INTRODUCTION
The red squat lobster, Pleuroncodes monodon, belongs to the Munididae family (Poore et al. 2011) and is distributed from southern Mexico (16°00ʹ N) to an area off Peru (6°25ʹ S) and Chile (41°00ʹ S) (Flores et al. 2020). In Chile, P. monodon inhabits muddy and hard bottoms on the continental shelf, from the upper edge of the slope to 450 m depth (Bahamonde et al. 2002), and has been recorded both in shallow waters and in abyssal sectors associated with anoxic bottoms (Gallardo et al. 2017). Pleuroncodes monodon has a long reproductive period, with the presence of ovigerous females from February to December (Palma and Arana 1997) and multiple spawnings in synchrony between embryonic development and gonadal development (Flores et al. 2020).
Pleuroncodes monodon is of commercial importance in the demersal crustacean fishery of central Chile. The P. monodon fishery is administratively composed of the Northern Fishery Unit (NFU), from 23°21ʹ00ʺ S to 32°10ʹ23ʺ S, and the Southern Fishery Unit (SFU), from 32°10ʹ23ʺ S to 38°28ʹ35ʺ S (Cavieres et al. 2018). Since the early 1970s, this fishery has exerted strong pressure on the stock of P. monodon (Cavieres 2017, Zilleruelo et al. 2020). Their fishing has been regulated through the implementation of annual global quotas, individual fishing quotas per shipowner, and maximum catch limits per shipowner (Canales et al. 1997, Párraga et al. 2012), but the main regulatory measure has been the prolonged extractive bans; in particular, those in the years from 1980 to 1982, 1989 to 1991, and 2001 to 2005 (Acuña et al. 2005). Furthermore, since 1997, there have been biological closure seasons in January-February, to protect the individual growth of P. monodon specimens, and reproductive bans in September (Cavieres 2017). However, the set of regulatory measures for the exploitation and conservation of P. monodon has been insufficient to allow a clear recovery (Ibarra and Yáñez 2021). At present, the NFU is in a state of overexploitation, and the SFU, in a recovery regime, after being overfished from 2000 to 2017, although with a reduction of the indicator of the reproductive potential levels from 55% (2019) to 48% (Ibarra and Yáñez 2021).
The reproductive potential is defined as the ability of a population to produce viable eggs and larvae that can then be incorporated into the fishery or stock as recruits (Trippel 1999). Therefore, it is related to maturity, fecundity, egg quality, and spawning time (Wright 2013). Nevertheless, the use of this index instead of the spawning stock biomass in stock assessments has been questioned (Marshall et al. 2006, Cerviño et al. 2013); Kell et al. (2016) demonstrated that both parameters describe different dynamics. The inclusion of the reproductive potential improves the perception of productivity. Kell et al. (2016) based their conclusion on the comparative analysis of time series of 3 North Sea stocks (Gadus morhua cod, Clupea harengus herring, and Pleuronectes platessa plaice). Therefore, we consider it important to monitor the reproductive potential of the P. monodon female stock and, thus, analyze their behavior in space and time.
This study includes the spatial component in the functioning and structure of marine populations, an aspect that has been emphasized in recent decades (Cressie et al. 1993, McKinley and Atkinson 2020). This provides a better understanding of population dynamics than only including the temporal view of the population (Dunning et al. 1995). Ignoring spatial structure leads to biases in stock assessment and fishery management, which can lead to overfishing, imbalances in the stock size structure, and population collapse and/or failure in stock reconstruction (Cadrin 2020), especially if there is overfishing in areas recognized as renewal or breeding areas for a resource.
Roa et al. (1995) identified a P. monodon breeding area, which was made up of 2 interconnected adult populations, an extensive one located between 35°10ʹ S and 36°15ʹ S (Achira population) and a smaller one located between 36°35ʹ S and 36°50ʹ S (Biobío population) (Roa and Bahamonde 1993, Roa and Tapia 2000). From there, the distribution of P. monodon extends north and south over the continental shelf of central Chile, from 34.0° S to 37.0° S. The breeding area is responsible for recruit turnover. This area is important for the recovery of the stock of P. monodon and could potentially be affected by fishing pressure, as has occurred with the reproductive potential.
This work aimed to estimate the reproductive potential of the P. monodon female stock in the central-southern zone of Chile (32.0° S-37.0° S) and to evaluate, through maps, its spatial and temporal distribution from 2005 to 2018.
