INTRODUCTION
The Mexican axolotl (Ambystoma mexicanum) is a neotenic urodele amphibian microendemic of the Mexican central region. A. mexicanum has been categorized as an endangered species by the Official Mexican Standard NOM-059-SEMARNAT-2010 (SEMARNAT 2010) and in critically endangered status by the International Union for Conservation of Nature (Zambrano et al. 2010). The alarming decline in the wild population density of A. mexicanum keep continues to be reported. According to the most recent census (2014), only 0.58 % remains of the original population reported in the 1998 census (Voss et al. 2015).
The axolotl is endemic of the lacustrine area of Xochimilco, a water system between rural and urban areas characterized by canals, ‘islands’ called chinampas, and temporary wetlands affected by urbanization (Contreras et al. 2009, Ayala et al. 2019). Within this region, there are vast agricultural areas that are very important for the economic activity of the region (González-Carmona et al. 2014). As a consequence, the natural habitat of A. mexicanum is being highly impacted, especially by the use of pesticides that affect other non-target organisms and can contaminate soil and water (Aktar et al. 2009, Badii and Varela 2015).
The water quality in the Xochimilco canals has been compromised by the intensive application of organophosphorus pesticides (OPPs) to control plagues in croplands and the urban area (Robles-Mendoza et al. 2009, Mercado et al. 2015). Malathion (MLT) and dichlorvos (DDVP) are the most common OPPs in Mexico (Blanco-Jarvio et al. 2011, Mercado et al. 2015, Bejarano-González 2017). It has been reported that MLT concentrations in the Xochimilco water canals (0.1 µg/L) surpass those established by the U.S. National Oceanic and Atmospheric Administration as indicators of chronic damage (Mercado-Borrayo et al. 2015). This implies a severe conservation issue for axolotls, whose survival is highly associated with water-quality parameters (Voss et al. 2015).
Therefore, we describe the detrimental effects of commercial-grade MLT and DVPP on the axolotl embryo survival, hatching period and early larvae size. Additionally, we contextualize the results in face of the direct impact of these pesticides in the axolotl habitat, regarding their conservation.
MATERIALS AND METHODS
Maintenance of eggs and larvae of A. mexicanum
One clutch of 210 fertilized eggs of A. mexicanum was used to reduce variability among the experiments. The eggs were obtained during the months of September-October 2009 from the herpetology laboratory of the Facultad de Estudios Superiores Iztacala, Universidad Nacional Autónoma de México. The eggs and larvae were maintained in a medium consisting of 0.095 g of NaHCO3, 0.06 g of CaSO4, 0.06 g of MsSO4, and 0.002 g of KCl in 1L H2O (Chaparro-Herrera et al. 2013). The experimental conditions corresponded to the season´s environmental temperature and photoperiod; dissolved oxygen was maintained at saturation through an air pump. The temperature was measured daily in the morning before and after hatching. Hatched larvae were fed daily with Artemia nauplii.
Pesticides exposure
Commercial OPPs malathion (C10H19O6PS2; 57 % purity, MLT) and dichlorvos (C4H7Cl2O4P; 76 % purity, DDVP) were obtained from El Semillero, Mexico. Commercial grade OPPs were chosen to evaluate formulations commonly used in the practice (as discussed below). Given the lack of information of commercial pesticides in relation to A. mexicanum, we used concentrations falling within the range of a similar report from Robles-Mendoza et al. (2009) using axolotls as a model, with the difference that they evaluated the effect of analytical grade OPPs and a maximum testing concentration of 30 mg/L. The latter was done in order to gain novel understanding of the possible effects of commercial grade OPPs in the context of the current literature. Eggs of A. mexicanum of no more than 24 h post-fertilization (hpf) were exposed to three MLT concentrations (10 eggs per group in triplicate): 5.7, 17.5, and 5.7 mg/L for the depuration treatment (D); and to three DDPV treatment levels (10 eggs per group in triplicate): 1.5,1.9, and 1.9 mg/L for the depuration treatment (D). The solutions were prepared by diluting the correspondent OPP solution in an Environmentral Protection Agency (USEPA) medium. The control group was placed in EPA medium without OPPs. The depuration treatment consisted of exposing the embryos to OPPs only during the first 96 h of development. Following the exposure, the eggs were transferred to a clean EPA medium. The EPA medium with its respective treatment was replaced every 48 h in all groups.
