INTRODUCTION
Species in the Gracilariaceae family, including those within the genera Gracilaria Greville, Crassiphycus Guiry, Gurgel, J.N. Norris et Fredericq, and Agarophyton Gurgel, J.N. Norris et Fredericq, are among the most commonly used algae in the production of agar (Oliveira et al. 2000, Kim et al. 2017). Globally, the production of this phycocolloid comes from species that are harvested from natural populations; however, some species in this family have been cultivated on a commercial scale for several years in countries such as Chile and Indonesia (Bixler and Porse 2011). In Brazil, agar is produced mainly from species that are extracted from natural populations since, despite the efforts, there have been no promising results in cultivating these agarophytes on a commercial scale. However, extractive practices have been combined with artisanal culturing, a joint practice that has played an important role in the economy of coastal communities (Carneiro et al. 2011).
Algae are highly mutable organisms (Lobban and Harrison 1994), a feature that can affect phenotypic expressions such as growth, morphology, and color. In this context, thalli of the same species with different colors are frequent in the wild, possibly as a result of expressions of adaptation to the environment or a genotypic pigment variant (Navarro 2015). Pigment variants have been reported in several species of red macroalgae, including Hypnea musciformis (Wulfen) J.V. Lamouroux (Yokoya et al. 2003), Palmaria palmata (Linnaeus) Weber et Mohr (Pueschel and van der Meer 1984), Kappaphycus alvarezii (Doty) Doty (De Paula et al. 1999, Aguirre-von-Wobeser et al. 2001), Mazzaella laminarioides (Bory) Fredericq (Navarro 2015), Gracilaria domingensis (Kützing) Sonder ex Dickie (Plastino et al. 1999), Crassiphycus birdiae (Plastino et E.C. Oliveira) Gurgel, J.N. Norris et Fredericq (Plastino et al. 2004), Crassiphycus caudatus (J. Agardh) Gurgel, J.N. Norris et Fredericq (Faria and Plastino 2016), and Crassiphycus corneus (Ferreira et al. 2006). The study of different strains of Gracilariaceae species on the Brazilian coast has contributed to knowledge on the functional diversity of each species and to the selection of strains most suitable for culture (Ferreira et al. 2006, Ursi et al. 2013, Araújo et al. 2014).
Crassiphycus corneus is distributed in the western Atlantic Ocean from Bermuda, across the Gulf of Mexico, and down to Cabo Frio, Brazil (Bird et al. 1986). Given the quality and yield of its agar (Freile-Pelegrín et al. 2002), C. corneus together with C. birdiae has been used as a main source of raw material for agar production in Brazil (Plastino and Oliveira 2002). Some of its physiological aspects have been studied: growth under laboratory conditions (Navarro- Angulo and Robledo 1999), photosynthesis and pigments (Dawes et al. 1999), ultraviolet-absorbing compounds (Sinha et al. 2000), agar quality (Freile-Pelegrín et al. 2002), and reproduction (Guzmán-Urióstegui and Robledo 1999, Orduña-Rojas et al. 2002). However, none of these studies have approached intraspecific diversity. On the northeastern coast of Brazil, the C. corneus populations are composed of mostly individuals of the wild red color strain and some individuals of the green color strain. Since it was first discovered, the green color strain has been scarcely studied in relation to its physiological performance under different temperature and irradiance conditions.
