INTRODUCTION
Ectoparasites are important in avian host populations because they can affect health condition, regulate population dynamics and alter interspecific competition (Proctor & Owens, 2000; Hudson et al., 2006; Jephcott et al., 2016). Ectoparasites include different groups of arthropods such as, mites, ticks, fleas, flies, hemipterans and lice (anoplurans); however, there are other groups such as feather mites, which include many species considered as commensal, that are also important for birds, because they can enhance their health condition indirectly eliminating skin remains (Blanco et al., 2001; Proctor, 2003). Parasitism is now considered a serious threat for several species affecting adults and nestlings´ health condition and survival (Whiteman & Parker, 2004a; Skoruppa et al., 2006; Liébana et al., 2011), modifying the population dynamics of wild birds (Price et al., 2003; Patz et al., 2004; Collinge, 2009), theirs patterns of distribution (Loye & Carroll, 1995) and reproductive parameters of their hosts (Merino & Potti, 1995; Magalhães et al., 2014).
Studies of ectoparasites in wild raptors are scarce, mainly focusing in looking for the presence of parasites in rehabilitation centers (Pérez et al., 1996; Morishita et al., 2001; Miller et al., 2004, González-Acuña et al., 2006; Oliveira et al., 2011) and scientific collections (Pfaffenberger & Rosero, 1984; Hunter et al., 1994; González-Acuña et al., 2006; Bush et al., 2012). Only few studies have focused on ectoparasites in raptors at natural environments (Hunter et al., 1994; Morishita et al., 1998; Rohner et al., 2000; Whiteman & Parker, 2004a, 2004b; Scott et al., 2017). In nocturnal raptors few studies have been made on ectoparasites prevalence and diversity (Rohner et al., 2000; Morishita et al., 2001). Known ectoparasites in the Great Horned Owl Bubo virgininanus include bugs (Usinger, 1966; Wilson & Oliver, 1978), lice (Kellogg & Chapman, 1902; Malcomson, 1960; Emerson, 1961; Price & Beer, 1963a; Carriker, 1966; Pfaffenberger & Rosero, 1984; Morishita et al., 2001), mites (Schulz et al., 1989) and black flies (Hunter et al., 1997; Rohner et al., 2000; Morishita et al., 2001). Most studies only show records of presences in North America (USA, Canada), including boreal forest, alpine areas, tundra, forest, woodland and grassland. For example, a juvenile Great Horned Owl was found having hyperkeratosis caused by the species mites Knemicokoptes mutans (Schulz et al., 1989); also, three species of chewing lice (Phthiraptera): Strigiphilus oculatus, Strigiphilus acutifrons and Kurodaia edwardsi (Emerson, 1961), and six species of black flies (Diptera) have been reported on this owl species (Rohner et al., 2000). However, to the best our knowledge no studies have investigated the infestation levels of ectoparasites associated with the Great Horned Owls at arid environments and nor in fragmented landscapes.
The Great Horned Owl is the largest North American Owl (length 46-63 cm, average weight 1,304g males, 1,706g females). This owl is widely distributed, from Canada to Chile, being present in almost all kind of ecosystems including croplands (Houston et al., 1998; Artuso et al., 2013). The Great Horned Owl is a top-order predator that has declined in North America about 33% between 1966-2015 mainly due to habitat loss and prey availability declines; other causes are secondary poisoning by accumulation of pesticides from prey, collisions with vehicles and electrocutions; parasitism in not included as a threat (Artuso et al., 2013). In México, this owl is a resident species and is distributed throughout all the country, except in the humid southeast region (Howell & Webb, 1995). Here, we present an evaluation of species richness, prevalence and intensity of ectoparasites in Great Horned Owl fledglings in a fragmented arid landscape, a modified ecosystem for which information on ectoparasites community does not exist. Information on the ectoparasites community in birds at human transformed habitats is almost non-existent. This baseline information could be useful in the future to document the impacts that land use changes and habitat fragmentation can produce in ectoparasites species of a raptor, the Great Horned owl.
