INTRODUCTION
Farmers have implemented measures to increase the production of food due to its growing demand, including the use of chemical substances, such as herbicides (FAO 1997). In the Hydrological Region 28 of Papaloapan, Veracruz (SINA 2021), diuron is an agrochemical used in agriculture (Zuleta-Rodríguez and Vázquez-Torres 1992).
In the state of Veracruz, shrimp production comes from artisanal capture and aquaculture (CONA-PESCA 2018). However, the production of shrimp presents various problems that make it an activity vulnerable to external factors such as water quality in collection sites and cultivation activities (Gil-Díaz 2020).
The herbicide diuron (3-(3,4-dichlorophenyl)-1,1-dimethylurea [DCMU]) belongs to the group of nitrogenous cyclodiene pesticides. Its economic importance is due to its use in the control of broadleaf weeds, grasses, cotton, banana, corn, sorghum, pineapple, citrus, and mainly sugarcane (UNA 2021). It is a potential contaminant of groundwater due to transport from farmland (INECC 2019, UNA 2021).
Studies in the field about the dissipation of diuron showed that the maximum amount in the sediment at the bottom was 0.76 mg/L immediately after application, which declined to 0.13 mg/L after four days and was detected at 0.066 mg/L in samples taken 256 days after application (Turner 2003). In analyses of water, Proia et al. (2011) reported pulses of up to 0.134 mg/L in the Llobregat river (NE Spain).
In Mexico, about 44 commercial brands contain the active ingredient in percentages of around 80 %. diuron is highly persistent (up to one year) in aquatic environments, and microorganisms are the main agents responsible for degrading diuron. It is classified as toxic to birds, amphibians, and crustaceans, as it is soluble in water, absorbed in sandy soils, and available for organisms associated with benthos (INECC 2019), such as prawn shrimp of the genus Macrobrachium.
Some studies have been carried out on nitrogenous compounds on postlarvae of the species of the genus Macrobrachium. In this regard, an LC50 of 4.5 NO2-N mg/Lthe was determined for the acute toxicity of nitrite to larvae of the giant Malaysian prawn, M. rosenbergii De Man, 1879 (Armstrong et al. 1976). Alternatively, LC50 values of 21.14 and 21.65 mg/L were determined for the acute toxicity of ammonia to various life stages of the Amazon River prawn, M. amazonicum, Heller, 1862 (Dutra et al. 2016). Regarding the species used in this study, the acute toxicity of carbofuran in M. olfersii was assessed by Barbieri et al. (2016), who determined an LC50 of 0.42 mg/L. In addition, the acute toxicity of the herbicide Dasurquat (paraquat) in the shrimp was assessed, and a LC50 of 0.31 µL/L (3.8 mg/L) was found (Gil-Díaz 2020). However, there are no studies about the use of diuron on the species M. acanthururs and M. olfersii.
An LC50 of 12 to 16 mg/L was obtained in other crustaceans with tests of acute toxicity for diuron, (Turner 2003, Koutsaftis and Aoyama 2008, Alyürük and Çavaş 2013, Shaalaa et al. 2015). Therefore, diuron produces mortality at high concentrations. However, lower concentrations can alter the neurotransmitters, hormones, immune response, reproduction, physiology, morphology, or behavior (Relyea and Hoverman 2006).
The prawn M. acanthurus (Wiegmann 1836) is distributed from North Carolina in the United States to Rio Grande do Sul in Brazil (García et al. 2013). The information available for this species is scattered, fragmented, or covers very specific aspects (Hernández-Hernández 2016). On the other hand, M. olfersii (Wiegmann 1836) is distributed from the Baluarte River, Sinaloa to El Naranjo, Chiapas, and along the Atlantic slope from St. Agustine Florida to Santa Catarina, Brazil (Hernández 2007). For larvae and postlarvae of M. acanthurus and M. olfersii in the study area, only the studies of Salas-de la Rosa (2018) and Arias-Martínez (2020) are known. Given the above, the present study aimed to establish the mean lethal concentration (LC50) in the postlarvae of the species M. acanthurus and M. olfersii to exposure to the agrochemical Karmex (diuron) used in crops in the Río Jamapa, Veracruz region.