Embryo hatching monitoring and counting
Egg hatching and survival, as well as larvae survival, were monitored daily excluding weekends. The embryo mortality was assessed when the eggs became pale-colored. Dead organisms were removed from the medium.
Early larvae measuring and monitoring
The size of hatched individuals was measured upon eclosion under a stereoscopic microscope (five animals per group). Early larvae were monitored daily until the 10th day post-hatching. Larvae mortality was considered after the animal became unresponsive to physical stimulus.
Statistical analysis
All data were analyzed using GraphPad prism v. 8.3 (San Diego, California USA, www.graphpad.com). Statistical significance was tested using χ2 with an α = 0, comparing the control (expected) against the treatments as a contingency table. The Levine’s test for equality of variances did not meet the assumption of equality, hence a Kruskal-Wallis followed by a Dunn’s test for multiple comparisons was performed where indicated in the results and figures.
RESULTS
Organophosphorus pesticides (MLT and DDVP) induce early hatching and larvae mortality
Embryo survival was defined as the embryo’s ability to hatch. The temperature was recorded at 17 ºC on the morning of hatching, which was minimally affected in the group exposed to MLT at all concentrations. Of the organisms, 96.6 % hatched in both the MLT 5.7 and 17.5 mg/L groups, and 90 % in the 5.7 mg/L (D) group. Regarding DDVP, 60 % of the organisms hatched in the 1.5 mg/L group, 66 % in the 1.9 mg/L group, and 93.3 % in the 1.5 mg/L (D) group (Fig. 1a). The control group presented 100 % of hatched organisms.
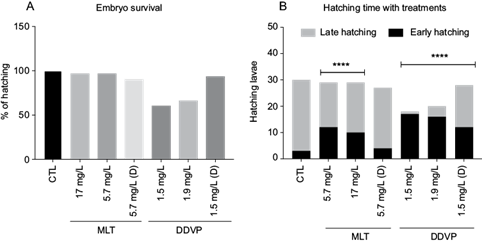
Fig. 1 Effects of organophosphorus pesticides on embryo survival (hatching). (a) Percentage of hatched organisms between the different concentrations of malathion and dichlorvos treatments. (b) Distribution of larvae that hatched early (≤ 14 d) or late (≥ 14 d) between the different treatments. X2 (6, 14) = 54, **** p < 0.001. CTL: control, MLT: malathion, DDVP: dichlorvos, (D): depurated.
The hatching time was also recorded. The MLT and DDVP treated groups started to hatch at day 11 in high proportion; in contrast, the control group started to hatch until day 14. Thus, we grouped and compared each experimental group as early hatched individuals (≤ 14 d) and late hatched individuals (≥ 14 d). As a result, we observed that for all treatments except for MLT 5.7 mg/L (D), the development time until hatching was significantly faster than in the control group (Fig. 1b; χ2, p < 0.0001).
Next, we investigated the OPPs effect on early larvae mortality. The larvae could not survive under continuous exposure to MLT nor DDVP pesticides for a long period after hatching (Figs. 2a and 3a). On one hand, the MLT 17.5 mg/L mortality rate reached 100 % at day 3, while in MLT 5.7 mg/L it reached 100 % at day 5 (Fig. 2a). On the other hand, all DDVP groups reached 100 % mortality by day 7 (Fig. 3a). When compared to the the control, all groups presented a significant difference in the dead vs alive rate on day 10 (χ2 p < 0.0001) (Figs. 2b and 3b).
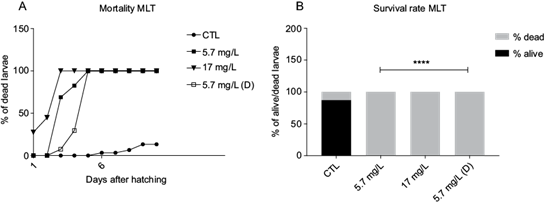
Fig. 2 Larvae mortality after exposure to malathion. (a) Percentage of dead larvae through days after hatching at different malathion concentrations (mg/L). (b) Rate of dead vs alive in percentage at different malathion concentrations (mg/L). X2 (3, 8) = 333.5, **** p < 0.0001. CTL: control, MLT: malathion, (D): depurated.
