INTRODUCTION
Coral reefs are some of the richest marine ecosystems on earth and are essential for maintaining ecological balance (Anthony et al. 2020). Millepora species (phylum Cnidaria, class Hydrozoa), which are recognized as the second most important reef-forming organisms, are extensively distributed in the Caribbean, which contains the second largest barrier reef on earth, the Mesoamerican Reef (Rojas-Molina et al. 2012). Reef-forming organisms engage in mutualistic symbiosis with unicellular dinoflagellate algae of the Symbiodiniaceae family, which is critical for the formation of coral reef structures (Fransolet et al. 2012).
Unfortunately, coral reefs are seriously threatened by environmental stressors, primarily global warming and ocean acidification. These stressors cause bleaching events in which coral and hydrocoral tissues lose their symbionts or pigments, which exposes their white calcium carbonate exoskeletons (McLachlan et al. 2020). In recent years, the frequency and severity of bleaching events have increased, and massive bleaching events have been documented in all tropical regions of the world (Suggett and Smith 2020). Notably, the thermal stress of the 2015-2016 El Niño was unprecedented in the Caribbean region over the period of 1871-2017. Indeed, 2017 was the warmest non-El Niño year ever recorded, which resulted in very high coral mortality and the rapid deterioration of reef structures, with far-reaching environmental impacts (Hughes et al. 2018, Lough et al. 2018, Eakin et al. 2019).
Thermally induced bleaching events have caused worldwide damage to coral reefs, with serious consequences for the biodiversity of tropical marine regions (Foster and Attrill 2021). A large number of studies have evaluated the consequences of thermal stress and bleaching and the resilience and tolerance of reef-forming anthozoans (Bay and Palumbi 2015; Ainsworth et al. 2016; Ricaurte et al. 2016; Hillyer et al. 2017, 2018; Oakley et al. 2017; Ruiz-Jones and Palumbi 2017; Mayfield et al. 2018). The results of these studies have linked tolerance and the capacity to recover after bleaching events with the energetic reserves present in cnidarians (Grottoli et al. 2014, Schoepf et al. 2015, Aichelman et al. 2016, Levas et al. 2016, Tremblay et al. 2016) and their ability to replace symbionts with more thermo-tolerant algal partners, either before or after a bleaching event (Sampayo et al. 2008, Baker et al. 2017, Swain et al. 2018). However, little is known regarding the consequences of thermal stress on reef-forming hydrozoans (García-Arredondo et al. 2011, Hernández-Elizárraga et al. 2019, Olguín-López et al. 2019). Thus, the aim of the present study was to conduct a comparative analysis of symbiont cell density, exoskeleton structure, and the biochemical composition of unbleached and bleached Millepora alcicornis, a fire coral, affected by the 2015-2016 El Niño in the Mexican Caribbean.
MATERIALS AND METHODS
Sample collection
Millepora alcicornis fragments were collected from 3 unbleached and 3 bleached colonies at depths of 4-10 m by scuba diving. To reduce the possibility of sampling genetic clones, at least 10 m separated the fragments that were collected. Sampling was conducted in November 2016 during the third-largest, global-scale mass bleaching event ever documented (Hughes et al. 2018) in the area known as “La Bocana Chica” in Parque Nacional Arrecife de Puerto Morelos (Quintana Roo, Mexico). A field permit (PPF/DGOPA-139/15) was granted by the Ministry of Agriculture, Rural Development, Fisheries, and Food (SAGARPA, for its Spanish acronym) for specimen collection. The specimens were frozen in liquid nitrogen and transported to the laboratory at the Autonomous University of Queretaro.
