Pineapple (Ananas comosus) is the third most important tropical crop worldwide (Sanewski et al., 2018). Curvularia clavata is the causal agent of leaf spot in pineapple plants with incidences from 35 to 58% in China (Zhong et al., 2016) and C. eragrostidis causes a post-harvest loss in pineapple fruit in Brazil (Ferreira et al., 2014), and Curvularia sp. causes leaf spot in pineapple crop fields in Nicaragua (García-Osorio and Orozco-Gomez, 2016). Mexico is the 7th producer of pineapple (INEGI, 2019) around the world. Recently, Maldonado-Michel et al. (2021) reported C. eragrostidis as the causal agent of pineapple leaf-spot in Colima state (Pacific Center of Mexico). However, this study lacks molecular data for accurate species identification. They also tested seed extracts of Swietenia humilis for antifungal activity. However, the regular local management of fungal diseases includes chemical fungicides, despite the fact that the main side-effects of these products are related to damage to human health, severe environmental impact, and development of resistant strains (Heydari and Pessarakli, 2010). Therefore, there is a need to evaluate alternative methods such as biological control agents (BCA) and plant extracts (PE), which have shown to be safer and efficient (Cerqueira-Sales et al., 2016) against C. eragrostidis. In Colima, Mexico some BCA and PE bio fungicides are available but have not been locally and properly tested; the main goal of this research were to identify the causal agent of the leaf-spot disease in pineapple and to evaluate the in vitro effectiveness of five commercial biological products against C. eragrostidis.
Plants with leaf spot symptoms were sampled randomly in a pineapple (cv. MD2) plantation in Tecoman, Colima, Mexico (18°47’41.3” N; 103°51’26.9” W). Ten samples were collected in 2 ha. Fungal isolation was carried out according to Orozco-Santos et al. (2004); infected leaves were cut, washed with 1% NaClO for 3 min, rinsed with autoclaved distilled water, and dried on sterile filter paper. Disinfected plant tissue was ground in liquid nitrogen and then lyophilized. This served as an inoculum in 400 mL of Czapek broth shaken at 2 Hz at room temperature for 3 d. After that, 50, 100, and 150 μL of culture were inoculated in Petri dishes with PDA and incubated at 31 °C for 3 d. Individual colonies were transferred and incubated for 5 d and used as inoculum for microcultures in slides with PDA (Zhong et al., 2016).
Morphological identification was carried out following Ferreira et al. (2014), Manamgoda et al. (2012) and Rocha-Santos et al. (2018). Samples of monoconidial cultures were used for molecular identification though sequencing of the ITS partial region with the ITS1 and ITS4 primers (White et al., 1990). The sequences were inspected by chromatograms on the SequencherSoftware™ v. 5.2.3 and curated sequence was used for BLASTN query at NCBI’s GenBank. Then, a MegaBlast was performed, and 48 highly similar Curvularia sequences (ex-types, Figure 1) and two outgroup sequences were downloaded from GenBank, aligned using the CLUSTALW algorithm with default parameters (Thompson et al., 2003), and manually edited using MEGA X software suite (Tamura et al., 2013). The molecular phylogenetic analysis consisted of a Maximum likelihood (ML) phylogenetic analysis, with the Kimura-2-parameter+G+I model (Kimura, 1980) with gaps treated as partial deletions with a 95% of coverage, using an NNI heuristic method for topology improvement with 1000 bootstrap replicates. The trees were rooted using Bipolaris maydis (=Cochliobolus heterostrophus) as sister clade and Alternaria alternata as outgroup (Manamgoda et al., 2012; Tan et al., 2018).
