Introduction
Spatial and temporal changes in ecological and environmental factors alter abundance of animals’ populations (Cloudsley-Thompson, 1962; Kühnelt, 1963; Andrewartha & Birch, 1982; Levings, 1983; Richards & Windsor, 2007; Pequeno et al., 2017). In terrestrial arthropods the environmental heterogeneity could affect differently the different stages, possibly more than in other groups of animals (e.g., birds and mammals). Because the life cycle of most terrestrial arthropods includes several stages, each with quite different ecological requirements (Randolph, 2014).
Tick abundance is known to vary across habitats and seasons often experiencing a peak during a certain period of the year. This temporal dynamic in ticks commonly correlates with climatic and microclimatic conditions, which are often determined by seasonal whether fluctuation and the structure of the vegetation (Milne, 1950; Perret et al., 2004; Labruna et al., 2009). Ticks spend a relatively large portion of their life cycle off-host, living free in their habitat. These are critical periods, because microclimatic conditions may have a strong effect on survival and development of these arthropods. Previous studies have shown that abundance of different tick stages is influenced by temperature, as well as humidity, rainfall, vegetation type (or habitat structure), soil water content and host availability to which individuals of each stage are exposed (Szabó et al., 2007; Tack et al., 2012).
The microclimatic conditions in some, but not all, habitats permit host-seeking ticks to survive and persist long enough to access a host. It is known that ticks, especially eggs and larvae, are highly vulnerable to dehydration in hot and drier climates, while extremely rainy conditions are also associated with high mortality (Milne, 1950; Tack et al., 2012). For example, more exposed sites, with scattered or no vegetation, have conditions with high ambient temperatures that may accelerate the molting process, but may also increase the chance of eggs and ticks’ dehydration (Needham & Teel, 1991). These conditions also affect host-seeking ticks’ activity, their questing behavior, and their chance to attach to a host, and thus their abundance (Kaiser et al., 1988; Perret et al., 2004; Randolph, 2014). Hot and dry conditions could drastically reduce activity of host-seeking ticks present in a particular habitat (Hubálek et al., 2003). Hence, evaluating the effect of different types of vegetation (habitats) and climatic conditions on abundance of different host-seeking tick species is crucial to understand the spatial and temporal fluctuation in their populations.
There are many studies estimating the abundance of host-seeking ticks in temperate zones (Barandika et al., 2006; Schulz et al., 2014; Mathews‑Martin et al., 2020), but systematic studies on abundance of ticks across habitats in the neotropics and particularly the Mesoamerica are scarce (Szabó et al., 2007). Such information is important for understanding the population dynamic of ticks, since abundance is a population parameter that is affected by tick mortality and likely by ticks’ hosts occurrence. Hence, considering the little information available on host-seeking ticks, we here compare the effect of habitat vegetation-structure and climate (temperature, humidity, and rainfall patterns) on their abundance in a Costa Rican dry forest.
Materials and methods
Study area. We conducted this study from February 2012 to February 2013, in the Palo Verde National Park, Guanacaste province (10° 21' N, 85° 21’ W, 50-250 m elevation) Costa Rica (Fig. 1). Palo Verde has an extension of 19,800 ha that includes part of the remaining southernmost fragments of the Mesoamerican tropical dry forest (Janzen, 1988a; Quesada & Stoner, 2004), with a mean annual precipitation of 1700 mm and temperatures that oscillate between 22 °C and 32 °C. The precipitation on the region occurs from June through November with a long dry season from December through May, with a short dry period between July-August.
The park includes different types of vegetation: deciduous dry forest, evergreen forest growing along rivers and permanent and seasonal streams, thorn scrublands, grasslands, mangroves, and disturbed areas caused by anthropogenic practices developed in the area (mainly livestock) before being declared a National Park in 1978. These types of vegetation have been previously identified and categorized (Slud, 1980; Vaughan et al., 1982; Hartshorn, 1983), and maintained over time by frequent intentional fires (Rozario et al., 2018). The park also includes seasonal wetlands, swamps, and several waterholes that are used as major water sources by the wildlife, especially during the dry season.