METHOD
Study Area. Our study was performed in fragmented area in south Baja California peninsula, México (25° 05’ N, 111° 41’W) (Fig. 1). The 1200 km2 study area is highly fragmented with only eight percent of native vegetation remaining (Munguia-Vega et al., 2012). This is a valley with an altitude of less than 100 m a.s.l. (Cardona et al., 2004). Desert fragments are inserted into an agricultural matrix with some livestock activity (Barret, 1974).
The native vegetation is typical of the Sonoran Desert region (Wiggins, 1980; Rebman & Roberts, 2012), with sarcocaulescent scrub vegetation composed predominantly by giant cardon cactus (Pachycereus pringlei), organpipe cactus (Sternocereus thurberi), mesquite (Prosopis glandulosa), torote (Bursera microphylla), palo verde (Cerdidium floridum), and Adam´s tree (Fouquieria spp.). The fragments are surrounded by agricultural areas where cultivated crops were sorghum, safflower, vegetables, alfalfa, potatoes, and maize and orange fruit trees too. Climate is dry, with summer rains and a mean annual temperature of 24 °C, with a maximum of 40 °C. Mean annual precipitation is around 140 mm (Cardona et al., 2004).
Sampling collection. Great Horned owl nests were located between January and May 2015 and 2017. Although we found active nests in both conditions, fragmented and non-fragmented (continuous) areas, predation on chicks was very high in continuous areas; thus, we were unable to get enough information in this study in non-fragmented condition (Bolaños-García & Rodríguez-Estrella, submitted). For this reason, statistical analysis was not performed comparing fragmented versus non-fragmented area because sample size was very low. We comment on this information later.
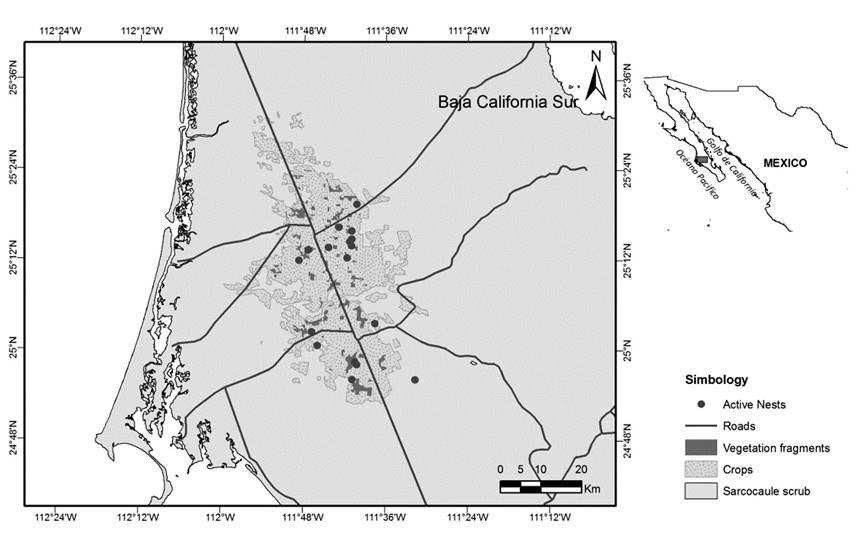
Figure 1 Study area with location of fragments and nesting sites in Santo Domingo Valley, Baja California Sur, Mexico.
Sampling of parasites from owls was done when they were nestlings approximately 40 days old, before fledged. The fledglings were put over a clean white surface and carefully examined for five minutes, the whole plumage was profusely and systematically surveyed to collect all possible ectoparasites (following Clayton & Drown, 2001). For feather mites, each wing was fully extended and reviewed for 30 seconds. Subsequently, brushing was performed for 60 seconds to collect skin mites (Clayton & Walther, 1997). Louse flies, were collected by hand following a standard method described in Young et al. (1993). All ectoparasites, feathers and remnants of skin detached from fledglings were fixed and conserved in vials containing ethanol 90% until processing under stereoscope in CIBNOR (Centro de Investigaciones Biológicas del Noroeste) laboratories.