Temperature and irradiance play a crucial role in different biological processes at different levels and determine the vertical and latitudinal distribution of algae (Lobban and Harrison 1994). Irradiance governs growth (provides energy for photosynthesis) and has photoperiodic and photomorphogenic effects (Hurd et al. 2014). Temperature, on the other hand, affects processes such as photosynthesis and respiration because of its direct influence on the speed of all enzymatic, electron transport, and solute movement reactions in algal cells and its influence on the properties of cellular components such as lipids, proteins, and carbohydrates (Davison 1991, Hurd et al. 2014). Knowing the requirements and tolerance of a given species regarding these factors is an important aspect of cultivation. In the case of color variants, the study of temperature and light requirements and tolerance levels could reveal valuable information on the presence of strains that are resistant to environmental variations. While some studies suggest that most pigment variants have lower biological adaptation than wild specimens, as variants are less robust and have lower growth and survival rates (van der Meer 1990), other studies have shown that variants and wild strains have similar physiological capabilities when exposed to the same conditions of irradiance, nutrients, temperature, etc. (Aguirre-von-Wobeser et al. 2001, Ferreira et al. 2006, Ursi et al. 2013). Given the commercial value of C. corneus and the importance of color strains to the study of genetics and intraspecific variability, this study aimed to characterize and compare the physiological performance of the green variant and the wild red strains of C. corneus under different temperature and irradiance conditions in the laboratory. The tested hypothesis was that the green strain would exhibit a similar physiological performance as specimens of the red color strain under different temperature and irradiance conditions.
MATERIALS AND METHODS
General culture conditions
Green variant and wild red strain tetrasporophytes of C. corneus from Meireles Beach(Fig. S1)(3°32’S, 41°42’’W), Fortaleza, Ceará State, northeastern Brazil, were obtained from the Gracilariaceae Germplasm Bank in the Édison José de Paula Seaweed Laboratory, Institute of Biosciences, University of São Paulo, Brazil. Biological material was cultured in sterile seawater (absolute salinity of 32) enriched with 12.5% von Stosch solution (Ursi and Plastino 2001). Algae were kept in a controlled environment at 25 ± 1 °C and a photoperiod of 14 h light. Photosynthetically active radiation, provided by fluorescent lamps (Osram 40W), was maintained at 200 μmol photon· m-2·s-1. Algae were kept under alternating aeration periods of 30 min. These conditions were used after having observed the results obtained for other Gracilariaceae species in our laboratory. Algae were kept under these conditions for 8 months in order to obtain enough material to start the experiments.
Experimental design
Three experiments were performed considering both the green and red strains. The first aimed to observe and compare the physiological performance of both strains at 3 different temperatures (18, 25, and 35 °C; 60 μmol photon·m m-2·s-1); the second aimed to assess the growth of both strains at 25 °C under high irradiance (500 μmol photon·m-2·s-1); and the third aimed to determine the photosynthetic rates of both strains under the conditions of the second experiment and to compare the data with the values obtained for specimens exposed to 25 °C and 200 μmol photon m-2·s-1
Growth at different temperatures
Eighteen 800-mL flasks containing 0.01 g (five 3-cm long apical segments per flasks) of both green and red strains were prepared (3 replicas per strain for each temperature treatment). These experimental units were cultured during 21 d at different temperatures (18, 25, and 35 ± 1 °C) and 60 μmol photon· m-2·s-1, with a 14-h photoperiod; the culture medium was changed weekly. Fresh biomass was measured for each strain in each treatment at the beginning of the experiment and then weekly to calculate the daily relative growth rate (described below).
Growth at 25 °C and high irradiance
Both strains were subjected to 25 °C and a high irradiance of 500 μmol photon· m-2·s-1under the same general laboratory conditions. Ten 800-mL flasks (5 replicates per strain) containing five 1.5-cm long apical segments each were prepared (0.06 g of each strain per flask). Fresh biomass was recorded at the beginning of the experiment and then weekly (during 21 d) to calculate RGR. At the end of the experiment, the contents of chlorophyll a, phycoerythrin, and phycocyanin were determined for each strain.
Photosynthesis and respiration
For this experiment, both strains were previously cultured at 25 °C and 500 μmol photon m-2·s-1 during 21 d (experiment 2). The light and dark bottle method (Littler and Arnold 1985, Thomas 1988) was used to measure gross and net photosynthesis (μmol O2·g-1 FW·h-1; FW, fresh weight) and respiration rates after algae were cultivated for a certain amount of time. The incubation bottles were prepared using 300 mg of fresh biomass, 170 mL of filtered seawater, and 2 mL of NaHCO3. Light and dark bottles were distributed into 2 irradiance treatments: 200 and 500 μmol photon· m-2·s-1 (3 replicates per treatment). Bottles with no algae were used as the control. The incubation period was 35 min at 25 ± 1 °C. Before and after this incubation period, the dissolved O2 value was recorded using a portable oximeter (YSI 58) with an automatic electrode (YSI 5905). The initial O2 value in each bottle was adjusted to 4 mg of O2 per liter of seawater.