Collection and identification of ticks. We collected host-seeking ticks monthly (from February 2012 to February 2013) in seven different sites. We selected these sites to include part of the terrestrial habitats that have been previously characterized by Vaughan et al. (1982) and Hartshorn (1983). A detailed description of the vegetation is included in the Table 1. In each forest type we established a 300m2 transect that was repeatedly sampled each month during the study period. Ticks in a particular habitat may remain inactive under drastic climatic conditions (Hubálek et al., 2003), so that abundance estimation of ticks is likely biased to host-seeking ticks. To reduce this potential effect, we collected ticks on a 1 m × 150m belt at each side of each transect (300 m2 total area) from 06:30 to 11:00 and 14:30 to 17: 30 hours to avoid the hottest periods of the day when ticks could be inactive (Hubálek et al., 2003).
Table 1 Characterization of sites sampled in the Palo Verde National Park, Guanacaste.
| Sites sampled | Description |
| Sendero Guayacán (SG) | Evergreen forest dominated by large trees of Brosimun alicastrum growing on limestone soil surrounding a small seasonal water spring on a slope of about 20 degrees. The undergrowth is not very dense where Garcia nutans and Sapranthus palanga are the most common shrubs. Herbaceous layer is dominated by some bambusoids and some Acantaceae herbs. |
| Ojo de agua (OA) | Permanent water spring surrounding by old second ground forest. Herbaceous layer is very dense, dominated by Acantacea and grasses. The canopy is dominated by trees of the species, Hura crepitans, Manilkara zapota y Coccoloba uvifera. |
| Sendero Mapache (SM) | Rocky limestone soil, with little dense undergrowth, with some shrubs of Vachellia (= Acacia)and large limestone rocks that cover much of the area. Trees of Sterculia apetala y Brosimun allicastrum are abundant. |
| Los Mangos (LM) | Altered area with the ground covered by a thick layer of litter and grasses. This site includes a camping area for visitors and 11 large mango trees Mangifera indica. |
| Camino Principal border (CP) | It includes a heterogeneous vegetation. One side of the road is covered with herbaceous vegetation and forested patches, the opposite site is covered with herbaceous vegetation and second growth patches and border a large seasonal lagoon that maintain water for most of the dry season. |
| La Cantera (LC) | Flat trail with a herbaceous layer mainly composed of grasses and a shrub layer dominated by Vachellia (= Acacia) and deciduous trees (e.g., Guazuma ulmifolia). |
| Laguna (LA) | Edge of a seasonal lagoon with some abundant tree species such as Pithecellobium lanceolatum y Parkinsonia aculeata trees. Herbaceous layer is dominated by Acanthacea and grasses. |
To collect the host-seeking ticks, we dragged a piece of white cotton fabric (a flag of 1 m × 1.5 m) on the litter and herbaceous layer (0-50 cm above the ground). During flagging, we walked at slow steady pace dragging the flag on the litter and herbaceous layer. Every 2.5 m we carefully examined visually the flag and collected manually all ticks adhere or walking on the flag and stored them in tubes containing ethanol 80%. In the laboratory, we counted individually adults, nymphs, and larvae. The flagging method is an effective and economical way for gathering large numbers of ticks and determining their seasonal activity patterns (Gray, 1985; Schulz et al., 2014; Domínguez et al., 2019; Montenegro et al., 2021). Only adults were identified morphologically to species level using the taxonomic keys of Bermúdez et al. (2018). We used keys from Fairchild et al. (1966), Keirans & Durden (1998), and Vargas (2006) to identify larvae and nymphs to genus level. Identification of tick immature stages to the species level is difficult, but it is feasible to identify them to the genus level, a procedure followed by other authors (Barandika et al., 2006; Domínguez et al., 2019; Montenegro et al., 2021). We identified two of the species, based on adult specimens, as Amblyomma oblongoguttatum Koch, 1844, and A. parvum Aragão, 1908. However, it has been suggested, based on molecular data, that the South American and the Central American-Mexican clades of these ticks represent different species (Nava et al., 2014; Lado et al., 2016; Lopes et al., 2016). Therefore, we prefer to call these species as A. cf. oblongoguttatum, and A. cf. parvum until further evidence is available. We deposited voucher specimens in the Museo de Zoología, Escuela de Biología, Universidad de Costa Rica.