MATERIALS AND METHODS
Study area
The estuary of the Jamapa River is formed by the confluence of the Cotaxtla and Jamapa rivers. The Jamapa River arises at the limit of the states of Puebla and Veracruz with the name of Barranca de Coscomatepec. It continues its course towards the east, crosses farmlands, and forms meanders and alluvial terraces up to the the Ixcualco stream. From there, it flows into the Cotaxtla River, which flows to the Moreno River and the Mandinga Grande Lagoon. Finally, it empties into the Gulf of Mexico in the town of Boca del Río, Veracruz. The Jamapa River basin is located between 18º45’ and 19º14’ north latitude, and between 95º56’ and 97º17’ west longitude. The area has a warm subhumid climate with a higher annual average temperature of 22 ºC, and the temperature of the coldest month is 18 ºC. The precipitation of the driest month is from 0 to 60 mm, and greater than 55.3 mm during the rainy season. In the season of cold fronts, the rain is from 5 to 10.2 % of the annual total. The main types of soil are Regosol and Vertisol (Fuentes-Mariles et al. 2014).
Specifically, the mouth of the Jamapa River is an estuarine system whose water discharges are rich in nutrients and reach the Sistema Arrecifal Veracruzano (SAV) (Leaño-Carrera et al. 2019). The level of the river in its estuarine part has a micro-tidal modulation (< 2 m) with a semi-diurnal (~ 12 h), diurnal (~ 24 h), and lunisolar component every two weeks (~ 15 days) (Salas-Monreal et al. 2019). The Jamapa River has a navigation channel in the southern part that generates important changes in the estuary dynamics (Salas-Monreal et al. 2019). The shipping channel produces stronger currents (> 0.5 m/s) and a continuous exchange of brackish water with the ocean (~ 12 h). The southern area is more dynamic (the exchange of water from the river and the ocean is continuous) than the northern area, where the water can remain static for periods longer than 24 h, due to its low speeds and the continuous supply of water from Arroyo Moreno, showing greater pollution from the urban discharges that it receives directly (Salas-Monreal et al. 2020).
Fieldwork
The organisms were collected using light traps made with plastic boxes; their dimensions were 0.40 m long, 0.25 m wide, and 0.30 m high. The traps had four 0.025 m diameter perforations. In the lower part, a sample receiver was placed, which consisted of a mesh with a 330-µm aperture. Inside the box, a plastic grid with a mesh opening of 0.05 m and a white light lamp were placed (Cházaro-Olvera et al. 2018).
Night samplings were carried out considering the full moon phase. The traps were placed in three contiguous sites at 20:00 LT on the first day of sampling and were collected at 08:00 LT the next day. From the in vivo sample, prawns were selected for the bioassays. The environmental factors of temperature (º C), salinity (practical salinity units, PSU), dissolved oxygen (mg/L), and pH were measured in situ at 0.50 m of depth, using a Hanna HI9828 multiparameter meter equipped with HI769828DO and HI769828PH probes.
Laboratory work
The identification of the postlarvae of Macrobrachium was realized following the descriptions of Holthuis (1952): Macrobrachium acanthurus presents a straight rostrum that reaches slightly beyond the scaphocerite. The dorsal margin bears nine to 11 teeth, of which two are behind the orbit margin. The second tooth is sometimes placed partly over the posterior margin of the orbit. The lower margin bears four to seven (generally six) teeth, which proximally are placed closer together than distally. The carapace is smooth; it only bears short hairs, especially in the anterolateral region. The second legs are equal; they reach with the carpus or with a small part of the merus beyond the scaphocerite. The fingers are slender, close over their whole length and are only slightly shorter than the palm. The pleura of the fifth segment ends in an acute point. The sixth segment is 1.5 times as long as the fifth. The telson is 1.5 times as long as the 6th abdominal segment. Macrobrachium olfersii presents a straight rostrum, reaching the third joint of the antennular peduncle. The dorsal margin bears 14 to 15 teeth, of which two to three are behind the orbital margin. The ventral margin bears three to four teeth. The second pair of pereiopods with chelas unequal in shape and size. The hepatic spine is smaller than the antennal. The second legs are very unequal; the larger reaches with the entire carpus and a small part of the merus beyond the scaphocerite; outer surface with smooth central area surrounded by spines and ornamented with bristles and pubescence. The pleura of the fifth segment has a rectangular top or is slightly acute. The 6th segment is slightly longer than the fifth. The telson is 1.5 times as long as the 6th abdominal segment.
