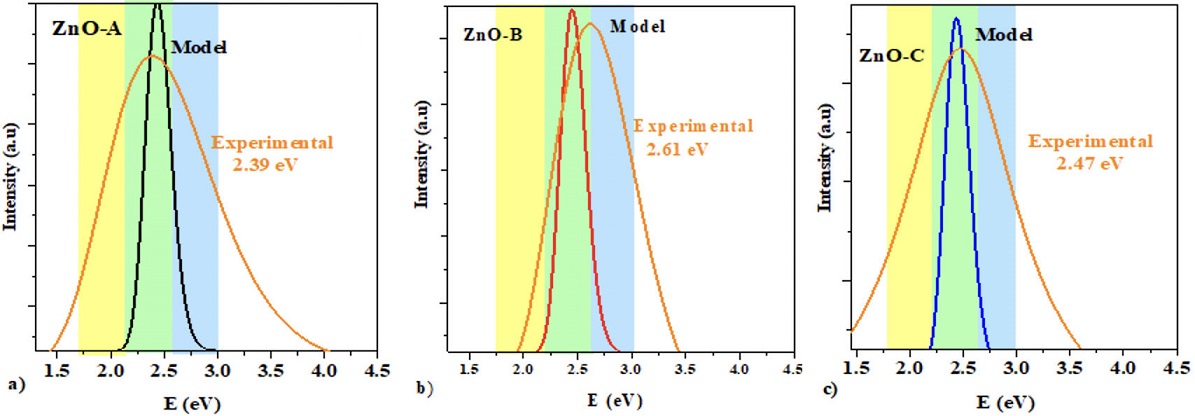
Figure 5 Theoretical and experimental PL spectra of a) Zn-A, b) Zn-B and c)
Zn-C thin solid film The distribution of particles is reflected by time
resolved, in this equation the absorption coefficient and the
Maxwell-Boltzmann statistical function Eq. (17) provided with
exponential term, with describes the energy variation of the density
traps. The MaxwellBoltzmann distribution of carriers was reflected in
the time resolved where is the PL intensity, is the energy dependent
absorption coefficient, is the bandgap energy of ~ 3.0 eV and k is the
Boltzmann constant. The Maxwell-Boltzmann stastical fiting energy
function cand be obtained by
Maxwell Boltzmann theoretical model (MBM), shown in Fig. 6. Table I presents the trap
density
Table I Trap density
| Sample | Density traps (cm-3) |
|---|---|
| ZnO-A | 8.9 × 1013 |
| Zno-B | 9.3 × 1013 |
| ZnO-C | 9.9 × 1013 |
Trap density can be obtained by the following equations:
where
These experimental results are generally concerned with high lifetimes
[15,16]. The presence of a detectable PL emission
bands in the nanocristal, characterized by the presence of
4 Conclusions
The search for the optimal conditions to prepare ZnO in a direct and simple way, present a greater scientific advance every day. Applying the appropriate technique in the synthesis of ZnO, the main objective is to have interesting morphological, structural and optical properties for its possible application. Considering the optical property, it is found that the PL signals recorded in the Vis-region of the spectra are associated with native defects. These are in turn associated with vacancies, intertices. It is well known that the origin of the aforementioned PL emission bands is still in a deep scientific debate. However, from the multiple reports on ZnO made by various research groups, they are identified and carefully examined through theoretical-experimental studies. We found a drastic and marked difference in the theoretical-experimental PL emission signals, with respect to those recorded by the MBM. In this theoretical model, the effect of other defects was not considered. However, it is applied here as a first approximation.











 nueva página del texto (beta)
nueva página del texto (beta)