Statistical analyses. We compared the abundance of larvae, nymphs, and adult host-seeking ticks among the seven sites previously defined using General Linear Mixed Models (library lmerTest; Kuznetsova et al., 2017) with a Gaussian distribution of errors. In addition to sites, we included in the models the monthly variation in climatic conditions as fixed factors and sampled date as a random factor to account for repeated samplings (n = 13) in each site. We evaluated normality and homoscedasticity of residuals in all models.
We obtained climatic data from the climatic station located in the Palo Verde National Park, from OTS (http://www.tropicalstudies.org). The distance from the climatic station to the sampling sites vary from 50m to 2km. We selected monthly minimum and maximum temperature, monthly rainfall, and relative humidity, as the climatic variables that could affect abundance of different tick stages. We then calculated a Principal Component Analysis for the climatic variables of each month and selected the first component (PC1) that account for 89% of the total variance encompassed by the four climatic variables. Next, we calculated the PC1 scores for each month, and used them as a unified climatic variable in the models. The four original climatic variables strongly correlated (> 0.92) with the PC1 scores, positively with rainfall and relative humidity, but negatively with maximum and minimum temperature.
We used chi-squared tests to compare the total of adult ticks of each species among sites. We used this test, instead of a GLMM, because some of the tick species were not collected in several months in some sites, and this undefine the model since the variance cannot be calculated for some factors. Similarly, we used one sample Kolmogorov-Smirnov test (D) to asses if abundance of each tick species shows a defined peak.
To analyze the temporal variation in abundance for each tick stage, we constructed a model that included month as the only fixed factor and site as a random factor. We did not collect either larvae, nymphs or adult ticks in some months at any particular site, and this condition preclude us to include the interaction month*site as fixed factor in the model, since this condition undefine the model. We neither include the PC1 in these models, because this variable has a strong collinearity with the fixed effect month, producing an overfitting of the model. To indirectly evaluate the temporal changes in abundance we correlated the mean monthly abundance of each tick stage with the PC1.
Additionally, we used contrasts (library emmeans; Lenth et al., 2022) to compare the means for those significant factors detected in the models. We used the R statistical Language (version 4.02: R Core Team 2020) for all statistical and graphical analyses.
Results
Species richness and abundance. We collected 5,852 free-living ticks in the seven sampled sites in the Palo Verde National Park. From this total, 87% corresponded to larvae, 9.5% to nymphs, and 3.5 % to adults (Table 2). We identified all larvae and nymphs to genus level, which corresponded to Amblyomma spp. and four different species of adult ticks: A. mixtum Koch, 1844, A. cf. oblongoguttatum Koch, 1844, A. cf. parvum Aragão, 1908, and A. dissimile Koch, 1844. A. mixtum was the most common species, followed by A. cf. oblongoguttatum and both were present in all seven sites, while A. cf. parvum was collected in all but the two most disturbed sites, Cantera and Laguna (Table 3). We collected only one female A. dissimile from the Ojo de Agua site (OA).