MATERIALS AND METHODS
Data source
For this study, we used the database of fishing sets of direct evaluation surveys from 2005 to 2018 of red squat lobster (P. monodon) and yellow squat lobster (Cervimunida johni). Between 1993 and 2004, the direct evaluations done used various methodological criteria, both to obtain and to process the data, which was standardized from the year 2005; therefore, those years were excluded in this work.
The surveys were carried out in commercial squat lobster trawling vessels that operate with scientific personnel on board. Within the 5 nautical miles of the Area Reserved for Small-scale Fishing (ARPA, for its acronym in Spanish), motor boats with average characteristics of 17-m length, 41-t gross register tonnage, and 53-m3 hold capacity were used. Outside the ARPA, high seas fishing vessels with average characteristics of 21-m length, 84-t gross register tonnage, and 116-m3 hold capacity were used (Párraga et al. 2012). In both cases, the actual fishing sets lasted approximately 15 min, depending on the environmental conditions and bottom topography.
This work analyzes information and data from 14 evaluation campaigns and 9,601 fishing sets, which contained georeferenced information by administrative region, depth (m), catch (kg) by species, and other data for each set. The size distribution (cephalothorax length [CL], in millimeters) of P. monodon was obtained from survey reports (Acuña et al. 2006). Stratified sampling by cell was applied in the surveys; this was intensified in the historically successful fishing areas to evaluate the abundance and density of the foci in the fishing grounds. The catch per set was recorded and standardized (Acuña et al. 2006).
Study area
The study area covers the central-southern zone of Chile from 32.0° S to 37.0° S, which covers 556 linear kilometers (Fig. 1). The area was divided into a northern zone (32.5° S-35.5° S) and a southern zone (35.5° S-37.0° S). The criterion applied was the distribution of the foci of abundance of P. monodon (Acuña et al. 2012, 2014).
Estimation of reproductive potential
The distribution of P. monodon is clumped and highly aggregated (Acuña et al. 2008, Queirolo et al. 2017) and is made up of abundance aggregations of variable size (Ahumada et al. 2013). A focus of abundance was defined as the aggregation of ovigerous females of P. monodon that meet the following characteristics: (1) greater number of potential eggs per unit area and (2) persistence of at least 7 years. This period was considered because it is the time in which the stock of P. monodon reaches the complete size structure, which is necessary for the population to expand its geographical distribution (Roa and Bahamonde 1993). The 30% percentile (P = 30) was chosen, that is, 70% of aggregations that reached densities ≥44.7 million potential eggs per square kilometer. Spearman’s rank test (Sokal and Rolf 2012) was applied to test the relationship between the size and density of aggregations for each year. To estimate the reproductive potential of P. monodon, the weight (g), CL (mm), and maturity status of 452,087 specimens were used; of these, only ovigerous females (133,440) were considered, particularly the mature ones, according to Palma and Arana (1997).
The reproductive potential was estimated for foci (individually) from 2005 to 2018 with the spatial position indexing model (Kell et al. 2016), whose expression is as follows:
where E is the number of eggs per area unit for each year, Z f(x,y) is the number density of females at position (x, y), P(x, y) is the fraction of females that carry eggs at position (x, y), and G(x, y) is the number of eggs per female at position (x, y), expressed as millions of potential eggs per square kilometer (referred to in this work as mpe·km-2). The number of eggs per female was estimated with different methods, depending on the work group that carried out the evaluation; thus, Acuña et al. (2004, 2008, 2010, 2012) and Queirolo et al. (2015, 2016) used the gravimetric method, whereas Queirolo et al. (2017) used the autodiametric method. Several evaluations reported the estimation of the fecundity-length or fecundity-weight function that was used in those years. In the cases where fecundity was not determined, the closest year was used because the size composition of the stock and the proportion of mature females were not available for all years.