Bioassays
Live specimens for bioassays were placed in transparent 500-mL containers with 100 mL of water from the site previously filtered and constant aeration using Elite 802 air pumps.To carry out the bioassays, 10 postlarvae were used for each 500-mL plastic container following the criteria of Mohapatra and Rengarajan (1995). The agrochemical used was obtained from the commercial brand Karmex (diuron: 3-(3,4-dichlorophenyl)-1,1-dimethylurea [DCMU] with 80 % active ingredient). Preliminary 96 h static bioassays were carried out to determine the concentration ranges of the definitive bioassays. The concentrations used were 0, 1.75, 3.5, 6.96, 14, and 28 mg/L (Castro-Castro et al. 2005). In each bioassay, three replicates were used. Mortality readings were taken after 1, 2, 4, 8, 18, 24, 36, 48, and 96 h of exposure (Uc-Peraza and Delgado-Blas 2012). For the acute toxicity bioassays, the guidelines of the US Environmental Protection Agency (USEPA 2016a) were followed for ecological effects tests (USEPA 2016b). On the other hand, to complement the information about toxicity of Karmex (diuron) on Macrobrachim, the LD50 with Probit-Log(dose) lineal regression models were obtained. Subsequently, ANOVA tests to assess significance and goodness of fit to the regression models were applied (p < 0.05) (Bustos-Obregón and Vargas 2010). To test if LD50 were significantly lower than LC50 a paired t test was applied (Ross and Willson 2017).
Statistical analysis
To determine the statistical significance of the concentration variability of Karmex (diuron) for the two species of Macrobrachium, a one-way analysis of variance (ANOVA) and the Tukey comparisons test were used (Zar 1999). The mean lethal concentration (LC50) was determined with the statistical program Minitab v. 18.1 (Minitab, LLC, State College PA, USA), and the 95 % confidence intervals were obtained. The LD50 linear regression models and the respective graphics were obtained with the statistical program Microsoft Excel 365. The paired t test was applied with PAST software v. 3.26 (Harmer et al. 2001).
RESULTS AND DISCUSSION
The dissolved oxygen in the Río Jamapa estuary was 6.7 mg/L, pH was 8.7, temperature was 27 ºC, and salinity was 0.72 ± 0.18 and 13.89 ± 5.48 PSU in March and October, respectively. For the bioassays, the values of the environmental parameters were maintained following the collection values (Table I). The values of the parameters are consistent with those recorded in the study area by Houbron (2010), Jasso-Montoya (2012), Aké-Castillo et al. (2016)), Castañeda-Chávez et al. (2017), González-Vázquez et al. (2019) and Salas-Monreal et al. (2020).
TABLE I PHYSICAL AND CHEMICAL FACTORS: ENVIRONMENTAL CONDITIONS FOR THE COLLECTION OF Macrobrachium acanthurus AND M. olfersii IN THE BOCA DEL RÍO, VERACRUZ, ESTUARY, AND ENVIRONMENTAL CONDITIONS IN THE LABORATORY DURING THE BIOASSAYS.
| Environmental conditions in the Boca del Río estuary | ||
| Environmental factors | March | October |
| Dissolved oxygen (mg/L) | 6.63 ± 0.22 | 6.69 ± 0.12 |
| pH | 8.6 ± 0.21 | 8.61 ± 0.11 |
| Temperature (ºC) | 25.11 ± 0.12 | 25.08 ± 1.02 |
| Salinity (PSU) | 0.70 ± 0.01 | 14.09 ± 0.58 |
| Environmental conditions in the laboratory during the bioassays | ||
| Environmental factors | March | October |
| Dissolved oxygen (mg/L) | 6.72 ± 0.12 | 6.73 ± 0.15 |
| pH | 8.70 ± 0.32 | 8.71 ± 0.32 |
| Temperature (ºC) | 27.41 ± 0.45 | 27.38 ± 1.09 |
| Salinity (PSU) | 0.72 ± 0.18 | 13.99 ± 5.48 |
PSU: practical salinity units.
The differences in mortality among the concentrations of Karmex (diuron) for the two species of Macrobrachium determined by ANOVA are greater than would be expected by chance; there is a statistically significant difference (p < 0.05), which estimated with Tukey’s test was found between all concentrations (p < 0.05), except for 1.75 and 3.5 mg/L (p = 0.056).