Table 2 Total number of ticks collected in the seven sites sampled in Palo Verde, Guanacaste.
| Site/ Instar | Guayacán | Ojo de agua | Mapache | Mangos | Camino | Cantera | Laguna |
| Larvae | 889 | 541 | 544 | 116 | 814 | 1358 | 871 |
| Nymphs | 164 | 114 | 88 | 59 | 30 | 38 | 23 |
| Adults | 72 | 34 | 22 | 12 | 47 | 14 | 2 |
| Total | 1125 | 689 | 654 | 187 | 891 | 1410 | 896 |
Table 3 Adult Amblyomma species collected in the seven sampled sites in Palo Verde, Guanacaste.
| Specie/ site | A. mixtum | A. cf. oblongoguttatum | A. cf. parvum | |||
| Male | Fitalicale | Male | Fitalicale | Male | Fitalicale | |
| Guayacán | 39 | 51 | 15 | 11 | 3 | 2 |
| Ojo de agua | 50 | 25 | 8 | 5 | 7 | 6 |
| Mapache | 22 | 16 | 11 | 13 | 6 | 3 |
| Mangos | 11 | 16 | 1 | 2 | 9 | 7 |
| Camino | 25 | 25 | 6 | 1 | 4 | 0 |
| Cantera | 5 | 3 | 3 | 3 | 0 | 0 |
| Laguna | 1 | 2 | 3 | 0 | 0 | 0 |
| Total | 153 | 138 | 47 | 35 | 29 | 18 |
Sites abundance. The abundance of larvae did not differ among sites (Table 4A; Fig. 2a). On the contrary, the abundance of nymphs and adults differed among sites. The Sendero Guayacán (SG), the most forested site, had the highest abundance of nymphs (Fig. 2b). The abundance of nymphs in this site was not significantly different from that of Ojo de Agua (OA), but it was significantly higher than the abundance of the other five sites (Table 4B; Fig. 2b); nymphs in the OA site were significantly more abundant than in the three most disturbed sites (CP, LC, and LA; Fig. 2b). For adult ticks, we found the highest abundance in SG, which did not statistically differ from that found at La Cantera site (LC: Table 4C; Fig. 2c); the abundance of adult ticks in LC was only significantly higher than that found in Los Mangos (LM) and La laguna (LA) sites (Fig. 2c).
Table 4 Comparison of the number of larva (A), nymph (B), and adult (C) ticks between sites, based on GLMM. The site and PC1 (combination of environmental variables) were included as predictor variables and sampling date as random factor. SE- standard error, df- degrees of freedom, T- t-student value, P- probability.
| A | Larvae | ||||
| Sites | Estimated | SE | df | T | P |
| Intercept | |||||
| Ojo de agua | -26.69 | 56.47 | 72 | -0.47 | 0.638 |
| Mapache | -26.45 | 56.47 | 72 | -0.47 | 0.641 |
| Mangos | -59.35 | 56.47 | 72 | -1.05 | 0.297 |
| Camino | -5.70 | 56.47 | 72 | -0.10 | 0.920 |
| Cantera | 36.15 | 56.47 | 72 | 0.64 | 0.524 |
| Laguna | -1.31 | 56.47 | 72 | -0.02 | 0.982 |