The density in number of females was calculated with the following expression:
where Z
Wf
is the weight density (t·km-2) of females at point (x, y) and
where Z
tot
(x, y) is the total density (males and females);
Spatial distribution of reproductive potential
A geostatistical analysis was applied (Petitgas 1993, Rivoirard et al. 2000). This methodology has been used in Chile since 1993 to study the spatial distribution of squat lobster population density (Roa and Tapia 1998, Acuña et al. 2012, Queirolo et al. 2018, Ossa et al. 2019). It is based on 2 aspects: (1) the density of potential eggs per surface unit measured locally, which is the execution of a random variable that rigorously explains the error in its determination, and (2) the average of the random process of the reproductive potential is constant in the study area and the covariance between 2 sampling points depends only on their relative distances (Isaaks and Srivastava 1989). The analysis was based on a spatial covariance model or semivariogram (Journel and Huijbregts 1978). Therefore, for each year the standardized experimental semivariogram was estimated, and it was used in all the analyses to obtain a more efficient model. It was calculated in 2 directions, north-south (90°) and east-west (0°), to observe the directional differences in the structure of the process, i.e., anisotropy. Models were fitted using the least squares method (Cressie 1993), and the observed structure was related to the assumed generating process. The models considered for the densities were the following: Matérn, spherical, exponential, and Gaussian (Matérn 1987).
Ordinary point kriging was used as the interpolation method to estimate the mean density of the reproductive potential of P. monodon over each distribution area of the population abundance. The average minimum distance between sampling stations was considered as the internodal distance of the interpolation grid. Annual distribution maps of the mean densities of the reproductive potential were made with PBSmapping (Schnute et al. 2010; https://www.r-project.org, accessed January 31, 2020) and Surfer v-9.9.
Spatiotemporal analysis of the distribution of the reproductive potential
The spatial variation of the reproductive potential of P. monodon females was determined with 2 indices as follows:
(1) center of gravity (CG): indicates the dispersion of the annual foci of reproductive potential (egg·km-2), and was located as follows:
(2) inertia of the center of gravity (I): determines the spatiotemporal variation range of the reproductive potential density, and was obtained as follows:
CG and I were used to analyze the evolution and displacement of the reproductive foci of P. monodon.
RESULTS
Sex ratio and fraction of mature females of Pleuroncodes monodon
The proportion of females for the entire study area was less than 50% between 2004 and 2014 (Fig. 2a), with a significant increase in their proportion (>50%) between 2015 and 2018. This increase coincided with the highest proportion of ovigerous females (78%; Fig. 2b).
Reproductive potential and reproductive potential density
The average annual reproductive potential of P. monodon females from 2005 to 2018 was 78,055.0 million potential eggs and the estimated average density was 74.0 mpe·km-2 (standard deviation = 47.4).
Potential egg density differed between zones (Fig. 2). The northern zone had densities between 195.0 mpe·km-2 in 2012 and 33.0 mpe·km-2 in 2014. The southern zone had lower densities, between 12.0 mpe·km-2 in 2006 and 141.0 mpe·km-2 in 2011.
In general, the average area covered by the density of the reproductive potential differed between the northern and southern zones. A greater area was covered in the southern zone (1,045 km2) than in the northern zone (754 km2) (Fig. 3). In addition, in the southern zone, a positive and moderately strong association was observed between the area covered and the density of the reproductive potential, whereas in the northern zone, the relationship was weak or non-existent (estimated Spearman’s rank correlation test, respectively, for each zone: r sSZ = 0.79, P = 0.05; r sNZ = 0.31, P = 0.05).
Foci identification
For the northern and southern zones, annual aggregations of the reproductive potential density were observed. The number of aggregations was greater in the northern zone, from 3 (in 2017) to 14 (in 2008) aggregations, than in the southern zone, from 3 (in 2005) to 8 (in 2012) aggregations (Fig. 4). Only aggregations located between 35°00ʹ S and 36°20ʹ S were recognized as foci of reproductive potential of red squat lobster females. In this sector, we estimated average reproductive potential densities close to 100.0 mpe·km-2. These values were above the 30% percentile; furthermore, they were persistent from 2006 to 2018.
Spatial distribution of female Pleuroncodes monodon
Geostatistical analysis
The calculation of the unidirectional standardized experimental semivariograms revealed the absence of an anisotropic effect in the spatial distribution of the aggregations for each year and for each zone (Table 1).