Relative growth rate
Daily relative growth rate (RGR) was calculated accor ding to the following equation:
where Bf is final biomass, Bi is initial biomass, and T is time (Yong et al. 2013).
Pigment content
Samples 300 mg in weight (FW, n = 3) were ground in liquid nitrogen in darkness. The powder was dissolved in 2 mL of phosphate buffer (0.05 M, pH 5.5) at 4 °C. The crude extracts were then centrifuged at 44,000 × g for 20 min at 4 °C, and the supernatant, which contained phycobiliproteins, was analyzed with a spectrophotometer (HP8452A). The pellet, which contained chlorophyll a, was dissolved in 90% acetone and centrifuged at 12,000 × g for 15 min. The supernatant was analyzed with a spectrophotometer. Phycocyanin (PC) and phycoerythrin (PE) concentrations were calculated according to Kursar et al. (1983):
Chlorophyll a (Chla) was calculated according to Ritchie (2008):
Photosynthetic rates
Photosynthetic rates were calculated using the O2 concentrations in each incubation bottle according to the following equations
where Np is net photosynthesis, GP is gross photosynthesis, and RE is respiration;
where Fc is the O2 level in the light bottle: (O2 final - O2 initial) × volume;
where Fo is the O2 concentration in the dark bottle: (O2 final - O2 initial) × volume.
Statistical analysis
The RGRs recorded in experiments 1 and 2 were analyzed with a repeated measures one-way analysis of variance (ANOVA). A two-way ANOVA (strain and irradiance) was used to determine differences between photosynthetic rates. In all cases, the Newman-Keuls test (95% confidence level) was used a posteriori to establish statistical differences. Before performing ANOVA, normality was tested using the Kolmogorov-Smirnov test, whereas the homogeneity and homoscedasticity of variances were tested using the Cochran test. Pigment concentration was analyzed considering the means using Student’s t-test. All statistical analyses were performed using Statistica 7 (Statsoft Inc; Tulsa, Oklahoma, USA).
RESULTS
Growth at different temperatures
Both the green and red strains of C. corneus were not tolerant to 35 °C, exhibiting necrosis and death in the first week of culture (Fig. 1). Both strains, on the other hand, were tolerant to 18 °C and showed similar RGRs (Table 1). Detailed analysis of RGRs (weeks 1-3) showed variations through time (Fig. 1). In the first 7 d green specimens exhibited higher RGR (8%) than red specimens (4%); however, this difference disappeared as the experiment continued. Higher RGRs were observed for both strains at 25 °C. At this temperature, no differences in the RGR of both strains were observed throughout the experiment (Fig. 1, Table 1).