The maximum concentration of Karmex (diuron) of 28 mg/L caused mortality rates of 100 % in both Macrobrachium species (Table II). With the Probit analysis, an LC50 of 8.77 mg/L of Karmex (diuron) for M. acanthurus at 96 h for March was found, with a 95 % confidence interval of 7.67 to 9.92 mg/L (Fig. 1a). For October, the LC50 was 9.04 mg/L, with a 95 % confidence interval of 7.92 to 10.23 mg/L (Fig. 1b).
TABLE II CUMULATIVE MORTALITY (%) AT 96 HOURS IN THE BIOASSAYS WITH Macrobrachium acanthurus AND M. olfersii CARRIED OUT ON TWO SAMPLING DATES (MARCH AND OCTOBER).
| Macrobrachium acanthurus | |||||||
| Initial date | Final date | Karmex (diuron) concentration (mg/L) | |||||
| 0 | 1.75 | 3.5 | 6.96 | 14 | 28 | ||
| Cumulative mortality (%) | |||||||
| 12/03/2019 | 17/03/2019 | 0 | 10 | 20 | 40 | 50 | 100 |
| 12/03/2019 | 17/03/2019 | 0 | 10 | 30 | 50 | 60 | 98 |
| 02/10/2019 | 06/10/2019 | 0 | 20 | 20 | 40 | 60 | 100 |
| 02/10/2019 | 06/10/2019 | 0 | 10 | 20 | 40 | 50 | 98 |
| Macrobrachium olfersii | |||||||
| Karmex (diuron) concentration (mg/L) | |||||||
| 12/03/2019 | 17/03/2019 | 0 | 10 | 20 | 50 | 60 | 100 |
| 12/03/2019 | 17/03/2019 | 0 | 10 | 30 | 50 | 60 | 98 |
| 02/10/2019 | 06/10/2019 | 0 | 20 | 20 | 60 | 80 | 100 |
| 02/10/2019 | 06/10/2019 | 0 | 10 | 20 | 40 | 60 | 98 |
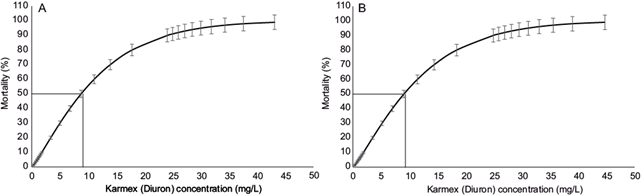
Fig. 1 Mean lethal concentration (LC50) of Karmex (diuron) for Macrobrachium acanthurus at 96 h with upper and lower 95 % confidence intervals: (a) March, (b) October.
With the Probit analysis, we found an LC50 of 8.14 mg/L for Karmex (diuron) in M. olfersii at 96 h for March, with a 95 % confidence interval of 7.14 to 9.24 mg/L (Fig. 2a). For October, the LC50 was 7.57 mg/L, with a 95 % confidence interval of 6.62 to 8.55 mg/L (Fig. 2b).
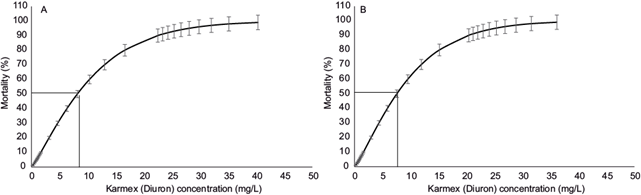
Fig. 2 Mean lethal concentration (LC50) of Karmex (diuron) for Macrobrachium olfersii at 96 h with upper and lower confidence intervals of 95 %: A, March, and B, October.
With the Probit-Log(dose) linear regression model, LD50 values of 7.974 and 8.221 mg/L for March and October, respectively, were obtained for M. acanthurus (Fig. 3a, b). For M. olfersii, LD50 values of 7.504 and 7.008 mg/L were obtained for March and October, respectively (Fig. 3c, d). The four correlation coefficients were statistically significant (p < 0.05). The values calculated for LC50 were higher than for LD50. Differences statistically significant between the LD50 and LC50 for M. acanthurus (t = 70.217, p = 0.009) and M. olfersii (t = 16.189, p = 0.0393) were obtained. Kamau et al. (2012) found an LD50 of 153.2 mg/L for Artemia salina with plant extracts as an alternative to synthetic insecticide. In this regard, Reed (2017) mentions that the smaller the LD50 values of a substance under test, the greater its toxicity.