The lice, bugs and fleas were cleared for about 18-48 hours in KOH 10% and put in distilled water. Following dehydration in a graded alcohol series, the specimens were mounted on slides in Canada balsam (Palma, 1978; Guzmán-Cornejo et al., 2012). Feather mites were cleared in lactophenol and mounted in Hoyer´s medium. Louse flies, were preserved in vials with ethanol.
All ectoparasites were identified at the highest taxonomic level of resolution possible; using specialized keys for each group of arthropod (Ewing & Fox, 1943; Price & Beer, 1963a; Price & Beer, 1963b; Clay, 1969; 1970; Price & Hellenthal, 1988; Lewis, 2000; Acosta & Morrone, 2003; Price et al., 2003; Wood, 2010; Santos et al., 2014). For bugs and feather mites, a specialist for each taxon was consulted. A light microscope Olympus® BX50 with differential interference contrast illumination was used to observe morphological characteristics. Photomicrographs were taken using a digital camera Olympus® Model PROVIS AX 70 with digital capture 5mp camera media cybernetic, with a stereoscopic microscope ZEISS® Model Stemi SV digital capture 24 mp Canon® EOS Rebel 7Ti camera and using Scanning electron micrograph.
Species richness was determined by total number of species of ectoparasites occurring on the host, prevalence and mean intensity (± standard error) were calculated according with Bush et al. (1997). Finally, to determine the proportion of each taxa in the parasite community Shannon and Weaner diversity index H’ was estimated.
A spatial autocorrelation analysis (Moran´s I) was performed to determine whether the proximity of Great Horned owl’ nests to each other within the study area influence the prevalence and the abundance of ectoparasites. The spatial autocorrelation helps to understand the degree to which one parasitized nest is similar to another nearby nest. We used the Moran´s I statistic to measure spatial autocorrelation and used the K-Nearest Neighbor with order 4, (GeoDa 1.12.1.59 software, 2017). While a positive Moran´s I indicate data is clustered, a negative Moran´s I implies data is dispersed. The absence of autocorrelation implies data are independent.
RESULTS
A total of 34 owl fledglings were sampled. About 61% (n= 22) had at least one species of ectoparasite; 13 fledglings with ectoparasites were raised from 10 nests located in fragments and five from three nests on trees in cultivated area (farms). We decided that a nest with two fledglings located at 0.7 km from the edge of fragmented area into continue vegetation should be considered in the edge of fragmented area, given the short distance from the influence of cultivate area. Only one nest with two fledglings was found and sampled in non-fragmented natural area and was excluded from statistical analysis. Most ectoparasites were blood-feeding species (Table 1).
Table 1 Ectoparasites associated with the Great Horned Owl (Bubo virginianus) fledglings in fragmented arid desert of south Baja California peninsula, México. In bold, the highest prevalence and mean intensity. SE: standard error.
| INSECTA | SPECIES | PH | TP | % | MI ± SE |
|---|---|---|---|---|---|
| Phthiraptera | Colpocephalum pectinatum Osborn, 1902 | 7 | 29 | 20.5 | 4.8 ± 0.41 |
| Geomydoecus telli Price & Hellenthal, 1988 | 1 | 5 | 2.9 | 5 ± 0.15 | |
| Neohaematopinus sciurinus (Mjöberg, 1910) | 1 | 1 | 2.9 | 1 ± 0.03 | |
| Hemiptera | Cimicidae gen. sp. | 1 | 1 | 2.9 | 1 ± 0.03 |
| Siphonaptera | Orchopea sp. | 1 | 1 | 2.9 | 1 ± 0.03 |
| Diptera | Icosta americana (Leach 1817) | 9 | 12 | 26.4 | 1.3 ± 0.11 |
| ACARI | |||||
| Mesostigmata | Ornithonysus sylviarum (Canestrini & Fanzago, 1877) | 2 | 25 | 5.8 | 12.5 ± 0.53 |
| Acariformes | Glaucalges cf. attenuatus (Buchholz, 1869) | 3 | 7 | 8.8 | 2.3 ± 0.14 |
PH= Parasitized hosts. TP= Total of parasites, %= Prevalence, MI= Mean Intensity
A total of 81 individual epizoic species were collected, 69 hematophagous and 12 commensals. Eight different taxa were recorded, the epizoic species diversity was H’ = 1.56. Hippoboscidae flies were present in 26% of fledglings while Phthiraptera lice (Amblycera) and Mesostigmata mites had a prevalence of 23% and 6%, respectively. Others were rare, as Siphonaptera and Hemiptera (Table 1, Fig. 2). Of these ectoparasites species, six were blood-feeding species: Diptera (Icosta americana); Hemiptera (Cimicidae gen. sp.); Phthiraptera (Neohaematopinus sciurinus, Colpocephalum pectinatum); Siphonaptera (Orchopea sp.) and Mesostigmata (Ornihtonysus sylviarum). Hippoboscidae flies I. americana had the highest prevalence 26.5% in fledglings, while the chewing lice C. pectinatum had a prevalence of 20.5. For N. sciurinus, Orchopea sp. and Cimicidae gen. sp. only one specimen was obtained (Table 1, Fig. 2).