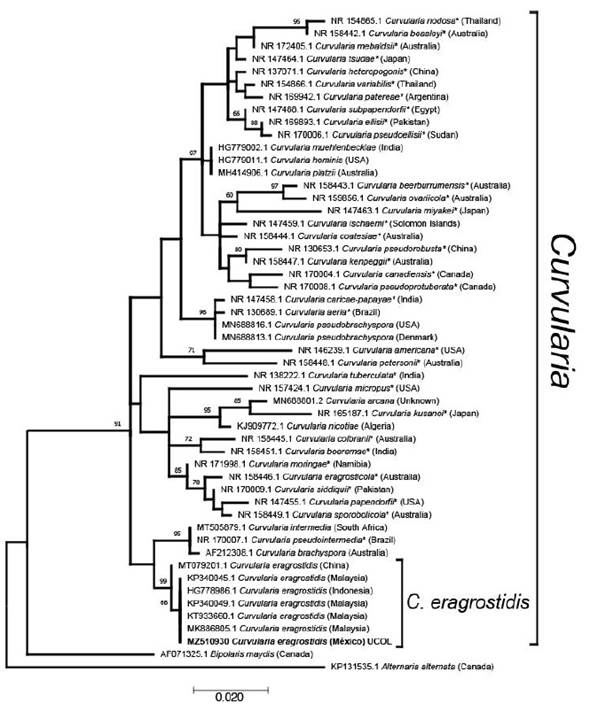
Figure 1 Molecular phylogenetic analysis of Curvularia species based on the Maximum likelihood method using the Kimura 2-parameter (G+I) model. The tree with the highest log likelihood (-1962.89) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches, based on 1000 bootstrap replicates. Accession number is indicated in each taxon. Taxa names marked with * are ex-type material. Country of origin of isolates are in parenthesis
Commercial biofungicides are detailed in Table 1. Based on their concentrations, four doses were evaluated: 1.0, 0.5, 0.25 and 0.125% (v/v or w/v) in 20 mL of PDA + biofungicide. Solid products were expressed in mg L-1 and liquid products were expressed in mL L-1. Amended PDA Petri dishes were obtained in two ways: 1) PE-based, and 2) BCA-based. PE products (System Max® / Sedric 4X®) were added to PDA before solidification and then served in Petri dishes. BCA products were mixed in the PDA and 20 mL were verted in Petri dishes. In each Petri dish, a disk of 9 mm in diameter of fungal pathogen that was 7 d old was inoculated and incubated at 29 °C for 10 d.
Table 1 Characteristics of the biological products evaluated in the doses response bioassay.
| Commercial name | Active ingredient | Recommended doses | Concentration |
|---|---|---|---|
| System Max® (Plantoria) | Mimosa tenuiflora extract + Quercus robur extract | 4.5 mL L-1 | 60 and 30 % |
| Sedric 4X® (BioCampo) | Yucca schidigera extract | 3 L ha-1 | 2.16 % |
| BliteFree® (Altus Biopharm) | Streptomyces spp. | 1.5 L ha-1 | 60 % |
| BioFungus Clean® (Syme Agroinsumo) | Bacillus subtilis + B. thuringiensis + Trichoderma harzianum + T. viride | 2 L ha-1 | 1.0, 1.0, 1.0 and 1.0 % |
| Tonka® (Novigo Natura) | B. subtilis + T. harzianum + Streptomyces lydicus | 1.5 kg ha-1 | 1x108 UFC g-1 1x107 UFC g-1 and 1x106 UFC g-1 |
Daily growth rate (DGR) was calculated by measuring pathogen colony diameter each 24 h for 5 d using the formula DGR = [(R1 - R0) / (T1 - T0)], where R1 and R0 is the colony diameter growth (mm) and T1 and T0 is the time (days) (Bahekar et al., 2017). Mycelial Growth Inhibition of the pathogen (% MGI) was calculated % MGI = [(C - T)/C] x 100% where C = Control growth diameter (mm) and T = Treatment growth diameter (mm) (Bahekar et al., 2017). Fifty and ninety effective concentrations (EC50 and EC90) were calculated using a Probit analysis in SAS 9.0 (Manzo-Sánchez et al., 2018). The experimental design consisted of a completely randomized design with the combinatorial factorial arrangement, being A the biological products brand and B the different doses of each product. Five biological products, four doses each, and a control (no product); each treatment had six repetitions, with a total of 126 experimental units. Data analysis of DGR was carried out with an ANOVA and mean comparison using the minimum significant difference (MSD) with p=0.05. Percentage of MGI data were transformed using the arcsen equation (% MGI)½ to obtain an approximately normal distribution and posterior analysis. The CL50 was calculated with a Probit analysis. Finally, a linear regression analysis (Y=mX+b) between the concentration of the product (X) and percentage of MGI (Y) were performed.