Fig. 3 Lethal dose (LD50) of Karmex (diuron) with the Probit-Log(dose) lineal regression model for Macrobrachium acanthurus: (a) March, (b) October; and M. olfersii: (c) March, (d) October.
Regarding the toxicity bioassays with 80 % diuron, an LC50 of 84 to 300 mg/L for Lepomis macrochirus Rafinesque, 1819, 16 to 23.8 mg/L for Oncorhynchus mykiss (Walbaum 1792), and 0.5 to 6.0 mg/L for Morone saxatilis (Walbaum, 1792) were found. The Pesticide Properties Database (PPDB 2021) determined that the LC50 for aquatic crustaceans is 1.1 mg/L; however, Shaalaa et al. (2015) found that for Artemia salina (Linnaeus, 1758) nauplii, the increase in diuron concentration increases mortality since they determined diuron LC50 values of 23.27 mg/L, 12.19 mg/L, and 6.00 mg/L after 24, 48, and 72 h of exposure, respectively. Likewise, Alyürük and Çavaş (2013) found that in A. salina larvae, the EC50 value for diuron was 12.01 mg/L. Meanwhile, Koutsaftis and Aoyama (2008) reported that the LC50 value for diuron in A. franciscana (Kellog, 1906), was 12.5 mg/L. With an active ingredient concentration of 80-95 %, LC50 values of 0.16 to 0.7 mg/L were found for Gammarus fasciatus, 0.16 mg/L for Gammarus lacustris, 47 (26 h) to 8.4 (48 h) mg/L for Daphnia magna, and 15.5 mg/L for Asellus brevicauda (Turner 2003). Jinlin et al. (2020) report that the LC50 of diuron (48 h) to Daphnia magna was 17.1 mg/L and Sipcam (2020) mentions that for the same species it is 6.3-13 mg/L.
The LC50 of diuron found in this study for both M. acanthurus at 96 h (10.23 mg/L) and M. olfersii (9.24 mg/L) were slightly lower than that found for Artemia species and is consistent with species of the Cladocera suborder and peracarids.
No change in the appearance of the organisms of the two Macrobrachium species was observed. Regarding the behavior, a decrease in movement was recorded, since organisms remained for a longer time at the bottom of the container with concentrations of 6.96 to 28 mg/L; at lower concentrations, active movement of the organisms was observed throughout the container.
It is important to mention that since 1980, diuron has been used in the state of Veracruz, Mexico at a concentration of 2.0 kg/ha (Ramírez-Mora et al. 2018). This compound, like other pesticides, has the potential to enter water bodies through rain that flows down streams and rivers into estuaries and oceans (NPIC 2021). Giacomazzi and Cochet (2004) reported that diuron is a biologically active pollutant present and persistent in water and sediments. In a three-year study, Xu et al. (2013) found that the annual average amount of diuron in seawater and sediments presented a slow-growing trend, indicating that diuron poses a potential risk to the aquatic ecosystems, like in our case, the estuary of the Jamapa River.
Thus, according to the previous values, there are differences in the resistance of exposure to diuron among species, which highlights the importance of knowing the effect of concentration of contaminants (such as herbicides) in species used in aquaculture.
According to the above and considering the studies of toxicity realized by Barbieri et al. (2016) with carbofuran and Gil-Díaz (2020) with paraquat in M. olfersii, as well as Marques et al. (2021) with chlorpyrifos in M. acanthurus, it is possible to consider that the postlarvae of Macrobrachium can be used as a suitable test organisms to evaluate the toxicity of diuron and other pesticides. Given the relevant role of prawn shrimp in aquatic environments and its use for human consumption, its inclusion in ecotoxicological studies is recommended.
CONCLUSIONS
The lethal concentrations of Karmex (diuron) for two species of Macrobrachium were greater than 3.5 mg/L. The maximum concentration of diuron (28 mg/L) caused a 100 % mortality rate in both Macrobrachium species. The LC50 and LD50 of diuron found in this study for both M. acanthurus (LC50 = 10.23 mg/L, LD50 = 8.221 mg/L) and M. olfersii (LC50 = 9.24 mg/L, LD50 = 7.504 mg/L) at 96 h were lower than that found for Artemia species and are consistent with the values for species of the Cladocera suborder. Considering the above, Macrobrachium postlarvae can be used as test organisms to evaluate the toxicity of Karmex (diuron).











 nueva página del texto (beta)
nueva página del texto (beta)