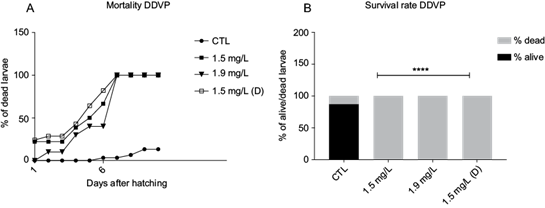
Fig. 3 Larvae mortality after exposure to dichlorvos. (a) Percentage of dead larvae through days after hatching at different dichlorvos concentrations (mg/L). (b) Rate of dead vs alive in percentage at different dichlorvos concentrations (mg/L). X2 (3, 8) = 333.5, **** p < 0.0001. CTL: control, DDVP: dichlorvos, (D): depurated.
In the case of MLT and DDVP depurated groups, mortality in both groups reached 100 % after 5 and 7 days, respectively, suggesting that the effect of OPPs in early stages is irreversible, even at the smallest concentration tested.
OPPs effect on larvae size and morphology
After hatching, OPPs-treated larvae presented clear morphological abnormalities in contrast to the unexposed control group. The control group presented a straight elongated body, while both MLT and DDVP treated groups presented featured lordosis (Fig. 4a). Regarding size, the organisms presented a shorter body size in both OPP treatments. A Kruskal-Wallis test provided evidence of a significant difference (p < 0.01) between the mean ranks. Dunn’s pairwise test revealed a significant reduction in body size in the organisms treated with MLT 17.5 mg/L and 5.7 mg/L (D), DVPP 1.5 mg/L and DVPP 1.9 mg/L in comparison to the control group (Fig. 4b, c). Nevertheless, our data was not sufficient to provide evidence of differences between the treatments for both OPPs.
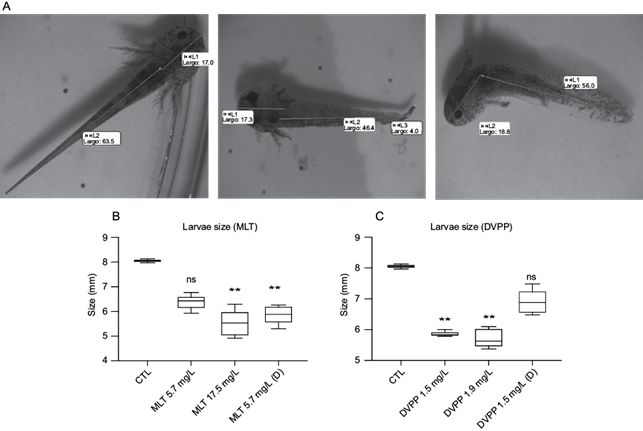
Fig. 4 Morphological abnormalities caused by organophosphorus pesticides. (a) Example of abnormal phenotype. (b, c) Larvae size in millimeters after treatments with different concentrations (mg/L) of malathion and dichlorvos, respectively (n = 5). Krustal-Wallis followed by Dunn’s test, ** p < 0.01. CTL: control, MLT: malathion, DDVP: dichlorvos, (D): depurated.
The results suggest that OPPs exposure from early developmental stages can cause an irreversible abnormal morphology as shown in larvae immediately after hatching.
DISCUSSION
The contribution of OPPs to the decline of several amphibian species including the axolotl has been reported in the literature (Slooff et al. 1983, Aktar et al. 2009). Despite these and other efforts to communicate the detrimental effects of pesticides, axolotl populations continue to decline in their natural habitats, tagging them as critically endangered (Frías-Álvarez et al. 2010) after more than 10 years from the first report on the effect of OPPs on A. mexicanum (Robles-Mendoza et al. 2009). In this study, we have recapitulated the effects of malathion, the most commonly used OPP, and dichlorvos, another widely used, persistent and toxic OPP. Most importantly, we have evaluated the effect of commercial-grade OPPs on A. mexicanum for the first time, in contrast with studies using analytical grade pesticides. The former would likely resemble better their effect in the wild.