The isolate showed macro and microscopic morphological characteristics matching with Curvularia eragrostidis, such as a cottony grayish colony (7.7 cm, 5 d of incubation) turning into velvety-black with regular edges. Ellipsoidal distoseptate conidia, 21.6-24.9 × 11.6-13.3 µm. Grouped and septate brown conidiophores. Mycelium is composed of dark septate hyphae. Molecular phylogenetic analysis of Curvularia species based on the maximum likelihood method, grouped the sequence with the aforementioned with high support, confirming the identity of the studied isolate as C. eragrostidis. As expected, Chinese material is slightly different from the isolates from Malaysia, Indonesia and Mexico. C. eragrostidis closest subclade, includes C. intermedia, C. pseudointermedia and C. brachyspora (Figure 1). All sequences were grouped within the monophyletic genus Curvularia with high support, separated from Bipolaris maydis, a sister anamorphic clade, which matches with Manamgoda et al. (2012) and Tan et al. (2018).
C. eragrostidis daily growth was affected by biological products with highly significant differences (P≤0.0001, Table 2). The DGR of the control group was 1.6 cm day-1, while Tonka® showed the lowest growth rate in all four evaluated concentrations with a DGR mean of 0.015 cm day-1, which is 106.6 times lower than the control group. The BioFungus Clean® activity had a similar effect in the first three concentrations (1.0, 0.5, and 0.25%) with a mean of 0.082 cm day-1. SystemMax® and Sedric 4X®, only reduced the DGR of C. eragrostidis from 0.25% and beyond with 1.47 and 0.27 cm day-1; however, at 1.0% (0.048 cm day-1) was statistically equal between Tonka®, Biofungus Clean® and BliteFree® with 0.02, 0.04 and 0.021 cm day-1, respectively. The% MGI achieved significant differences (P≤0.0001) at 5 d after inoculation. Sedric 4X® showed the highest inhibition percentage at 1% concentration (86.8%), 2.5 times higher than System Max® and 1.3 times higher than BliteFree® (63.7%) at the same concentration. This value was significantly higher (P≤0.0001) than the other concentrations of the same product (Table 3). On the other hand, Tonka® was effective in every concentration with no statistical differences (P≤0.2657) between all four doses, with a mean of 68.97% of inhibition. Similarly, Biofungus Clean® had the same inhibition effect (P≤0.0871) in all concentrations with 1.2 times lower inhibition than Tonka®. BliteFree® registered the highest inhibition rate (P≤0.0002) with 63.7% at the highest concentration, while lower ones between 55.8 and 58.6%. On the contrary, System Max® had a poor effect against C. eragrostidis, 5.1 times lower than Tonka® (0.125%). Control group had a radial mycelial growth of 7.7 cm. The results of the inhibition assays with the five products at four doses in 5 d are showed in Figure 2.
Table 2 In vitro daily growth rate (cm day-1) of Curvularia eragrostidis under different doses of biological products.
| Commercial products | Doses (%) | |||
|---|---|---|---|---|
| 0.125 | 0.25 | 0.5 | 1.0 | |
| Control | 1.635±0.018 b | 1.635±0.018 e | 1.635±0.018 c | 1.635±0.018 c |
| System Max® | 1.698±0.007 b | 1.476±0.015 d | 1.298±0.031 b | 1.186±0.057 b |
| Sedric 4X® | 1.571±0.056 b | 0.27±0.016 c | 0.173±0.008 a | 0.048±0.007 a |
| Tonka® | 0.01±0.006 a | 0.028±0.006 a | 0.013±0.008 a | 0.018±0.013 a |
| BioFungus Clean® | 0.026±0.009 a | 0.12±0.054 b | 0.086±0.030 a | 0.04±0.010 a |
| Blite Free® | 0.036±0.004 a | 0.026±0.009 a | 0.181±0.169 a | 0.021±0.010 a |
| P-value | 0.00001 | 0.00001 | 0.00001 | 0.00001 |
| F= | 1135.94 | 523.85 | 46.12 | 353.54 |
Means (± SE) with different letters in the same column indicates significant differences (LSD, P≤0.05
Table 3 Mycelial growth inhibition (%) of Curvularia eragrostidis under different doses of biological products.