Table 1 Parameters of the theoretical variogram adjusted to the density of the reproductive potential of Pleuroncodes monodon. S, south; N, north; Gaus, Gaussian; Sphe, spherical; Mat, Matérn.
| Year | Zone | Model | Nugget | Sill | Range (km) | Unexplained variance (%) | Anistropy |
| 2005 | S | Gaus | 56.9 | 362.5 | 26.8 | 13.6 | 0; 30 |
| 2006 | S | Sphe | 57.3 | 96.7 | 11.3 | 37.2 | No |
| 2007 | S | Gaus | 94.0 | 2,130.8 | 28.0 | 4.2 | No |
| 2008 | N | Sphe | 344.8 | 419.7 | 138.8 | 45.1 | No |
| S | Gaus | 1,735.3 | 4,758.4 | 28.2 | 26.7 | 45; 30 | |
| 2009 | N | Gaus | 290.8 | 138.1 | 111.1 | 67.8 | No |
| S | Sphe | 0.0 | 9,190.7 | 10.8 | 0.0 | 45; 30 | |
| 2011 | N | Gaus | 2,246.3 | 6,542.8 | 71.4 | 25.6 | No |
| S | Mat | 913.7 | 13,932.3 | 60.7 | 6.2 | 45; 30 | |
| 2012 | N | Mat | 5,076.3 | 24,230.0 | 9.9 | 17.3 | 45; 30 |
| S | Sphe | 3,941.1 | 18,020.7 | 107.3 | 18.0 | 45; 30 | |
| 2013 | N | Mat | 1,341.7 | 5,130.4 | 13.7 | 20.7 | 0; 30 |
| S | Sphe | 3,204.0 | 9,508.5 | 18.0 | 25.2 | No | |
| 2014 | N | Sphe | 78.9 | 229.8 | 203.4 | 25.6 | No |
| S | Gaus | 1,626.4 | 569.4 | 26.6 | 74.1 | No | |
| 2015 | N | Sphe | 142.5 | 491.7 | 89.2 | 22.5 | 45; 30 |
| S | All | 149.7 | 283.3 | 2.0 | 34.6 | No | |
| 2016 | N | Sphe | 592.4 | 518.0 | 109.0 | 53.4 | No |
| S | Sphe | 1,916.9 | 1,219.9 | 33.9 | 61.1 | No | |
| 2017 | N | Gaus | 412.9 | 770.6 | 76.9 | 34.9 | 45; 30 |
| S | Sphe | 5,708.1 | 5,201.0 | 49.1 | 52.3 | No | |
| 2018 | N | Sphe | 279.0 | 406.4 | 100.8 | 40.7 | No |
| S | Gaus | 63.2 | 1,716.1 | 161.4 | 3.6 | No |
Of the 15 years analyzed, 39% indicated the presence of anisotropy in a northwest-southeast direction (NW-SE, 45°) with respect to the study area and coast orientation. In those cases, the theoretical semivariogram model that obtained the smallest residual sum of squares was chosen. When there was no anisotropic effect, 60% of the distribution of the patches conformed to the spherical model, and the range of distances between patches per zone varied. Table 1 shows the fits and the unexplained variance (UEV) of the parameters of the theoretical variogram. In the northern zone, the range of the distance between patches was between 10 km (UEV = 17.3%) and 203 km (UEV = 25.6%); for the southern zone, the range was between 2 km (UEV = 34.6%) and 161 km (UEV = 3.6%), which indicated that the aggregations in the northern zone were more independent among themselves than in the southern zone (Fig. 5).
Spatial analysis
The analyses of the CG and the I suggest that the displacement of the densities of the reproductive potential was from 36°20ʹ52ʺ S (in 2005) to 34°18ʹ39ʺ S (in 2012), that is, a displacement greater than 2° toward the north, which is equivalent to 120 nautical miles, with an average distance of 16 km·yr-1. However, from 2005 to 2007, the CG remained close to 36.0° S, with an average dispersal range of 0.08° (~3 km·yr-1). Starting in 2008, the dispersal range increased to values over 13 km·yr-1 (Fig. 6).
Mapping the distribution of the reproductive potential of Pleuroncodes monodon
In the annual distribution map of the reproductive potential densities of P. monodon females (Fig. 7), 2 well-marked sectors with high values were identified, one off San Antonio (33°30ʹ S to 34°00ʹ S) and the other between Point Achira and Biobío (34°40ʹ S to 36°20ʹ S), which were present for 6 years or more. For the sector off San Antonio, between 2005 and 2009, male specimens predominated in the study area and the proportion of females reached its lowest value in the entire series (~20% in 2006). Starting in 2008, the proportion of females increased and, with it, the reproductive potential, which reached average values of 194.5 mpe·km-2 between 2011 and 2013; then, the reproductive potential decreased drastically and remained at average values of 47.0 mpe·km-2 during the last few years. In the sector between Point Achira and Biobío, mature females showed higher concentrations throughout the study period, with the presence of 2 important maximums of reproductive potential with averages of 115.0 mpe·km-2 between 2011 and 2014. The reproductive potential decreased in 2015, but it picked up again with a third maximum in 2017 with averages close to 140.0 mpe·km-2.