In terms of the effects of OPP treatment at the embryo stage, our observations suggest that the exposure of eggs to MLT and DDVP causes only mild effects in the mortality rate, but affects significantly both the hatching time and larvae morphology, which is suggestive of an abnormal developmental process (as discussed below).
Seemingly, DVPP concentrations had a stronger effect in mortality, with a lowest survival rate of 60 % (DVPP 1.9 mg/L), while the lowest survival rate for MLT treatments (MLT 5.7 mg/L) was 90 %. Only a few critical reports describe the embryo mortality rate in presence of OPPs or an unexposed control group. For instance, Chaparro-Herrera et al. (2013) reported a rate of 75 % hatching in EPA medium conditions, while Robles-Mendoza et al. (2009) reported a 100 % hatching rate in similar clean water conditions. The latter is identical to our observations for the control group. In the presence of OPPs, Robles-Mendoza et al. (2009) observed a much higher mortality rate when exposed to MLT, showing that even the lowest concentration in their study (10 mg/L) led to a 0 % survival/hatching rate. In our experiments, the highest MLT concentration used was 17.5 mg/L, which contrary to them, presented a survival rate of 90 % in embryos.
It is important to highlight that commercial pesticides contain other components in their formulations that could affect their toxicity and therefore provide a more realistic scenario of their effects on the environment (Pereira et al. 2009). In this regard, conflicting evidence can be found in the literature in which analytical grade malathion has been reported to be more toxic than the commercial pesticide on Rana clamitans tadpoles (Puglis and Boone 2011), but less toxic when tested on Rana ribunda (Sayim 2008). It should be noted that the comparison of the mentioned reports should be taken with caution, because there are different species in different experimental set-ups. Similarly, our results of OPPs conditions cannot be accurately compared to those of Robles-Mendoza et al. (2009) due to the use of commercial pesticides in our experiments in contrast to the analytical grade pesticides in the mentioned study. Nevertheless, it is suggested that commercial grade pesticides could have a lesser detrimental effect in A. mexicanum.
In addition to the hatching efficiencies, we observed a severe effect of OPP treatments on the hatching time in comparison to the control group. Interestingly, some reports have shown the effect of pesticides on the hatching time of the Ambystoma genus, including the axolotl (Rohr et. al. 2003, Robles et al. 2009). Such reports indicate differences in the hatching time described as either delayed (Rohr et al. 2003) or early, similar to our study and the report from Robles-Mendoza et al. (2009). Altogether, these differences suggest that hatching of Ambystoma larvae depends on the pesticide they are exposed to, as well as the exposure time and the concentration used.
In relation to our results, the acceleration of the amphibian development (caused by unfavorable conditions) has been shown to carry physiological consequences that can translate into poor survival (Gómez-Mestre et al. 2013). Although we did not seek to observe embryo malformation, we could suggest the presence of altered embryo development at the time of neurulation (i.e., neural fold closure and notochord formation) as observed previously with MLT (Robles-Mendoza et al. 2009). We can suggest a similar effect with DVPP, although it should be tested in the future.
As implied above, A. mexicanum hatching timing and survival depend on the environmental conditions. Interestingly, our experiments were performed at ambient conditions (temperature and photoperiod) during the months of September-October, which seemingly resulted in differences with the current literature. For instance, the hatching reported by Robles et al. (2009) started at day 9 (controlled temperature and photoperiod), while our control individuals started hatching at day 12. Furthermore, different survival/hatching percentage has been reported independently: 75 % found by Chaparro-Herrera et al. (2013) in comparison to 100 % observed by Robles et al. (2009) and 100 % in the present report. This suggests mild differences in embryo timing that could be related to independent captivity environments (i.e., ambient temperature) (Chaparro-Herrera et al. 2018).