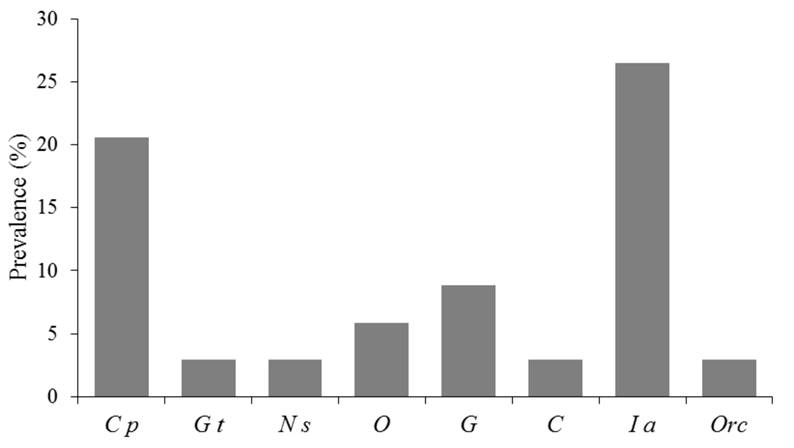
Figure 2 Percentage of parasitized of Great Horned Owl fledglings in scrub desert of Baja California peninsula. C p, Colpocephalum pectinatum; G t, Geomydoecus telli; N s, Neohaematopinus sciurinus; O, Ornihtonysus sylviarum; G, Glaucalges cf. attenuatus; C, Cimicidae gen. sp.; I a, Icosta americana; Orc, Orchopea sp.
We recorded O. sylviarum with a low prevalence (5.8%) but with the highest mean intensity (12.5) (Table 1). Adults of lice G. telli and N. sciurinus were unexpected for Great Horned Owl fledglings because these lice have been recorded parasitizing only rodents. A greater number of adult females was registered in all species of ectoparasites, except for chewing lice Geomydoecus telli, with more adult males. Feather mites Glaucalges spp. were also recorded but were uncommon.
A severe infestation was recorded in four fledglings of two nests, two from fragmented area parasitized by the chewing louse C. pectinatum, and the other two fledglings from the nest close to the edge of crops (0.7 km), parasitized by a mite Ornihtonysus sylviarum (Table 1, Fig. 3).
No spatial autocorrelation existed for the ectoparasites abundance and distance among nests (Moran´s I = 0.010; z = 0.16, P > 0.05; Fig. 4). Thus, the abundance of ectoparasites in one nest is independent of their abundance in neighbor nests.