| PC1 | -15.65 | 10.58 | 11 | -1.48 | 0.167 |
| B | Nymphs | ||||
| Intercept | 12.65 | 2.28 | 60 | 5.54 | <0.001 |
| Ojo de agua | -3.84 | 2.82 | 72 | -1.36 | 0.177 |
| Mapache | -5.87 | 2.82 | 72 | -2.08 | 0.041 |
| Mangos | -8.13 | 2.82 | 72 | -2.88 | 0.005 |
| Camino | -10.40 | 2.82 | 72 | -3.69 | <0.001 |
| Cantera | -9.72 | 2.82 | 72 | -3.45 | <0.001 |
| Laguna | -10.91 | 2.82 | 72 | -3.87 | <0.001 |
| PC1 | -1.95 | 0.71 | 11 | -2.76 | 0.019 |
| C | Adults | ||||
| Intercept | 5.54 | 1.26 | 33 | 4.40 | <0.001 |
| Ojo de agua | -2.91 | 1.29 | 72 | -2.26 | 0.027 |
| Mapache | -3.85 | 1.29 | 72 | -2.99 | 0.004 |
| Mangos | -4.58 | 1.29 | 72 | -3.56 | <0.001 |
| Camino | -1.89 | 1.29 | 72 | -1.47 | 0.146 |
| Cantera | -4.46 | 1.29 | 72 | -3.46 | <0.001 |
| Laguna | -5.41 | 1.29 | 72 | -4.20 | <0.001 |
| PC1 | 0.01 | 0.49 | 11 | 0.01 | 0.988 |
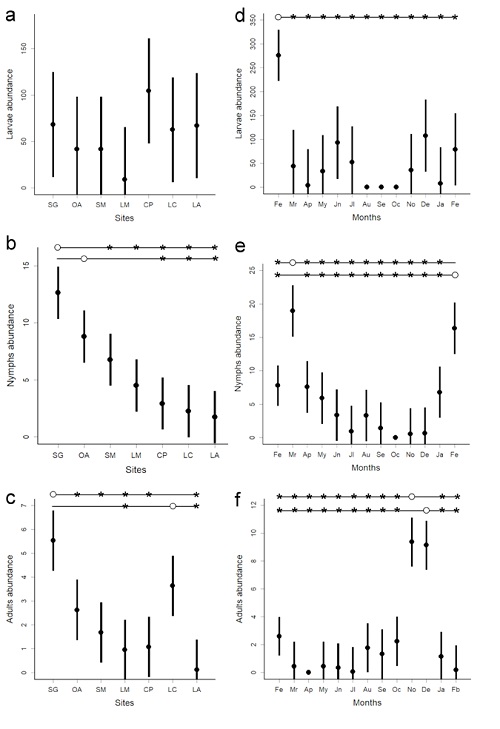
Figure 2 Sites and monthly variation in tick abundance. Abundance of larvae (a), nymphs (b), and adults (c) in each of the 7 sites sampled. Abundance of larvae (d), nymphs (e), and adults (f) over the 13-month period. For each site and month, the mean abundance is represented by black circle, and the line represents standard error above and below the mean. The horizontal line(s) on the superior section of some graphs inform on the significant difference between either sites or months. The open circle of a horizontal line indicates the focal site (or month) that is being compared with the others. An asterisk indicates that there is a significant difference between the focal site (or month) and that particular site (or month). The lack of an asterisk indicates no significant difference between the sites or months being compared. A graph without a horizontal line indicates that none of the comparisons was significant.
The abundance of nymphs correlated negatively with the PC1, indicating that low temperatures (maximum and minimum), low precipitation and low relative humidity correlate with high abundance of nymph ticks, but the PC1 did not correlate with the abundance of larvae nor adults (Table 4).
We compared the abundance of adults of the three most common tick species (A. mixtum, A. cf. oblongoguttatum, and A. cf. parvum) among sites. We collected more A. mixtum in the SG, CP, and OA (X 2 = 106.00, df = 6, p < 0.001; Fig. 3a); A. cf. oblongoguttatum in the SG (X 2 = 25.21, df = 6, p < 0.001; Fig. 3b); and the abundance of A. cf. parvum did not differ among sites (X 2 = 9.36, df = 6, p = 0.153; Fig. 3c).