Genetic identification of Symbiodiniaceae algae
Total DNA samples were obtained from the hydrocoral tissues using the following extraction buffer: 100 mM Tris-HCl pH 8, 2% CTAB, 200 mM EDTA, and 1.4 M NaCl (Salgado et al. 2007). The Symbiodiniaceae species hosted by the hydrocorals were identified by PCR to the genus level. Specific primer sets targeting the internal transcribed spacer region ITS1 or domain 2 of the large-subunit rDNA (LSU) of Symbiodiniaceae genera (Symbiodinium [formerly Clade A], Breviolum [formerly Clade B], Cladocopium [formerly Clade C], and Durisdinium [formerly Clade D]) were obtained (Table 1; Mieog et al. 2007, Correa et al. 2009). The PCR reaction mixture (20 μL) contained 7.5 μL sterile water, 2.0 μL buffer (10×), 1.0 μL MgCl2, 2.0 μL DNA, 2.5 μL of each forward and reverse Symbiodinium primer (25 mM), 1.0 μL DNTp mix (2 mM), and 0.5 U of Taq polymerase (Taq DNA Polymerase Recombinant, Thermo Fisher Scientific, Waltham, MA, USA). Amplifications were performed on an iCycler thermocycler (Bio-Rad Laboratories, Hercules, CA, USA). The PCR products were analyzed in 2% agarose gels, and the gels were visualized using ChemiDoc MP (Bio-Rad Laboratories). The 1 Kb Plus DNA Ladder was used as a molecular weight marker (Invitrogen, Carlsbad, CA, USA). Dominant bands on each gel were excised and sequenced using a BigDye Terminator v.3.1 cycle sequencing kit (Thermo Fisher Scientific) and a 3130XL Genetic Analyzer (Applied Biosystems, Waltham, MA, USA). Symbiont cell ratio densities were quantified from each DNA sample using qPCR. Primers and probes for qPCR assays targeting specific β-actin loci from M. alcicornis, Symbiodinium sp., Breviolum sp., Cladocopium sp., and Durisdinium sp. were used as controls. The qPCR amplification was performed in triplicate for each sample on a CFX96 Touch Real-Time PCR Detection System (Bio-Rad Laboratories, Table 1).
Table 1 Primers used in PCR for Symbiodiniaceae algae identification (Mieog et al. 2007, Correa et al. 2009)
| Primer | Sequence |
| ITS A-specific forward | 5’-CCTCTTGGACCTTCCACAAC-3’ |
| ITS A-specific reverse | 5’-GCATGCAGCAACACTGCTC -3’ |
| LSU-28S B-specific forward | 5’-GTCTTTGTGAGCCTTGAGC-3’ |
| LSU-28S B-specific reverse | 5’-GCACACTAACAAGTGTACCATG-3’ |
| nITS1 universal forward | 5′-AGGAGAAGTCGTAACAAGGTTTCC-3′ |
| nITS1 C-specific reverse | 5′-AAGCATCCCTCACAGCCAAA-3′ |
| nITS1 D-specific reverse | 5′-CACCGTAGTGGTTCACGTGTAATAG-3′ |
Alizarin red S staining
Fragments from unbleached and bleached M. alcicornis specimens collected from different colonies were stained with alizarin red S. The uptake and incorporation of alizarin red S into hydrocoral skeletons, which results in pink-red areas, is proportional to the calcification rate and consequently highly correlated with newly calcified zones (Le Tissier 1990, 1991; Frank et al. 1995; Tambutté et al. 2012). Hydrocoral fragments were placed in seawater containing 10-15 mg of vital stain alizarin red S for 12 h. Then, the skeletons were cleaned of overlying tissues in a 5% sodium hypochlorite solution for 30 min, rinsed in distilled water (12 h), and air-dried. The stained areas of the hydrocoral exoskeletons indicated zones in which calcium carbonate deposition occurred.
Scanning electron microscopy
Three fragments from unbleached colonies and 3 fragments from bleached colonies collected in November 2016 were cleaned of overlying tissues. Cross sections of the hydrocoral fragments were obtained using a 0.5-cm sander, and the microstructure of the exoskeleton was examined by scanning electron microscopy under low vacuum conditions (30 Pa; JSM 6060LV, Jeol, Tokyo, Japan).