DISCUSSION
An infestation by ectoparasites may result in the reduction of the host's ability to defend itself and compromises the health of young chicks (Collinge, 2009; Murillo & Mullens, 2017) resulting in stunted growth (Lehane, 2005) and may severely affect the body condition (Christensen et al., 2015). Ectoparasites affect the population dynamics of hosts, so it is important to determine their prevalence mean intensity and species diversity. However, these data on ectoparasites are scarce for owls and generally are only-presence records or have been obtained from small sample sizes (Hunter et al., 1994; Skoruppa et al., 2006). The ectoparasites of the Great Horned Owl are poorly known, and most records are from sporadic observations. This is the first study of the prevalence and intensity of ectoparasites in Great Horned Owl fledglings.
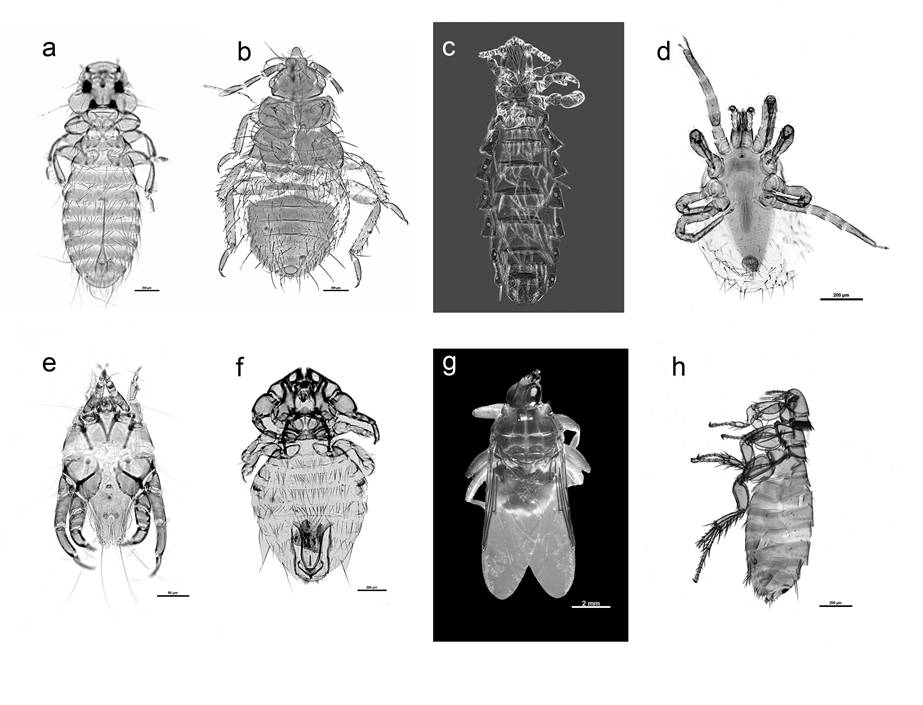
Figure 3 Ectoparasites found on Great Horned Owl nestlings; a) Colpocephalum pectinatum adult male ventral view, b) Geomidoecus telli adult male ventral view, c) Neohaematopinus sciurinus adult female ventral view, d) Ornihtonysus sylviarum adult ventral view, e) feather mite, Glaucalges cf. attenuates, f) Cimicidae gen. sp., nymph instars, g) louse flies Icosta americana, adult female dorsal view, h) Orchopea sp. adult female side view.
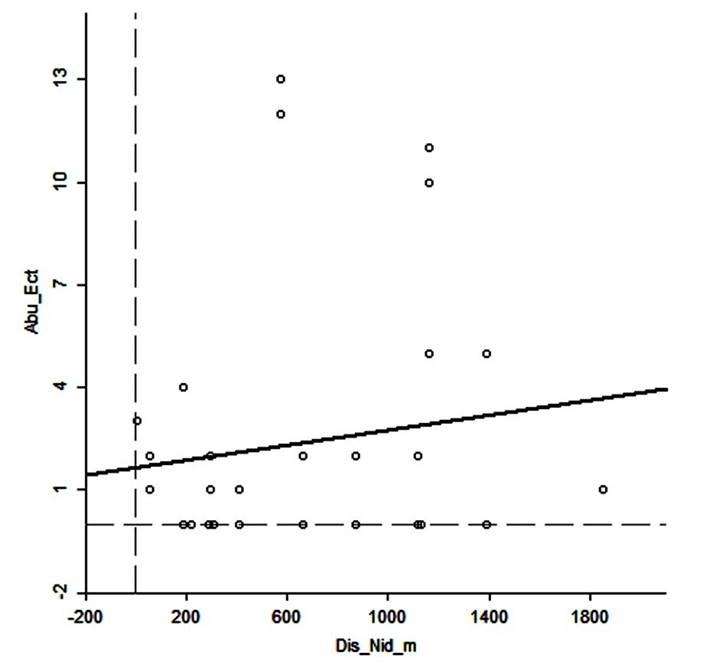
Figure 4 Spatial autocorrelation analysis between ectoparasites abundance (Abun_Ect) and distance among Great Horned Owl active nests (Dis_Nid (m)).