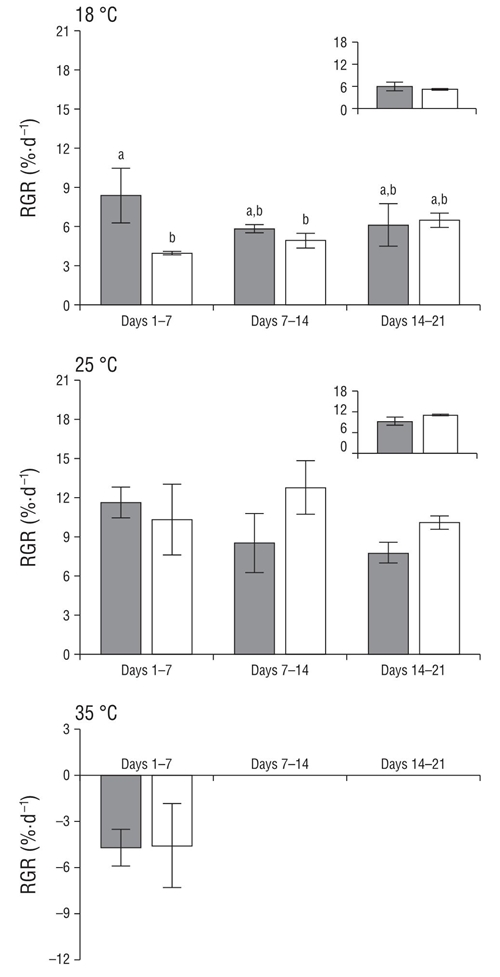
Figure 1 Daily relative growth rates (RGR, percent fresh weight per day) for red (white bars) and green (gray bars) strains of Crassiphycus corneus cultured under 3 temperature treatments (18, 25, and 35 °C) at 60 μmol photon·m-2·s-1 for 21 d. Insert shows total RGR during the experimental period in each treatment. Data are expressed as mean values (± standard deviation of the mean, n = 3). Different letters indicate differences at P < 0.05.
Table 1 Analysis of variance (ANOVA) and significance for the main effects and interactions between factors for daily Relative Growth Rate (RGR, in experiments 1 and 2) and for photosynthesis (experiment 3) for the green and red strains of Crassiphycus corneus. The repeated measures ANOVA was used to analyze the RGR over time in experiments 1 and 2, and the one-way ANOVA was used to determine differences between strains during the entire period (1-21 d). Two-way ANOVA was used to determine differences between the photosynthesis values (experiment 3). Bold values indicate differences at P < 0.05.
Experiment and variable |
Source of variation |
d.f. |
F-value |
P-value |
Growth (experiment 1) |
||||
RGR (18 ºC) |
Strain (A) |
1 |
24.770 |
0.007 |
Time (B) |
2 |
0.880 |
0.451 |
|
A × B |
2 |
5.440 |
0.003 |
|
RGR (18 ºC, 1–21 d) |
Strain |
1 |
1.180 |
0.338 |
RGR (25ºC) |
Strain (A) |
1 |
5.370 |
0.081 |
Time (B) |
2 |
2.060 |
0.189 |
|
A × B |
2 |
3.420 |
0.084 |
|
RGR (25 ºC, 1–21 d) |
Strain |
1 |
5.330 |
0.082 |
Growth (experiment 2) |
||||
RGR (25 ºC) |
Strain (A) |
1 |
12.640 |
0.007 |
Time (B) |
2 |
103.960 |
>0.001 |
|
A × B |
2 |
3.650 |
0.049 |
|
RGR (25 ºC, 1–21 d) |
Strain |
1 |
5.330 |
0.082 |
Photosynthesis under diff erent irradiances (experiment 3) |
||||
Irradiance (A) |
1 |
1.735 |
0.224 |
|
Strain (B) |
1 |
0.941 |
0.361 |
|
A × B |
1 |
3.245 |
0.109 |
|
Growth at 25 °C and high irradiance
The red strain showed higher growth rates than the green strain when they were cultured at 25 °C and 500 μmol photon·m-2·s-1 (P < 0.05, Fig. 2). Detailed analysis of RGRs (weeks 1-3) showed progressive decrease in RGR in both strains over time. Differences between both strains were observed in the second and third week, with the red strain showing the highest growth rate (Fig. 2). At the end of the experiment, no differences were observed in the chlorophyll a and phycobiliprotein concentrations between the green and red strains (Table 2).