| Commercial products | Doses (%) | |||
|---|---|---|---|---|
| 0.125 | 0.25 | 0.5 | 1.0 | |
| System Max® | 4.51 ± 0.77 d | 5.77 ± 0.75 d | 13.16 ± 1.78 c | 24.16 ± 1.29 e |
| Sedric 4X® | 7.13 ± 1.22 c | 57.48 ± 0.55 b | 68.87 ± 0.39 a | 74.96 ± 0.14 a |
| Tonka® | 65.31 ± 0.25 a | 65.77 ± 0.62 a | 66.91 ± 0.96 a | 66.72 ± 0.61 b |
| BioFungus Clean® | 53.47 ± 1.15 b | 47.64 ± 3.62 c | 51.57 ± 2.08 b | 56.22 ± 1.27d |
| Blite Free® | 53.90±0.93 b | 56.30 ± 0.64 b | 52.95 ± 2.13 b | 61.45 ± 1.02 c |
| P-value | 0.00001 | 0.00001 | 0.00001 | 0.00001 |
| F= | 677.44 | 178.68 | 137.32 | 353.54 |
Means (± SE) with different letters in the same column indicates significant differences (LSD, P≤0.05).
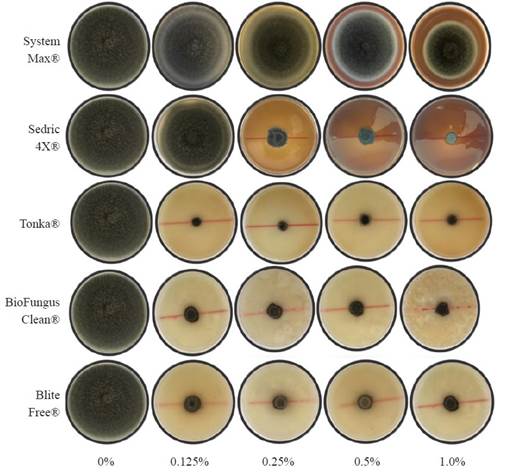
Figure 2 Mycelial growth of Curvularia eragrostidis in PDA amended with five doses of biological products.
Tonka® achieves a CL50 of 0.008%, which was the lowest value in comparison with the rest of the treatments, 32.2 times lower than Sedric 4X® (0.260%) and 449.8 times lower than System Max® (3.59%) (Table 4). However, BliteFree®, BioFungus Clean® also showed good values with 0.015% and 0.017%, respectively. In EC90, the best result was again for Tonka® with 0.040%, which was 9.7 times lower than BioFungus Clean® and 4.9 times lower than BliteFree®. The linear regression analysis between inhibition percentages and product doses (Figure 3) indicated a high correlation between System Max® concentrations (r=0.86, P≤0.0001) and Sedric 4X®, (r=0.69, P≤0.0001) and their inhibition percentages with highly significant differences. On the contrary, BliteFree® showed a low correlation between concentration and inhibition (r=0.40, P≤0.0008). On the other hand, Tonka® and BioFungus Clean® did not indicate a statistical difference (P≤0.1191 and P≤0.0939, Table 4).