Biochemical composition
Fragments (approximately 1 cm2) from unbleached and bleached M. alcicornis specimens were lyophilized, and their soluble protein, lipid, carbohydrate, and refractory material content were determined. To determine calcium carbonate content, the hydrocoral fragments were dried for 24 h in an oven at 60 °C. To estimate organic matter content, the dry weight (DW) of the sclerites was subtracted from the DW of calcium carbonate. The values of the biochemical components of each sample were expressed as the percentage of total DW (% g DW) and as the percentage of total organic matter (% g OM). Calcium carbonate, lipids, carbohydrates, and refractory material (insoluble protein content) were extracted and quantified from the lyophilized M. alcicornis fragments using the method described by Shirur et al. (2014).
Statistical analysis
The results are expressed as mean ± standard error of the mean (SEM; n = 3; symbiont cell ratio density and biochemical composition). Statistical analyses were performed in GraphPad Prism v.6.0 (GraphPad Software, San Diego, CA, USA). The means ± SEM were compared using unpaired Student t-tests. In all cases, a significance level of 95% (α = 0.05) was employed.
RESULTS
Unbleached and bleached fragments of M. alcicornis collected in the Mexican Caribbean during November 2016 are shown in Figure 1a and 1b, respectively. Figure 1c and 1d show unbleached and bleached M. alcicornis fragments stained with alizarin red S. Unbleached hydrocorals exhibited a pink-red color (hue saturation value [HSV]: 346%, 45%, and 66%), while the bleached hydrocorals exhibited a white-red color (HSV: 355%, 15%, and 96%), which indicated an elevated rate of calcification. Unbleached and bleached M. alcicornis fragments from different colonies harbored Symbiodinium sp., Breviolum sp., Cladocopium sp., and Durisdinium sp. Unbleached M. alcicornis specimens showed a higher distribution of Breviolum sp. and Durisdinium sp. compared to those of bleached specimens, which exhibited similar symbiont distributions (Fig. 2).
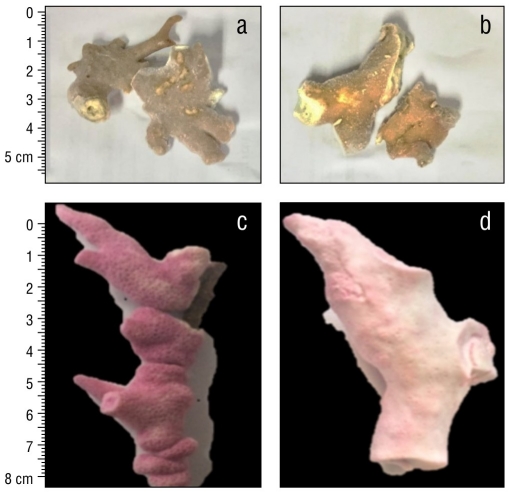
Figure 1 Unbleached (a) and bleached (b) Millepora alcicornis fragments collected in 2016 in the Mexican Caribbean. Bleached (c) and unbleached (d) M. alcicornis fragments stained with alizarin red S.
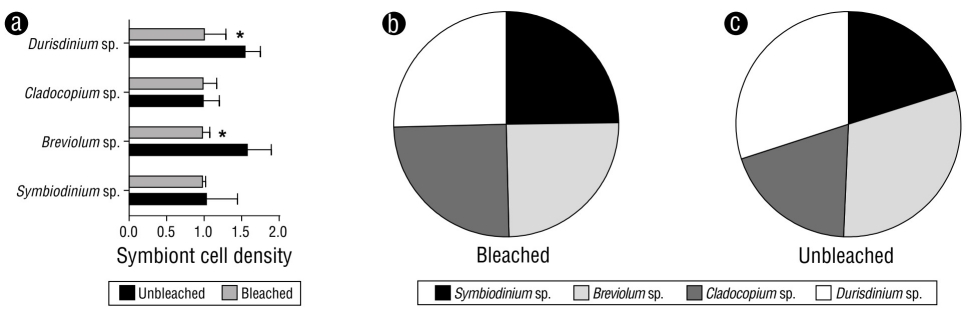
Figure 2 (a) Cell ratio densities of symbionts in unbleached and bleached Millepora alcicornis colonies. Values are reported as means ± standard error of the mean (SEM). Distribution of symbionts in bleached (b) and unbleached (c) specimens.