In our experiments, severe effects of OPPs were observed clearly in early larvae from treated organisms. All hatched MLT-treated eggs died 4 days post-hatching, even those who were in depuration, whereas larvae of DVPP-treated eggs survived until day 7 post-hatching, regardless of the depuration period. These results show the high susceptibility of axolotl embryos to commercial-grade OPPs. The mechanism of action of these pesticides is the inhibition of acetilcholinesterase, whose activity increases with development. Hence, it has been previously proposed that OPPs effect could be more adverse in larvae than embryos (Tahara et al. 2005, Arufe et al. 2007, Robles-Mendoza et al. 2009). Nevertheless, our results showed that even in eggs with a shortened exposure to OPPs, the removal of these compounds from the medium was not enough to increase survival in early larvae, suggesting important bioaccumulation in body tissues and irreversible damage early on. Further research should attempt to determine whether this effect is due to irreversible damage in axolotl embryos in a similar fashion to Sparus aurata in the larvae stage (Arufe et al. 2007) or is caused by OPPs prevalence (and continuous damage) in the tissue as observed in A. mexicanum elsewhere (de Llasera et al. 2009). The seemingly lack of effect of depuration (observed in the MLT depurated group) raises several concerns in conservation strategies that would need to consider effective bioremediation methods to increase survival opportunities.
Furthermore, early larvae presented morphological abnormalities and reduced size, as described previously in various amphibians including the axolotl (Bonfanti et al. 2004, Robles-Mendoza et al. 2009). Attribution to this trait has been given to an abnormal muscular myotomy structure, as a consequence of continuous action potentials occurring in the tail musculature, which leads to poor or abnormal reposition of muscle fibers during the development (Bonfanti et al. 2004, Colombo et al. 2005).
Noteworthy, an original report has shed light into realistic quantities of OPPs in the Xochimilco lake, ranging from non-detected to 2.7 µg/L of malathion in the water canals. These results in significant lower concentrations than those used in this and other reports in the mg/L range (Mercado-Borrayo et al. 2015). Nevertheless, even realistic concentrations of OPPs such as chlorpyrifos have been shown damage to early axolotl larvae (Robles-Mendoza et al. 2011). Hence, it is proposed that future work should attempt to test realistic OPP quantities to better assess and recapitulate their possible effects in the axolotl habitat.
Importantly, the present study is, for the best of our knowledge, the first attempt to describe the effect of DVPP on the embryo stage of a urodele amphibian species. Previous reports from anuran tadpoles support the detrimental effect of DVPP observed in the present study (Geng et al. 2005).
Efforts should be made to link ours and others laboratory observations to explain plausible scenarios of A. mexicanum disappearance from its natural environment, in order to help conservation strategies. For instance, suitable areas preferred by the species are in the local areas used for traditional agriculture (Contreras et al. 2009). Adding to these observations, microhabitat preferences have been proposed to explain the failure of efforts to ensure axolotl survival and conservation over the past years (Ayala et al. 2019).
For instance, Ortiz-Ordóñez et al. (2016) showed that sediment elutriates from Xochimilco lake were a source of pollutant accumulation with the ability to cause damage at biochemical, cellular and organic levels on A. mexicanum. Considering that axolotls often lay their eggs on the substrate (Chaparro-Herrera et al. 2013), it is likely that the accumulation of OPPs in the sediment contributes to the poor survival and conservation of the species.
Finally, although pesticides are used to control pests, their application can generate damages and alterations to any exposed organism. Axolotls could be considered as a model for the study of teratogenesis, the alteration of reproduction and development in humans when exposed to these pesticides (Eskenazi et al. 2007).
CONCLUSION
We have presented an analysis of the effect of MLT and DVPP on the survival, hatching, and morphology of axolotl in early stages, recapitulating previous reports describing the effects of the former, as well as reporting for the first time the effects of the latter in the axolotl embryo. The effects of these pesticides in early larvae size, sparsely described in previous reports, was included. Additionally, we analyzed such results in the context of the A. mexicanum habitat and how it could have partially influenced the dramatic population descent observed over time. It is relevant to take immediate conservation and restoration actions, in order to maintain the axolotl population in its natural habitat, as well as eco-friendly agriculture practices.











 nueva página del texto (beta)
nueva página del texto (beta)