Table 4 Mean effective concentration (EC50) and ninety (EC90) of biological products on Curvularia eragrostidis.
| Commercial products | EC50 (%) | FL (%) | Slope | Probit equation | Chi- X2 | P>Chi- X2 |
|---|---|---|---|---|---|---|
| System Max® | 3.599 | 2.32-7.01 | 0.8070 | y=0.8070(x)-0.4485 | 85.51 | ≤0.0001 |
| Sedric 4X® | 0.260 | 0.24-0.27 | 2.2153 | y=2.2153(x)+1.2974 | 536.62 | ≤0.0001 |
| Tonka® | 0.008 | NC | 0.0650 | y=0.0650(x)+0.5264 | 121.15 | ≤0.0001 |
| BioFungus Clean® | 0.017 | NC | 0.0921 | y=0.0921 (x)+0.1631 | 12.70 | ≤0.0004 |
| BliteFree® | 0.015 | NC | 0.1579 | y=0.1579 (x)+0.2876 | 38.79 | ≤0.0001 |
| Commercial products | EC90 (%) | FL (%) | Slope | Probit equation | Chi- X2 | P>Chi- X2 |
| System Max® | 139.405 | 47.22-758.51 | 0.8070 | y=0.8070(x)+(-0.4485) | 85.51 | ≤0.0001 |
| Sedric 4X® | 0.984 | 0.885-1.110 | 2.2153 | y=2.2153(x)+1.2974 | 536.62 | ≤0.0001 |
| Tonka® | 0.040 | NC | 0.0650 | y=0.0650(x)+0.5264 | 121.15 | ≤0.0001 |
| BioFungus Clean® | 0.388 | NC | 0.0921 | y=0.0921 (x)+0.1631 | 12.70 | ≤0.0004 |
| BliteFree® | 0.197 | NC | 0.1579 | y=0.1579 (x)+0.2876 | 38.79 | ≤0.0001 |
FL=fiducial limits, NC=no estimated by the model, X=biological product concentration, Y=inhibition percentage.
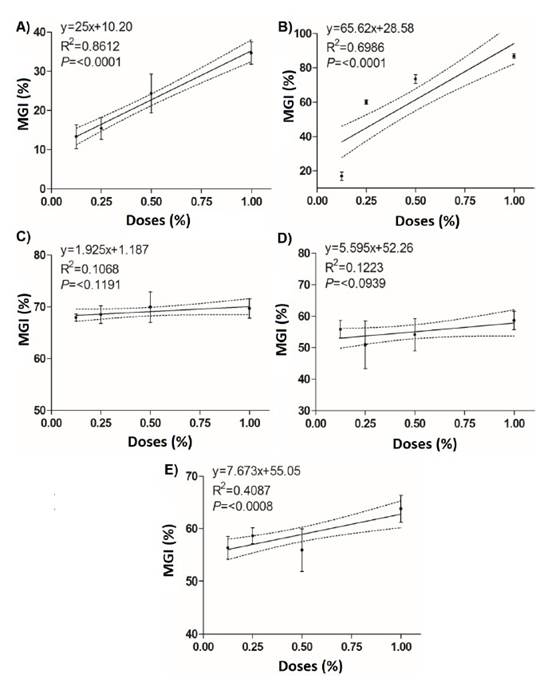
Figure 3 Linear regression between the mycelial growth inhibition (%) of Curvularia eragrostidis and the doses of the biological products. A) System Max®; B) Sedric 4X®; C) Tonka®; D) BioFungus Clean® and E) BliteFree®.
Leaf spot disease caused by C. eragrostidis is an emerging disease of pineapple production in Colima, Mexico (Maldonado-Michel et al., 2021), the present study corroborates the identity of the causative agent morphologically and molecularly. In South America, it has been reported at C. eragrostidis as a plant pathogen in pineapple plants in similar features by Ferreira et al. (2014). In other hand, plant extracts have been used to control plant pathogens in biological and organic agriculture. La-Torre et al. (2014) evaluated the in vitro efficiency of a Mimoten®, a PB extract of Mimosa tenuiflora (80.0%) against Alternaria alternata and Botrytis cinerea with a maximum inhibition of 19.2% and 14.1%, respectively, at maximum doses (1.0%). There are no reports of the use of M. tenuiflora against C. eragrostidis; in this case, System Max® contains M. tenuiflora, but it is supplemented with Quercus sp. extract and gallic acid, which increased the inhibition up to 34.6%. Quercus extracts have been tested as antibacterial and antifungal activity, in this sense, Söhretoglu et al. (2007) tested methanolic extracts from four Quercus species, against gram (+) and (-) bacteria and three Candida spp. Extracts were more effective against fungi than bacteria. In filamentous fungi, Yeo et al. 2008 tested Q. mongolica in aqueous, methanolic and ethanolic extracts against Botrytis cinerea, being the aqueous extract more effective. Gallic acid and other phenolic compounds have been tested as an antifungal for human and plant pathogens.