Microstructural properties of the exoskeleton representative of unbleached and bleached M. alcicornis specimens are shown in Figure 3 and Table 2. These properties included gastropores and dactylopores in the skeletal surface, individual zooid pores, trabeculae, and pillar crystals. No significant differences were observed in the number or size of gastropores, dactylopores, or pores of the exoskeleton microstructure between unbleached (Fig. 3a) and bleached (Fig. 3e) hydrocorals. However, images obtained from individual zooid pores indicated that these were significantly smaller in the exoskeletons of bleached specimens compared to those of unbleached specimens (Fig. 3f), and their shapes differed from those of unbleached hydrocorals (Fig. 3b). Moreover, trabecular (wall) thickness in the exoskeletons of unbleached specimens (Fig. 3c) was greater than that of bleached specimens (Fig. 3g). We compared the bars of unbleached and bleached specimens from M. alcicornis by the widths of one side of the bar. No differences were observed in the regular cross-linking of solid bars and aragonite supports between the exoskeletons of unbleached (Fig. 3d) and bleached (Fig. 3h) specimens.
Table 2 Measured characteristics (μm) of the exoskeleton structure from unbleached and bleached Millepora alcicornis specimens.
| Unbleached | Bleached | P value | |
| Gastropore | 325.000 | 350.000 | 0.3508 |
| Dactylopores | 166.700 | 178.600 | 0.4486 |
| Zooide | 90.150 | 79.120* | 0.0212 |
| Trabecular (wall) thickness | 24.700 | 14.480* | 0.0047 |
| Cross-linking of solid bars and aragonite supports | 1.085 | 1.088 | 0.9607 |
Each value is expressed as the mean ± standard error of the mean (n = 3). *P < 0.05 compared to control by student’s t-test.
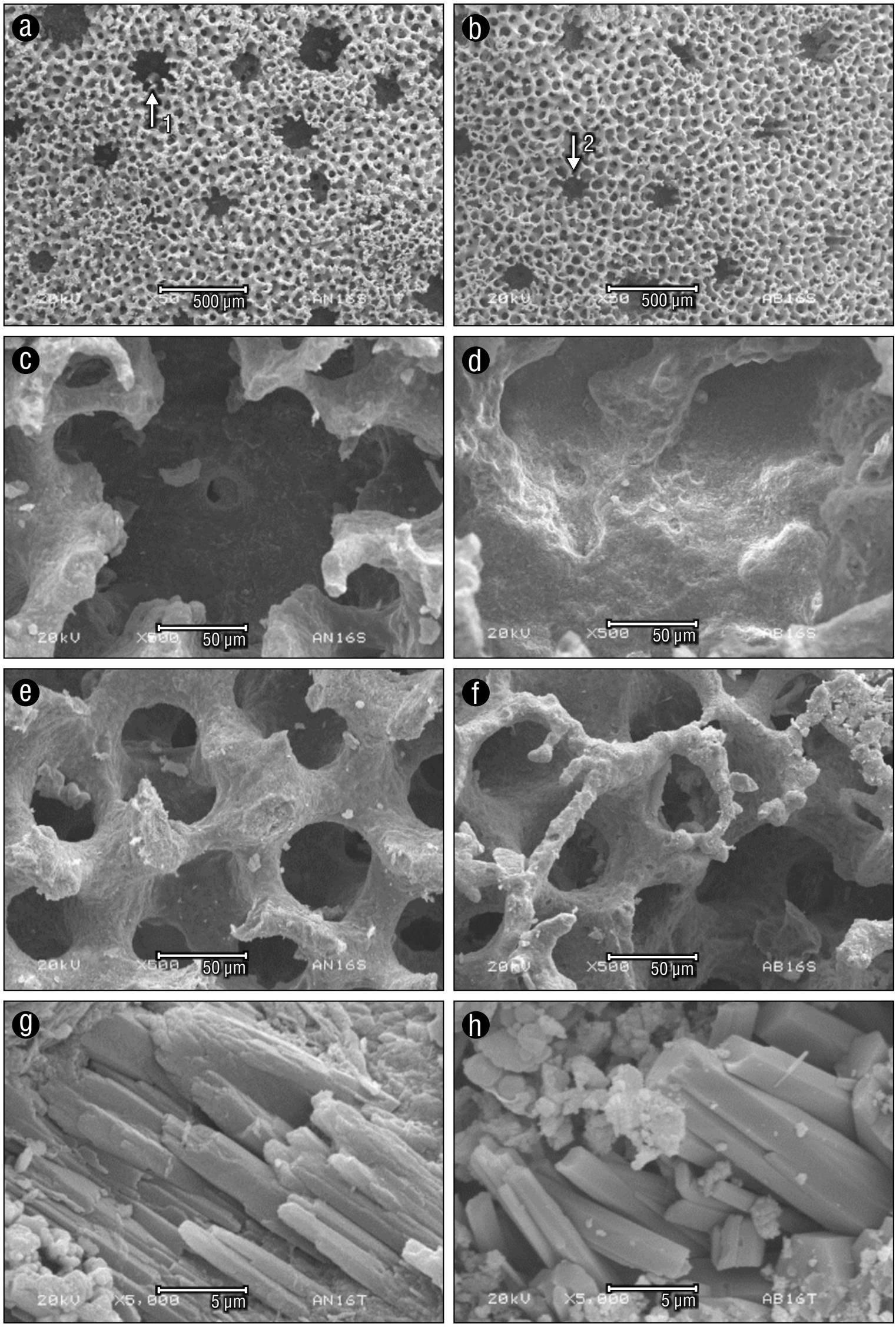