The occurrence of parasites is determined by environmental factors, some related to the host and others to the biology of parasites (Keesing et al., 2010). It has been also found that the variations in the level of infestation seem to be correlated with temperature and humidity, climate and age of hosts (Young et al., 1993; Skoruppa et al., 2006). For instance, prevalence of parasites and intensity of infestation in wild animals may be affected by habitat loss and fragmentation (Collinge, 2009), because host individuals concentrate in smaller areas than in continuous habitat, then favoring the exchange and transmission between hosts, and survival of ectoparasites as well (Greer & Collins, 2008; Lüdtke et al., 2013; Webstern et al., 2014).
Not information exists to compare the levels of infestation of ectoparasites (e.g. prevalence and mean intensity) in the Great Horned Owl, and certainly no for fragmented desert. In our study, we were unable to determine if habitat fragmentation promotes an increase in species richness and abundance of ectoparasites in the Great Horned Owl because almost all nestlings were predated in non-fragmented condition, thus comparisons were not possible.
When comparing our results with other studies on raptors we found a high number of ectoparasites species in our owl population in fragmented habitat within a desert environment, in contrast with all other raptor species for which information exists and that have a small number of ectoparasites species and most of them with a relatively low prevalence as we will show below. Nevertheless, raptors under certain circumstances have higher ectoparasites prevalence. For example, the prevalence of C. pectinatum in Burrowing Owl Athene cunicularia was 50% and the mean intensity was 9.0 (Pfaffenberger & Wilson, 1985) much higher than in our study. It has been proposed that differences in prevalence and mean intensity may be age-related or due to habitat conditions (Moyer et al., 2002); in the A. cunicularia study all birds were adults, while in our work all hosts were fledglings. So, fledglings should have more ectoparasites than adults but when comparing with the results we found for the Great Horned Owl population, this hypothesis is contradicted. Thus, other explanations should be considered, such as differences in habitat conditions. In the study of A. cunicularia, habitat was plains and prairies dominated by short grass surrounded by agricultural areas. In wintering Burrowing Owl (Athene c. hypugaea) adults, a low prevalence infestation of two lice species (C. pectinatum and Strigiphilus speotyti) was recorded (27%). Authors propose that the low lice prevalence and abundance is due to low temperatures during the seasons of the year because during breeding season birds are more susceptible (Skoruppa et al., 2006). However, habitat could have a strong influence on these prevalences because most birds were located in agriculture areas and few in natural grassland (Skoruppa et al., 2006).
On the other hand, the host age seems to influence the ectoparasites prevalence too. For example, in natural environments of northwest California (e.g. forests), the prevalence of hippoboscid flies (Ornithomya anchineuria and I. americana) was greater in adults than in juveniles of Northern Spotted Owl, which however were parasitized in only 7% of individuals by these flies, with mean intensity of 2.4 (Young et al., 1993). The abundance of I. americana has been found to be regulated by climate because the pupa phase is more resistant to cold than to warm climates (Bennet, 1961; Young et al., 1993). Thus, the low mean intensity of I. costa in hosts at the Baja California peninsula arid desert could be due to the high temperatures from spring to fall seasons. In fact, a low prevalence of a louse fly species (Microlynchia pusilla 1%, n = 401 birds) was found in birds of Baja California peninsula and one of the factors proposed was climatic conditions (Tella et al., 2000).