In Trichophyton rubrum, gallic acid (50.0 μg mL-1) reduced the activity of sterol 14α-demethylase P450 (CYP51) and squalene epoxidase altering the fungal membrane (Li et al., 2017). In the present study, Sedric 4X®, PB extract of Y. schidigera showed 86% of inhibition at the highest concentration. Wulff et al. (2012) evaluated the effect of Y. schidigera extract against C. lunata from sorghum, no incidence (0%) was achieved when was used 10.0% (v/v) of Y. schidigera extract. These plants are rich in saponins, which can alter the cell membranes having a toxic effect on fungi. Maldonado-Michel et al. (2021) tested hexane, ethyl acetate dichloromethane and methanol PE of Swietenia humilis against this isolate of C. eragrostidis, being the etyl-acetate at 500 mg L-1 the most effective with 68.0% of MGI. The main secondary metabolites of S. humilis are limonoids, a kind of triterpenoids that are found in the seeds (Ovalle-Magallanes et al., 2015).
A complete inhibition (100.0%) of spore germination of C. lunata using Cinnamomum zeylanicum organic extracts at lowest concentration (50 μg mL-1) and a complete fungicidal activity at highest concentration (500 μg mL-1), but aqueous extracts were not efficient (Mishra et al., 2009). Otherwise, Akinbode (2010) evaluated in vitro the efficacy of leaf aqueous extracts of Gliricidia sepium, Tithonia diversifolia, Phyllanthus amarus and Morinda lucida to control C. lunata. All the extracts at 100% concentration, suppressed the growth of the pathogen.
In the other hand, BliteFree® achieved 55.6-67.7% inhibition against C. eragrostidis. These results are similar to those reported by Evangelista-Martínez (2014), who evaluated Streptomyces spp. against Curvularia sp. with a 55.0% of plant-pathogen growth inhibition. Wonglom et al. (2019) confronted C. lunata isolated from Brassica rapa with S. angustmyceticus (NR8-2) with inhibition of 69.0%. Some of the antagonism mechanisms of Trichoderma spp. against plant pathogens include antibiosis, mycoparasitism, induced resistance of the host cell, nutrient and niche competition (Ghazanfar et al., 2018). Tekade et al. (2017) evaluated T. viride and T. harzianum against C. lunata, presenting 60.8 and 50.7% of inhibition, respectively, in 7 d at 27 °C. These results are similar to BioFungus Clean® which caused 54.8% inhibition in 5 d against C. eragrostidis. Bacillus spp. is one of the most studied genera as BCA since they present a diverse biochemical activity against plant pathogens (Layton et al., 2011). It has been reported that Bacillus spp. induces cell membrane defects and cell death in fungal hyphae, when the cell membrane is destroyed the nucleus and protoplast are exuded. Among the secondary metabolites secreted by Bacillus spp. has been reported antimicrobial proteins with active components such as paeonol, ethyl palmitate and oxalic acid, which are able to inhibit the sporulation and mycelial growth in fungal plant pathogens (Ku et al., 2021).
Basha and Ulaganathan (2002) evaluated BC121, a chitinase producer Bacillus strain against C. lunata, with inhibition up to 60.0%. Scanning electron microscope images showed a clear hyphae lysis and degradation of fungal wall. Orberá-Ratón et al. (2012) tested five rhizosphere isolates of B. subtilis against C. lunata with inhibition up to 61.0% (SR/A-1) and Curvularia gudauskasii with inhibition up to 71.0% (SR/B-16), and observed vacuolization, bulb formation, hyphal swelling, growing and conidia formation inhibition. However, other strains show different results. For example, Fleitas-Centurion and Grabowsky-Ocampos (2015) evaluated a Bacillus sp. (500 uL) against Curvularia sp. with 31.0% of inhibition. These previously reported values are heterogeneous, as occurred with BioFungus Clean® and Tonka®.