Figure 3 Representative images of scanning electron micrographs (1: gastropore; 2: dactylopore) of the skeletal surface (a), individual zooid pores (b), trabeculae (c), and pillar crystal (d) from unbleached Millepora alcicornis. Scanning electron microscope images of the skeletal surface (e), individual zooid pores (f), trabeculae (g), and pillar crystal (h) from bleached M. alcicornis.
Figure 4 shows the biochemical composition (calcium carbonate, refractory material, and other organics) of the dry tissues obtained from unbleached and bleached M. alcicornis specimens. No significant differences in composition were observed between unbleached and bleached specimens. Hydrocoral dry tissue was composed mainly of calcium carbonate, which constituted approximately 90% of the DW of unbleached and bleached specimens. In addition, 6-7% and 2% of the DW of the specimens was refractory material and other organics, respectively (Table 3).
Table 3 Biochemical composition of dry tissues from unbleached (uMa) and bleached (bMa) Millepora alcicornis specimens. Values are reported as means ± standard error of the mean (SEM).
| Parameter (% g DW) | uMa | bMa | P value |
| Calcium carbonate | 91.23 ± 1.56 | 90.62 ± 1.67 | 0.8028 |
| Refractory material | 6.64 ± 1.12 | 7.987 ± 1.36 | 0.4881 |
| Protein | 0.27 ± 0.03 | 0.52 ± 0.10 | 0.0708 |
| Lipid | 1.31 ± 0.28 | 0.27 ± 0.04* | 0.0208 |
| Carbohydrate | 0.55 ± 0.16 | 0.59 ± 0.17 | 0.8517 |
Each value is expressed as the mean ± standard error of the mean (n = 3). *P < 0.05 compared to control by student’s t-test.
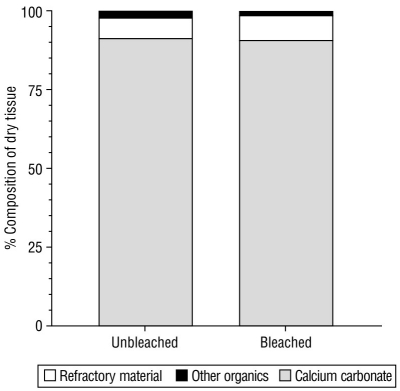
Figure 4 Biochemical composition of tissues from unbleached (uMa) and bleached (bMa) Millepora alcicornis specimens. Calcium carbonate (CaCO3), refractory material (insoluble protein), and other organic constituents (soluble proteins, lipids, and carbohydrates) are standardized to the dry weight (DW) percentage.