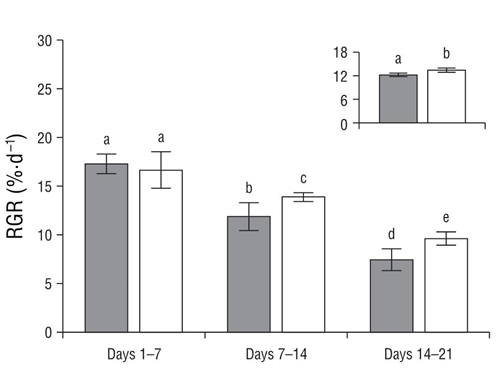
Figure 2 Daily relative growth rate (RGR, percent fresh weight per day) for red (white bars) and green (gray bars) strains of Crassiphycus corneus cultivated under 500 μmol photon·m-2·s-1 during 21 d at 25 °C. Insert shows total RGR during the experimental period. Data are expressed as mean values (± standard deviation of the mean, n = 5). Different letters indicate differences at P < 0.05.
Table 2 Pigment content (mg·g FW-1; FW, fresh weight) in the red and green strains of Crassiphycus corneus cultivated under 500 μmol photon·m-2·s-1 at 25 °C. Data are expressed as mean values (± standard deviation of the mean, n = 3). Student’s t-test was used to compare the means.
Strain |
|||||
Red |
Green |
d.f. |
t |
P |
|
Chlorophyll a |
0.092 (0.024) |
0.089 (0.013) |
4 |
–0.1325 |
0.9001 |
Phycoerythrin |
0.204 (0.071) |
0.274 (0.028) |
4 |
1.5832 |
0.1885 |
Phycocyanin |
0.105 (0.012) |
0.136 (0.006) |
4 |
2.1144 |
0.1020 |
Photosynthetic rates
Green and wild red strains showed no differences in photosynthetic rates between the irradiance treatments (Fig. 3, Table 1).
DISCUSSION
Green and red strains of C. corneus showed no differences in pigment content or in photosynthetic rates under the tested conditions. However, RGRs were different throughout the study, generally at 25 °C and under high irradiance. These results allow us to partially reject our hypothesis because physiological performance was similar under many of the tested conditions.
Photosynthetic rates in both the green and red strains were similar under the tested irradiances (200 and 500 μmol photon·m-2 s-1). These results are in agreement with those reported for red and green morphotypes of K. alvarezii, for which the photosynthesis-irradiance curves were similar (Aguirre-von-Wobeser et al. 2001). Similarity in photosynthetic rates could be related to the pigment concentration in both strains. In fact, red and green strains of C. corneus (this study), C. birdiae (Costa and Plastino 2011), and K. alvarezii (Aguirre-von-Wobeser et al. 2001) showed similar chlorophyll a contents. In the case of the red and green morphotypes of K. alvarezii, no differences were observed in PE content, but high allophycocyanin and PC contents were observed for the green morphotype (Aguirre-von-Wobeser et al. 2001). According to Aguirre-von-Wobeser et al. (2001), differences in phycobiliprotein contents could explain the absence of the typical red color of Rhodophyta in the green morphotype. On the other hand, Doty et al. (1987) suggested that the green color in K. alvarezii morphotypes is the result of its low PE concentration. Guimarães et al. (2003) and Costa and Plastino (2011) observed low PE contents in green strains of G. domingensis and C. birdiae, which could give them low viability, explaining the scarce presence of these strains in the wild. Likewise, van der Meer (1990) suggested that most color variants of Gracilaria tikvahiae have lower biological adaptation compared to wild specimens since color variants are less robust and have lower growth and survival rates. This seems to be in agreement with the results obtained for C. corneus, but only when cultured under high irradiance (500 μmol photon·m-1·s-1) at 25 °C. The tolerance to 18 °C should be noted, especially so for the green strain, which reached an RGR of 8% in the first 7 d of culture. According to Dawes et al. (1999), C. corneus is not tolerant to temperatures below 15 °C, because low temperatures promote low photosynthetic rates.