DISCUSSION
In this work, the annual average of potential eggs of P. monodon for the central-southern zone of Chile in the period 2005-2018 was estimated at 78,055 million and the annual average density at 73.7 mpe·km-2. The density levels estimated here are within the intervals reported by Palma and Arana (1997) for fishing foci between 36.0° S and 37.0° S (Point Achira and north of the Biobío Canyon), which were estimated from samples obtained during 15 monitoring surveys of P. monodon between August 1988 and October 1989. Palma and Arana (1997) indicated reproductive potential values between 1,808 and 33,966 eggs from ovigerous females of P. monodon of 22.0 to 43.9 mm CL. The present study used new estimates of annual areas using only ovigerous females for each year, and estimated densities between 8.5 and 159.0 mpe·km-2. These estimates are below the 9,000 million eggs per square kilometer reported by Tapia (1999) for the same area (34.0° S-37.0° S), with the same methodology. Their study was based on an evaluation survey done in “non-trawlable” bottoms in November 1996, which focused on areas close to the hard bottoms of the platform. According to Cadrin (2020), there can be a sequential expansion of resources to new areas, but a wrong specification of the spatial structure of a resource can play an important role in the decline of fisheries because it results in an overestimation of productivity in the stock assessment. In this sense, it is possible that the study by Tapia (1999) overestimated the reproductive potential of P. monodon. Furthermore, in 1996 the biomass of P. monodon was estimated at 122,000 and 132,000 t with direct and indirect methods, respectively (Roa et al. 1997); almost a decade later, these figures were analyzed by Acuña et al. (2005), who found that the biomass of P. monodon for that year did not exceed 98,000 t. Although, after closed seasons, the total biomass and the exploitable biomass increase, specifically in the central-southern zone of Chile, the estimated biomasses from the surveys do not exceed 90,000 t (Ibarra and Yáñez 2021), nor the landings, the 8,000 t.
In addition, observations indicate that, after a prolonged closed season of P. monodon, an expansion and recovery of the resource occurs as a result of the important income of the recruited year class (Roa et al. 1997, Cavieres et al. 2018), as it occurred after the closed seasons from 1989 to 1991 (Roa and Bahamonde 1993) and from 2001 to 2005 (Acuña et al. 2005). In both cases, good recruitment and an increase in total biomass and exploitable biomass were reported (Acuña et al. 2012, Cavieres et al. 2018).
In our geostatistical analysis for the years 2005-2007, male specimens predominated in the study area, and the proportion of females reached its lowest value in the entire series (~20% in 2006), which was reflected in the absence of the reproductive potential of P. monodon in the distribution map for that period. These results are consistent with the reports of Acuña et al. (2010) for the period from 2005 to 2007, who observed the entry of abundant recruitments that allowed a significant recovery of the biomass from 2006 to 2009, which was favored by the closed season implemented for the extraction of the resource between 2001 and 2005 (Acuña et al. 2005, Cavieres et al. 2018). This is how the cruise biomass went from 10,000 t in 2005 to 70,000 t in 2007. However, the catches showed an increase in the proportion of males and the predominance of females carrying eggs in the initial state of development (Acuña et al. 2010). Acuña et al. (2009) also reported male predominance in the captures for the year 2007. Although the observed changes in the sex ratio of P. monodon were not clear, Acuña et al. (2009) indicated that the predominance of males could have been associated with the dates on which the study was done, as it corresponded to the end of the reproductive period of this species.
In the present work, no seasonal analysis was done because the information came from research surveys done to estimate the standing stock of P. monodon and 2 other species of crustaceans that applied the swept area method, which uses the quantitative records of catches for each fishing haul. The Chilean Fund for Fisheries Research (https://www.fip.cl/proyectos) finances this type of project quasi-annually through public tenders, which are carried out exclusively for a few weeks in the winter months. However, the existence of seasonal variations in some population indicators is recognized, such as the extensive reproductive period (February to December) and the fraction of ovigerous females and their eggs, as reported by Bascur et al. (2017) for P. monodon in the central zone of Chile.