The biochemical composition of the organic matter (i.e., the percentage that represents the removal of calcium carbonate from total DW) of unbleached and bleached hydrocorals is presented in Figure 5. The highest percentage corresponded to refractory material (insoluble proteins), which was significantly higher in bleached specimens than in unbleached specimens, as was the case with the percentage of soluble proteins. In contrast, the percentage of lipids was significantly lower in bleached specimens than in unbleached specimens. The percentage of carbohydrates did not change as a result of bleaching (Table 4).
Table 4 Biochemical composition of organic matter (OM) from lyophilized tissues of unbleached (uMa) and bleached (bMa) Millepora alcicornis specimens. Values are reported as means ± standard error of the mean (SEM).
| Parameter (% g DW) | uMa | bMa | P value |
| Refractory material | 75.90 ± 0.89 | 85.39 ± 0.71* | 0.0012 |
| Protein | 3.23 ± 0.61 | 5.57 ± 0.39* | 0.0323 |
| Lipid | 14.77 ± 0.48 | 2.92 ± 0.11* | 0.0001 |
| Carbohydrate | 6.09 ± 0.76 | 6.11 ± 0.94 | 0.9820 |
Each value is expressed as the mean ± standard error of the mean (n = 3). *P < 0.05 compared to control by student’s t-test.
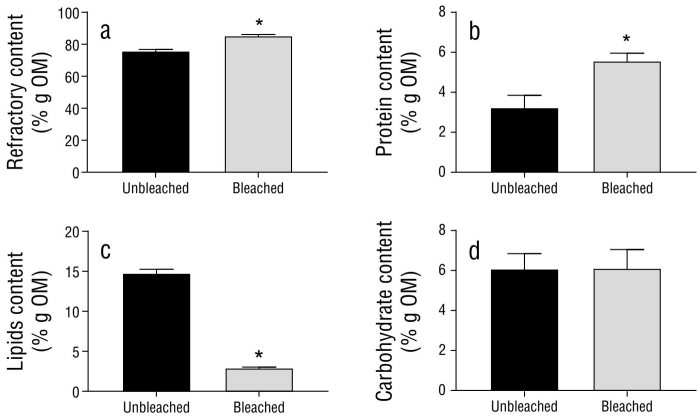
Figure 5 Biochemical composition of organic matter (OM) from unbleached (uMa) and bleached (bMa) Millepora alcicornis specimens. Plots show (a) refractory material, (b) NaOH-soluble protein, (c) lipid, and (d) carbohydrate contents. Variables are standardized to the weight of the organic matter (% g OM) within fragments. Values are reported as means ± standard error of the mean (SEM).
DISCUSSION
In recent years, massive bleaching events have increased in intensity and frequency, resulting in declines in coral cover worldwide (Anthony et al. 2020), with some of the most severe effects observed in Caribbean coral reefs. Hydrocorals are particularly vulnerable to thermal stress (Pereira-Dias and Gondim 2016). For example, M. alcicornis has experienced severe bleaching episodes over different periods in various locations, including summer 2005 in the Great Barrier Reef (Marshall and Baird 2000); summer 2006 and 2007 in the Florida Keys (Wagner et al. 2010); and 1987, 1993, 1995, 1998, 2003, 2005, 2009, 2010, and 2014-2017 in Puerto Rico and the Caribbean (Pereira-Dias and Gondim 2016, Eakin et al. 2019).
The Symbiodiniaceae genera Symbiobinium, Breviolum, Cladocopium, and Durisdinium have been previously identified in Millepora species (Rodríguez et al. 2019) and scleractinian corals in the Caribbean Sea (LaJeunesse 2002, Santos et al. 2004). Cladocopium species are the most common symbionts found in reef-forming corals, and Breviolum species are among the most dominant symbionts associated with reef-forming cnidarians in the Caribbean Sea (LaJeunesse 2004). In contrast, Symbiodinium and Durisdinium species are considerably less common, although certain species appear to be adapted to high irradiance and temperature conditions (Jones et al. 2008, Reynolds et al. 2008).