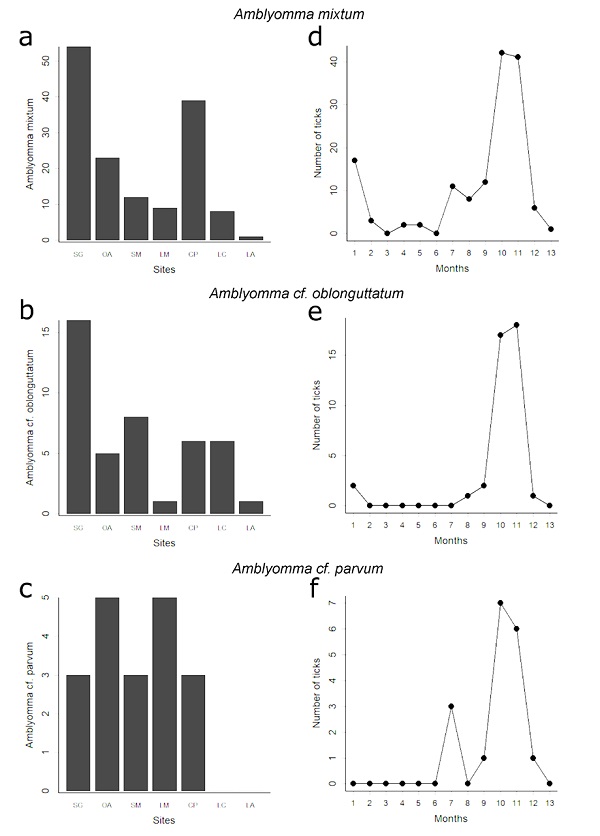
Figure 3 Number of adult ticks of Amblyomma mixtum, A. cf. oblongoguttatum, and A. cf. parvum collected in the seven study sites (a-c) and during 13 months study period (d-f).
Monthly abundance. The abundance of ticks also varied along the study period for all three stages. We found the highest abundance of larvae in February-2012, which differed from the abundance of all other months; the abundance of larvae was similar among the other months (Table 5A; Fig. 2d). For nymphs, March and February-2013 had the highest abundance, which did not differ between them. The abundance of nymphs registered in both months, March and February-2003, was significantly higher than that of the other months (Table 5B; Fig. 2e).
Table 5 Comparison of the number of larva (A), nymph (B), and adult (C) ticks between months, based on GLMM. The month was included as the predictor variable and site as random factor. SE- standard error, df- degrees of freedom, T- t-student value, P- probability.
| A | Larvae | ||||
| Month | Estimated | SE | df | T | P |
| Intercept | 276.30 | 53.47 | 78 | 5.17 | <0.001 |
| March | -232.30 | 75.62 | 78 | -3.07 | 0.003 |
| April | -272.34 | 75.62 | 78 | -3.60 | <0.001 |
| May | -242.90 | 75.62 | 78 | -3.21 | 0.002 |
| June | -182.96 | 75.62 | 78 | -2.42 | 0.018 |
| July | -224.21 | 75.62 | 78 | -2.96 | 0.004 |
| August | -276.30 | 75.62 | 78 | -3.65 | <0.001 |
| September | -276.30 | 75.62 | 78 | -3.65 | <0.001 |
| October | -276.30 | 75.62 | 78 | -3.65 | <0.001 |
| November | -240.80 | 75.62 | 78 | -3.18 | 0.002 |
| December | -168.33 | 75.62 | 78 | -2.23 | 0.029 |
| January | -268.80 | 75.62 | 78 | -3.55 | <0.001 |
| February | -197.14 | 75.62 | 78 | -2.61 | 0.011 |
| B | Nymphs | ||||
| Intercept | 7.77 | 3.02 | 55 | 2.58 | 0.013 |
| March | 11.18 | 3.84 | 72 | 2.91 | 0.005 |
| April | -0.20 | 3.84 | 72 | -0.05 | 0.959 |
| May | -1.87 | 3.84 | 72 | -0.49 | 0.628 |
| June | -4.42 | 3.84 | 72 | -1.15 | 0.254 |
| July | -6.83 | 3.84 | 72 | -1.77 | 0.080 |
| August | -4.47 | 3.84 | 72 | -1.16 | 0.249 |
| September | -6.36 | 3.84 | 72 | -1.65 | 0.103 |
| October | -7.77 | 3.84 | 72 | -2.02 | 0.047 |
| November | -7.22 | 3.84 | 72 | -1.88 | 0.064 |
| December | -7.10 | 3.84 | 72 | -1.85 | 0.069 |
| January | -0.98 | 3.84 | 72 | -0.25 | 0.800 |
| February | 8.59 | 3.84 | 72 | 2.23 | 0.029 |
| C | Adults | ||||
| Intercept | 2.59 | 1.38 | 53 | 1.87 | 0.067 |
| March | -2.15 | 1.76 | 72 | -1.22 | 0.225 |
| April | -2.59 | 1.76 | 72 | -1.48 | 0.144 |
| May | -2.14 | 1.76 | 72 | -1.22 | 0.227 |
| June | -2.26 | 1.76 | 72 | -1.29 | 0.202 |
| July | -2.54 | 1.76 | 72 | -1.45 | 0.152 |
| August | -0.82 | 1.76 | 72 | -0.46 | 0.643 |
| September | -1.26 | 1.76 | 72 | -0.72 | 0.474 |
| October | -0.35 | 1.76 | 72 | -0.20 | 0.842 |