Similar photosynthetic rates between the green and red strains of C. corneus could suggest similar growth rate patterns. In fact, in the first 7 d of culture at 25 °C under 60 μmol photon· m-1·s-1 (Fig. 1) and under 500 μmol photon m-1·s-1(Fig. 2), no differences in RGR were observed between the color strains. Nevertheless, longer culture periods promoted differences between both strains, with the green strain showing the lowest RGRs mainly under high irradiance. A similar pattern was reported for color variants of G. tikvahiae (van der Meer 1990), C. birdiae (Plastino et al. 2002), and K. alvarezii (Hurtado-Ponce 1995). However, K. alvarezii (Aguirre-von-Wobeser et al. 2001, Hayashi 2007) and C. birdiae (Ursi et al. 2013) cultures carried out in the field showed similar growth rates between the green variant and red specimens. In the case of C. corneus, the red and green strains cultured for 5 weeks showed similar growth rates when they were exposed to different nutrient concentrations and irradiances of 90 and 180 μmol photon·m-2·s-1 (Ferreira et al. 2006). A similar pattern was observed for M. laminarioides color strains cultured under low irradiance in the laboratory (Navarro 2015).
The highest RGRs in C. corneus were observed at 25 °C and 500 μmol photon··m-2·s-1. According to McLachlan and Bird (1986)), maximum growth potential for warm-water species like C. corneus is achieved at 25 to 30 °C. These authors also suggest that temperatures above 30 °C are lethal, which is consistent with our results for both color strains. Our results also concur with the average seawater temperatures in the geographic range of this species, which vary between 23 and 28 °C (Orduña-Rojas et al. 2002).
Growth in C. corneus also seems to depend on photon flux density, since RGR values for both strains did not exceed 13% at 60 μmol photon··m-2·s-1 (Fig. 1) but reached over 18% at a higher photon flux density (500 μmol photon··m-2·s-1) in the first 7 d of culture (Fig. 2). These differences remained until day 21 of the experiment, as RGRs did not exceed 11% at 60 μmol photon··m-2·s-1but were greater than 12% at 500 μmol photon··m-2·s-1. A direct correlation between photon flux density (90 and 180 μmol photon··m-2·s-1) and RGR was also reported for C. corneus by Ferreira et al. (2006). However, samples from natural populations have shown a saturation irradiance of 120 μmol photon··m-2·s-1 (Orduña-Rojas et al. 2002).
During the growth experiments, both strains of C. corneus showed a progressive decrease in RGR (Fig. 2). This decrease could be related to the increase in biomass in the same sea water volume and to the ensuing competition for nutrients and light (“shadow effect”). Ferreira et al. (2006)) suggested that C. corneus can adapt to low nutrient concentrations, which are common in tropical oligotrophic waters. The decrease in RGR could then be related to high algal density. High algal density can decrease photosynthetic activity and consequently affect growth (Figueroa et al. 2008).
Regardless of the observed differences in RGR between both strains, the high growth rates reached by the green strain (over 10%) when cultured at 25 °C and under high irradiance (500 μmol photon·m-2·s-1) are noteworthy. This result, however, should be taken with caution because algae are subject to many other biotic and abiotic factors in their natural environment, all which can act synergistically or antagonistically. Information on the minimum and optimal requirements for temperature, irradiance, and nutrients is thus needed to determine the best culturing conditions for each C. corneus strain. This should be obtained by means of factorial experiments. Furthermore, more complete studies on the properties of the green color strain of C. corneus could create new research fields exploring new biotechnological alternatives with the aim to obtain natural products with added value. On that matter, an unusual galactan with high antioxidant activity was reported in green specimens of Gigartina skottsbergii collected from natural populations in the southern region of Chile (Barahona et al. 2012). Also, in the case of C. birdiae, similarties between the green and red strains were observed not only in growth but also in agar yield and quality, indicating a potential use in mariculture (Ursi et al. 2013).
Finally, it is important to highlight that the study of color strains has contributed to the selection of models for the elucidation of physiological and biochemical processes in algae (Kursar et al. 1983, Zhang and van der Meer 1987, Santelices et al. 1996). In addition, population studies are needed to determine the frequency of strains (or the presence of others) and determine whether they have established in natural populations. Physiological studies should be conducted to understand strain performance in natural and stressful conditions, such as those predicted to arise with climate change (Navarro 2015)











 nueva página del texto (beta)
nueva página del texto (beta)