The comparison of the reproductive potential series of P. monodon females with the recruitment estimated by Cavieres et al. (2018) seems to indicate that the trend of good recruitment, like the one that occurred in 2004, is reflected in the trend of reproductive potential 6 years later (Fig. 8). However, it is a short period and, due to differences in methodological criteria, it was not possible to include information from the 1990s.
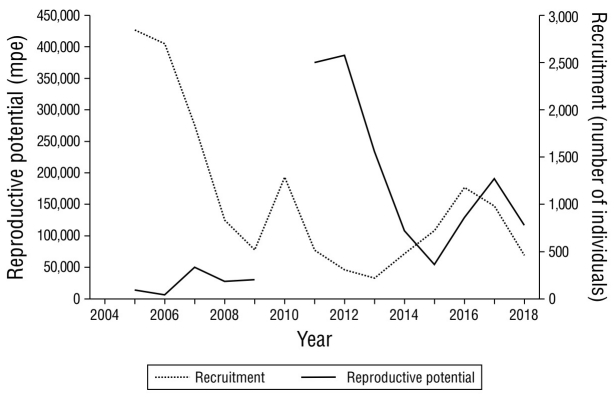
Figure 8 Recruitment (thousands of individuals) vs. reproductive potential (millions of potential eggs, mpe) for Pleuroncodes monodon from 2005 to 2018 in the central-southern zone of Chile.
Regarding the expansion of the resource, this study revealed an interesting finding, with the latitudinal displacement of the distribution of reproductive potential from 36°20ʹ52.5ʺ S to 34°18ʹ39.1ʺ S between 2005 and 2012, with an expansion of 226.4 km, which explains the existence of the northern sector of high reproductive potential mentioned above (Fig. 7). For P. monodon, Roa and Bahamonde (1993) recorded 60 km as their largest expansion interval between 1989 and 1991.
The center of gravity of the reproductive potential remained close to 36°19ʹ S between 2005 and 2007, with an average dispersal interval of 3 km·yr-1 (I = 0.08° S). However, starting in 2008, the interval increased to 13 km·yr-1 (I = 1.28° S) until reaching 34°18ʹ S in 2012 (see Fig. 5). These results are consistent with what was reported by Acuña et al. (2009), who indicated a concentration of red squat lobster population density near 36°19ʹ S, between 1999 and 2006, with little latitudinal dispersion (I < 0.17° S). It is possible that, after intense fishing pressure, this resource retreats from north to south, as fishing leads to sharp declines in egg production from the stock as older age classes are removed. After closed seasons, it slowly recovers and expands from the foci of the reproductive potential stock of ovigerous females found between 35°00ʹ and 36°50ʹ S. These foci with densities of reproductive potential close to 100 mpe·km-2 are the ones that expand to the north and south of the Chilean coast, which reinforces the hypothesis that the population of P. monodon comes from a single breeding area located between 35.5° and 36.5° S (Roa and Bahamonde 1993).
The use of the reproductive potential in the stock assessment of fishing resources has been controversial due to how it is estimated. However, it is important to include it because it allows building key spatiotemporal models to address conservation problems in the aggregation or breeding areas of this species. Through this approach, it was possible to identify and delimit the essential habitats for ovigerous females and breeding, which made it possible to design and prioritize marine protected areas with the aim of achieving responsible fishing capable of maintaining the renewal of not only P. monodon, but also of the species associated with its ecosystem.
Finally, geostatistics has been widely applied since the 1960s both in the estimation of mineral reserves and the characterization of oil deposits, and in the analysis of environmental problems of hydrology, ecology, climatology, and oceanography (McKinley and Atkinson 2020). Recently, in the study and management of fisheries, it is used to map essential habitats for fishes to monitor the conservation of ecosystems, specifically, to assess the variability of their distribution over time (Le Pape et al. 2014; Petitgas et al. 2018, 2020). In Chile, this methodology has been used since 1992 to assess crustacean populations, but little has been published on it. The growth and population expansion of P. monodon was analyzed after a 3-year closed season (Roa and Bahamonde 1993), and spatial differences in growth and maturity were observed (Roa and Tapia 2000). These studies supported the use of geostatistics and methodological standardization in the evaluation of crustaceans in Chile (Acuña et al. 2004, 2005; Queirolo et al. 2018). However, this is the first time that it was used to estimate the reproductive potential of P. monodon and observe its distribution over time.











 texto en
texto en 