| November | 6.78 | 1.76 | 72 | 3.86 | <0.001 |
| December | 6.55 | 1.76 | 72 | 3.73 | <0.001 |
| January | -1.44 | 1.76 | 72 | -0.82 | 0.415 |
| February | -2.40 | 1.76 | 72 | -1.36 | 0.177 |
The highest abundance of adults occurred in November and December, which was similar between these two months. The abundance of adults in these two months was significantly higher than the abundance in the other months (Table 5C; Fig. 2f); the abundance was similar among the other months. We found similar results when the monthly abundance of adults of the three most common species was analyzed. All three species showed a significant abundance peak between October and December (A. mixtum: D = 0.75, p<0.001, A. cf. oblongoguttatum: D = 0.50, p = 0.003, A. cf. parvum: D = 0.50, p = 0.003; Figs. 3d-f), like the results obtained for all adults combined (Fig. 2f). Furthermore, when we correlated the mean monthly abundance of each tick stage with the PC1, only nymphs correlated significantly with PC1 (r = -0.64, p = 0.019).
The results for each of the three most common species matches the general results of habitat and monthly variation in abundance for all adults combined. Therefore, we are confident to use the general results of adults in the discussion.
Discussion
The abundance of host-seeking ticks varied among the three stages, across habitats and through time. In general, adults and nymphs were more abundant in forested sites (Fig. 2 a-c), but larvae were common on all sites, with higher abundance (but not significant) in the CP, indicating that the physiognomy of the vegetation likely affect the abundance of host-seeking ticks. The structure of the vegetation is known to play an important role in determining the occurrence and abundance of different arthropod assemblages, because differences in vegetation offer different arrangements of environmental conditions and biotic interactions (Levings & Windsor, 1982; 1984). Our study suggests that forested habitats provide a better suit of environmental conditions for host-seeking hard ticks than more disturbed habitats (Table 4, Fig. 2). Ticks molting (larva to nymph, and nymph to adult) occur on the ground; thus, forested sites likely have the best suitable conditions for molting and surviving. One important condition is that trees in forested sites retain the leaves during the entire dry period (Hartshorn, 1983), so that the crown of trees drastically reduce the radiation and temperature at ground level, also maintaining a higher relative humidity at this level (Borchert, 1994; Schulz et al., 2014). The lack of association of larvae abundance with vegetation types suggests that habitat structure does not have a significant effect on their abundance (Tables 4-5; Fig 1a), or that gravid females do not have a site preference to abandon their hosts and lay eggs.