Hunter (1994) found two species of hippoboscids (Ornithoica vicina and I. americana) in Mexican Spotted Owl from mixed evergreen forest and montane forest with a prevalence of 33% and mean intensity of 4.2. In American Kestrel Falco sparverius chicks monitored in nest box, Carnus hemapterus (Diptera, Carnidae) was detected with a high prevalence (up to 50%) and moderate intensity of infestation, 2.5 (Dawson & Bortolotii, 1997). Carnus hemapterus was also observed in Barn Owl (Tyto alba) up to four weeks of age, but it was not detected on older nestlings or adult owls (Kirkpratrick & Colvin, 1989). In other birds, like ovenbird nestlings in mixed coniferous forest in Minnesota, a prevalence of 21% de infestation in nests by Bird Blow flies Trypocalliphora braueri was recorded (Streby et al., 2009). Thus, prevalence and mean intensity of parasites may be due to vegetation type, climatic conditions and man-made disturbances due to land use changes and human activity.
According to our spatial autocorrelation analysis our findings in fragmented condition do not support the idea that prevalence of parasites and mean parasite intensity in nests should be correlated to neighbor nests level of infestation. Habitat fragmentation (e.g. environmental factor) has been found that promotes a greater aggregation of hosts, then transmission of ectoparasites can increase (Bradley & Altizer, 2007; Webstern et al., 2014). We did not find a relationship between the abundance of ectoparasites in one nest and its abundance in neighbor nests. It could be certainly possible that habitat heterogeneity (diverse crops, ranch, farms and livestock) promotes the high species diversity we found in fragmented landscape.
Studies have shown that lice species have a high degree of specificity to their hosts (Price & Beer, 1963a; Morishita et al., 2001). Most genera of the Phthiraptera are restricted to particular taxa and some louse species parasitize only one host species (Clayton et al., 2008). Furthermore, lice might be the ectoparasites with the highest prevalence in raptors (Peréz et al., 1996). In our study, the chewing lice C. pectinatum, was clearly associated with nestlings in the study area, having a high prevalence and the highest mean parasite intensity of all ectoparasites in fragmented area. Also, this chewing louse was present in the two owl nestlings we sample in continuous vegetation area. Previous records of C. pectinatum in owl species were in A. cunicularia (Pfaffenberger & Wilson, 1985), Athene cunicularia hypugaea, Athene brama brama, Otus bakkamoena (Price & Beer, 1963a; Skoruppa et al., 2006) and A. noctua (Rak et al., 1975), Otus watsonii and Tyto alba (Price et al., 2003) but not in Great Horned Owls (Schulz et al., 1989; Price et al., 2003). Thus, the Great Horned Owl is a new host species for this ectoparasite.
Glaucalges attenuates, is a feather mite species reported for the Great Horned Owl and other owl species (Dabert et al., 2008). This mite is a permanent and obligatory symbionts-parasite that spends their entire life cycle on the bird host. These mites are commensals, neither harming nor benefiting the host but they feed on secretions that the host spreads on the feather while grooming (Galloway et al., 2014).
Colpocephalum pectinatum is a blood-feeding louse species that can be vector of endoparasites (Whiteman & Parker, 2004b). It is then very likely that this louse may decrease the body condition of Great horned owl fledglings that have moderate to high infestation levels but also can transmit endoparasites and pathogens which in turn may affect their survival.
The family Macronyssidae includes hematophagous mites that are parasites of birds and may be vectors of pathogens (Proctor & Ownes, 2000). The mites of most Strigiforms species are unknown, but these birds can host a diverse community of pathogenic and feather mites (Philips, 2000). Mites can cause irritation, severe dermatitis and anemia. We recorded a hematophagous mite Ornithonysus sylviarum in high numbers but with a low prevalence, two fledglings presented a severe infestation in the whole body. Due to its fast reproduction this mite can quickly infest a host (McCulloch & Owen, 2012). There are reports of cases in which this species may reach up to 50,000 mites per bird (McCulloch & Owen, 2012; Murillo & Mullens, 2017), high infestation levels can cause up to 6% blood loss per day (DeLoach & DeVaney, 1981). There is a suspected role of O. sylviarum mites in the spread of the Avian paramyxovirus type 1 Newcastle disease and Saint-Louis encephalitis virus (Flavirus) (Valiente Moro et al., 2005).