It has been widely documented that Symbiodiniaceae algae provide different physiological benefits to their coral hosts. Silverstein et al. (2015) found that Montastraea cavernosa colonies dominated by D1a Symbiodinium experienced less photo-damage and symbiont loss compared to corals that hosted only stress-sensitive symbionts (Symbiodinium C3). It has also been demonstrated that the distribution of Symbiodiniaceae can change after bleaching events due to a shift in symbiont dominance (Jones et al. 2008, Sampayo et al. 2008, Silverstein et al. 2015). Moreover, symbiont shuffling has also been observed prior to and during bleaching events (LaJeunesse et al. 2009).
In a previous study conducted by our research group, we found that M. alcicornis specimens exhibited a 40% decrease in symbiont density per square centimeter after the 2015-2016 El Niño (Olguín-López et al. 2019), which was classified as “moderate bleaching” according to the categories defined by ReefBase (Oliver et al. 2019). It is worth mentioning that the M. alcicornis specimens employed in the present study were selected from the hydrocoral samples collected by Oliver et al. (2019). In this study, we found that, although both unbleached and bleached M. alcicornis specimens hosted Symbiobinium, Breviolum, Cladocopium, and Durisdinium species, the abundance of thermotolerant Durisdinium species was higher in unbleached M. alcicornis specimens than in bleached specimens.
Durisdinium species, which are relatively rare yet globally distributed, have attracted increasing interest over recent decades, as they increase the thermal tolerance of their coral hosts (Oliver and Palumbi 2011, Kennedy et al. 2015). Similar to what we found in unbleached M. alcicornis in this study, a higher abundance of Durisdinium spp. has also been observed in Orbicella faveolata after a bleaching event (Kemp et al. 2014). Thus, M. alcicornis might be acquiring a certain degree of thermotolerance related to the presence of thermotolerant symbionts. Indeed, our results allow us to hypothesize that M. alcicornis colonies that were not bleached by the 2015-2016 El Niño in the Mexican Caribbean were more thermotolerant due to their associations with Durisdinium algae.
We used alizarin red S to assess calcification in unbleached and bleached M. alcicornis specimens. Unbleached hydrocorals exhibited greater calcification than bleached specimens given the intensity of alizarin red S incorporation. These findings were expected, as the products of photosynthesis conducted by Symbiodiniaceae algae play critical roles in the calcification process, and the photosynthetically fixed carbon pool that is supplied to the coral host significantly diminishes after bleaching events (Colombo-Pallotta et al. 2010, Muller-Parker et al. 2015, D’Olivo and McCulloch 2017). As has been observed with anthozoans, our findings suggest that thermal stress that provokes bleaching also affects hydrocoral calcification.
To determine if a decrease in calcification was reflected in changes in the microstructure of the hydrocoral exoskeleton, unbleached and bleached samples were analyzed by scanning electron microscopy. No apparent differences were detected in the number or size of gastropores or dactylopores, which are related to the number of polyps specialized in prey capture and heterotrophic feeding, between unbleached and bleached specimens. However, when analyzing individual zooid pores, a reduction in depth, size, and trabecular thickness was observed in the exoskeletons of bleached hydrocorals, which suggests that the deposition of calcium carbonate decreased. Not surprisingly, exoskeletons from bleached specimens showed no evident changes in the regular cross-linking of solid bars and aragonite crystals, as modifications in the calcification process were expected to be observed in the early mineralization centers and not in the fibrous aragonite crystals that were already formed.
It has been well demonstrated that thermal stress reduces calcification and causes a differential expression of genes and proteins involved in regulatory pathways associated with the biomineralization of coral exoskeletons in Acropora palmata, Montastraea faveolata, Acropora Millepora, Galaxea astreata, Porites astreoides, Porites divaricata, and O. faveolata (Desalvo et al. 2008, Rodriguez‐Lanetty et al. 2009, De Salvo et al. 2010, Moya et al. 2012, Ricaurte et al. 2016, D’Olivo and McCulloch 2017, Huang et al. 2018, Levas et al. 2018). Calcium carbonate comprised the bulk of the hydrocoral exoskeletons (DW) of both unbleached and bleached M. alcicornis specimens. Refractory material (insoluble proteins) constituted ~7% of the hydrocoral tissue samples (DW). No significant differences were observed in the content of either component between unbleached and bleached fragments.