Sunpapao et al. (2018) confronted C. oryzae against S. hygroscopicus, T. harzianum and an endophytic Trichoderma species (V76-12), the latter was the most effective treatment tested in reducing leaf spot disease of oil palm seedlings with 85.71% of MGI. The formulation of Tonka® consists of three species consortia: a fungus (T. harzianum), a Bacillus species (B. subtilis), and an actinobacteria (S. lydicus). These results are interesting, because BioFungus Clean® has four species, two bacteria (B. subtilis and B. thuringiensis) and two fungi (T. harzianum and T. viride), this situation suggest that is more efficient a higher phylogenetic, biochemical and ecological diverse composition than a high number of close-related microorganisms. This is coherent with the concept of suppressive soils, on which plant diseases do not develop even with the presence of a plant pathogen in a susceptible host and favorable conditions. Pathogens may or not establish, persist, cause no or low harm, or the disease may manifest but disappear over time, which is widely attributed to soil microbiomes. General suppression depends on a high diversity and abundance of microorganisms, competing for space and resources; while specific suppression on the effect of individuals or groups of microorganisms directly over the life cycle of a pathogen (Raaijmakers and Mazola, 2016).
Research of microorganisms with BCA potential is challenging because the in vitro behavior might be promising, but the application in agricultural lands results in fluctuations of effectiveness. Production of metabolites is a common phenomenon in antagonistic relationships; however, their synthesis carries an energetic cost, which must be compensated with the benefits and can be influenced by toxic substances and the proportion of strains. For example, bacterial antagonism rises with carbon source metabolism similarities (Russel et al., 2017). We can differentiate microorganisms into productive and sensitive strains, which interact according to microbial diversity and their environment (Kelsic et al., 2015). Production of metabolites and their related processes decreases the growth rate. Despite this, productive strains rise compared to sensitive ones, even with low growth rates. The carbon source is not a problem when strains are grown in vitro; however, in agricultural lands, organic matter fluctuates according to soil type and management. Also, the use of fungicides and bactericides affected native microorganisms and added BCA and their performance. In addition to studying the diversity of soil microorganisms to develop biological products; another alternative is the design of synthetic microorganism communities or “syncoms”. This highly diverse consortium results closer to a natural community, with higher complexity and resilience (Rábago-Aguilar et al., 2020). By other hand, this study provides the LC50 values for five biofungicides against C. eragrostidis, LC50 indicates the amount of biofungicide required to inhibit the 50% of fungal growth. There are no abundant reports of biological or biorational products with respect to the LC50 against Curvularia species. Therefore, the present study provides these values, which are useful to define doses to be evaluated under in situ conditions. In a previous study, Kumar et al. (2020) evaluated the antifungal activity of Cedrus deodara essential oil against C. lunata, Alternaria alternata and Bipolaris spicifera, reporting LC50 values of 2.22, 3.71 and 4.8 μL/mL for each fungus, respectively. By other hand, García-Ordaz et al. (2021) reported LC50 values of chemical fungicides against C. eragrostidis, the lowest values were achieved by System Cu®, Mancozeb® 80 WP and Tecto® 60 with 0.024, 0.066 and 0.076%, respectively. Future studies should be carried out to test the LC50 under field conditions in pineapple production.
In conclusion, the associated fungus to the leaf spot disease in pineapple plants corresponded at Curvularia eragrostidis according to the morphological and molecular analyses. Tonka® was the better biofungicide against C. eragrostidis, according to the EC50. While, Sedric 4X® achieved the highest% of MGI at the highest studied doses (1.0%).











 texto en
texto en 