Another factor associated to the larger abundance of adult and nymph of ticks in forested sites is the presence and abundance of hosts. The two most forested sites have small waterholes, whose water flow during the entire dry season; the other forested site is near to a waterhole and has a seasonal spring that dries out during part of the dry season. The presence of water, benign environmental conditions, and occurrence of some fleshy-fruiting trees (Frankie et al., 1974; Opler et al., 1980), whose fruits ripe during the dry season, made these sites attractive for many mammal and other potential hosts (e.g., white tailed deer, wild pigs, raccoons, coatis, birds, reptiles; Vaughan & Weis, 1999; Bermúdez et al., 2018; Montalvo et al., 2019). The CP presents a different situation. The large abundance of larvae and adults in this site may be related with the intense transit of animals (e.g., mammals and reptiles) between the forest and a large seasonal lagoon that serves as water supply for many animals for most of the dry season. Hence, differences in abundance of ticks of different stages between sites are also likely related with the activity of these hosts. Most animals, including mammals, are associated to forest patches with water sources (Janzen, 1988b; Montalvo et al., 2019). Hence, the patterns of tick abundance found in this study are likely related to the vegetation structure in the different sites, host preference, behavior of tick hosts (e.g., mobility and social structure), and environmental conditions.
The abundance of ticks also varied temporarily, but only nymphs correlated negatively with PC1. Adults (total and individual species) drastically increase their abundance at the onset of the dry period (November-December). The peak abundance of larvae and nymphs seems to follow that of adults, suggesting that the periods of drought in the dry forest are important for the reproduction and population dynamics of ticks (Fig. 2 d-f). The patterns (or lack of them) we detected in immature ticks could be the result of the confounding effect of different species-specific phenological patterns. However, there are two consistent patterns, the abundance of adults (general and species-specific) increases at the beginning of the dry season, and nymphs and larvae drastically decreased during the rainy season (nymphs in October and larvae in Aug, Sep, Oct) (Table 3, Fig 2 b, c). Heavy precipitations likely flush away and kill larvae and nymphs (Rawlins, 1979), so that the peaks of abundance observed in these stages could either be related to a lower mortality due to lower precipitation, or, in the case of larvae, that females adjust their reproductive events (laying eggs) to the dry periods. Our results on nymphs and larvae should be treated cautiously since we did not identify individuals of these stages to the species level.
There is some consensus among researchers that tick species have a single breeding annual period (Oorebeek & Kleindorfer, 2008; Szabó et al., 2007; Montenegro et al., 2021). Particularly, Labruna et al., (2009) state that Amblyomma cajennense, the most abundant species in their study, has one generation per year, based on behavioral diapause of larvae. In Costa Rican dry forest adults have a well-defined peak of abundance that likely correspond to the most important reproductive event. The peak of abundance of adults of the three species analyzed occurs during the same period of the year (Fig. 3a-c). But only adults of A. mixtum were present during the entire study period, which suggests that this species reproduce year-round, but has a defined reproductive peak that coincide with that of the other species. Differences in the reproductive patterns of tick species might also be influenced by the diversity and abundance of hosts they parasitized. For instance, A. mixtum is a host-generalist species that parasitized reptiles, birds, and a diverse group of mammals including domestic species (Bermúdez et al, 2018). On the contrary, A. dissimile is primarily found on toads and reptiles (Guglielmone & Nava, 2010), which are abundant in the study area, although abundance of toads drastically decreases during the dry season (Savage, 2002). Hence, host-generalists tick species are apparently more likely to reproduce year-round than host specialist species.
In conclusion, vegetation structure plays an important role in determining the spatial variation in abundance of the three host-seeking stages of Amblyomma spp. ticks in a Costa Rican dry forest. Larvae are abundant in all sites, but abundance of nymphs and adults is higher in forested sites, indicating that these sites offer more suitable conditions for molting and survivorship of ticks. In addition, these two sites concentrate a rich and abundant fauna (Janzen & Wilson,1983; Stoner & Timm, 2004), with many of the mammal species, and other animals that likely serve as hosts for the tick species found in this dry forest (Fairchild et al., 1966; Guglielmone & Nava, 2006; Montalvo et al., 2019). The reproduction of ticks in Costa Rican dry forest likely occurs at the onset of the dry season.











 nueva página del texto (beta)
nueva página del texto (beta)