Given that Symbiodinaceae algae carry out photosynthesis and transfer more than 50% of their photosynthetic products to their cnidarian hosts (Venn et al. 2008, Yellowlees et al. 2008, Davy et al. 2012, Fransolet et al. 2012), we explored whether a decline in symbiont density resulted in an increase in the consumption of energy sources, including stores of carbohydrates, lipids, and proteins. Carbohydrate content was not modified in bleached M. alcicornis specimens, which suggests that a carbohydrate deficit induced by the departure of symbionts could be attenuated by an increase in heterotrophic feeding. This result supports our previous observation that hemolytic and proteolytic activity and the activity of phospholipase A2 in the soluble proteome of bleached M. alcicornis were not modified, with bleached specimens showing a higher expression of cytolytic toxins potentially involved in prey capture and digestion than those of unbleached specimens (Olguín-López et al. 2019). Moreover, studies carried out in scleractinian corals have demonstrated that some corals are able to recover from bleaching events by increasing heterotrophic feeding rates and the percent contribution of heterotrophically acquired carbon to daily animal respiration (CHAR; Grottoli et al. 2006, Aichelman et al. 2016).
Lipids were the second most abundant organic compounds in unbleached M. alcicornis tissues (approximately 14.7%), while the lipid percentage in bleached specimens was only 2.9%. Previous studies have found that the lipid percentage in dry tissues of unbleached scleractinian corals varies from 12% in Porites porites to 30% in Orbicella annularis (Harland et al. 1992). As observed with M. alcicornis, lipid levels were significantly lower in bleached Porites divaricate and Porites compressa specimens when compared to those of unbleached specimens (Grottoli et al. 2004, Levas et al. 2018). High energy reserves may play a central role in long-term recovery from bleaching, as coral species that possess high energy reserves have better chances of overcoming the negative effects of bleaching events (Schoepf et al. 2015). Therefore, our results suggest that bleached M. alcicornis compensates for its nutritional deficiency by utilizing its lipid reserves.
Both refractory material (insoluble proteins) and soluble protein content were significantly higher in bleached M. alcicornis specimens than in unbleached specimens. Previous studies that have employed proteomic and transcriptomic approaches have demonstrated that corals show changes in protein synthesis and folding after bleaching events (Ricaurte et al. 2016, Hou et al. 2018, Mayfield et al. 2018). However, the results obtained to date related to the effects of thermal stress on the protein content in reef-forming cnidarians remain inconclusive. For example, Aiptasia pallida was found to accumulate amino acids and their intermediates after being exposed to thermal stress. However, bleached Acropora aspera specimens exhibited a decrease in the levels of amino acids and other metabolites (Hillyer et al. 2016, 2017). In the present study, the importance of high protein levels in bleached hydrocorals was unclear.
If resilience and recovery from bleaching events depends upon heterotrophic plasticity, shifts in endosymbiont types, and energy reserves (Grottoli et al. 2006, 2014; Anthony et al. 2009; Connolly et al. 2012), it is possible to hypothesize that an increase in heterotrophic feeding and the use of energy reserves and a higher abundance of thermotolerant Durisdinium symbionts may constitute some of the mechanisms of resilience developing within M. alcicornis that counteract the effects of global warming.
The present study provides evidence that the 2015-2016 El Niño induced a decrease in calcification and changes in the exoskeleton microstructure of M. alcicornis in the Mexican Caribbean. The increase in seawater temperatures modified the biochemical composition of these organisms, resulting in a notable decrease in lipid levels, which suggests that bleached hydrocorals use metabolic reserves to maintain cellular activities necessary for their survival, even at the expense of limiting important processes such as calcification. Symbiodinium, Breviolum, Cladocopium, and Durisdinium species were found in both unbleached and bleached specimens. The greater abundance of Durisdinium species in unbleached M. alcicornis specimens suggests that unbleached colonies were more thermotolerant due to their associations with these symbionts, which are tolerant to thermal stress.











 texto en
texto en 