The louse fly I. americana is the most common Hippoboscidae in Strigidae (Maa, 1969). This hematophagous louse fly can affect health condition of birds (Wood, 2010), is a vector of Western Nile virus (WNV) in raptors of North America (Farajollahi et al., 2005; Philips, 2007), and has been also recognized as a threat for nestlings due to their role as a vector of diseases (Proudfoot et al., 2006). The louse fly had the highest prevalence in Great Horned Owl nestlings in this study, so it is important to determine the potential effects of louse fly in this owl species.
Although in our work the prevalence of the hemipteran (Cimicidae gen. sp.) was very low, it has been found that the presence of large numbers of this kind of bugs (hematophagous) in nests can cause young birds to leave the infested nest prematurely (Grubb, 1986), nestlings die or the nest is abandoned during hatching by parents e.g. Falco mexicanus and Buteo jamaicensis (Grubb, 1986; Wilson & Oliver, 1978).
We recorded several ectoparasites species in Great Horned Owl nestlings, almost fledglings, in the fragmented area. The high ectoparasites richness species and diversity can indicate a risk to the health condition of this owl species. Birds living in arid environments tend to have fewer ectoparasites than similar birds in humid environments (Moyer et al., 2002; Clayton et al., 2008), e.g. the prevalence of lice for Mourning dove and Feral pigeon was lower in Arizona (3% and 47%) than in Texas (79% and 100%) respectively (Moyer et al., 2002). The abundance of lice showed the same trend.
The fragmented area with natural vegetation patches inserted into a matrix of crop fields, may favor an increase of diversity of ectoparasites because of habitat heterogeneity. Further studies are needed to evaluate the affectations in health condition of Great Horned Owl populations in this highly fragmented system. For instance, we found that the four fledglings showing severe infestations (>1000 lice or mites) were from nests located in highly degraded habitat containing livestock, garbage dumps, roads, dead animals and reduced cover vegetation. On the other hand, the use of chlorinated hydrocarbon insecticides in the agricultural zone (Jiménez et al., 2005) to control plant pests possibly have had an indirect effect on ectoparasites survival, this is the reason of the low prevalence of some species.
The probability transmission of ectoparasites between raptor species can be high in desert fragmented areas during reproduction, particularly when birds do not build their own nest so they use nests that were used by other birds which can be infected previously by ectoparasites remaining in these sites even when the host left the nest, especially when there is a limited availability of spaces for nesting in patches of highly fragmented systems (Phillips & Dindal, 1977; Collinge, 2009). Also, adults can transmit ectoparasites when contacts occur in the nest. On the other hand, it is important to remark that the flea, the chewing louse and the sucking lice (Anoplura) are strictly parasites of mammals (Price & Hellenthal, 1988; Durden & Musser, 1994; Lewis, 2000). The transmission of these ectoparasites to the owl fledglings into nests occurs when mammals are taken by adult birds and deliver prey to the nest; owls have been found infested with rodent fleas (Jellison, 1939; Rothschild & Clay, 1957). The bug inhabits into the nests (especially when nests are old) and walk or climb towards the host to feed on blood. Bugs of this kind can parasite birds in nests like the Great Horned Owl because this species does not replenish the spays of green material to avoid and eliminate parasites because the owl does not build nests (Wimberger, 1984).
We propose studies should be done to determine if pathogens are present in Great Horned Owls in the fragmented arid area and their effects. If so, further research on the parasites in top-order predator owls should be done to better understand the host-parasite interactions and how habitat fragmentation influences the interactions and the host survival.











 nueva página del texto (beta)
nueva página del texto (beta)


