INTRODUCTION
There is a great concern about arsenic (As) pollution, which is the foremost harmful and carcinogenic chemical substance and is regarded by the World Health Organization (WHO) as the first priority issue among the toxic compounds (Zhu et al. 2009, Ajiboye et al. 2021). Also, it has been recognized as a group 1 carcinogen by the International Agency for Research on Cancer (Hao et al. 2018).
In recent years, a number of methods have been utilized for the removal of As from contaminated water, including reverse osmosis, electrodialysis, ion exchanges, membrane filtration, precipitation, and adsorption (Chaudhry et al. 2017, Uddin and Jeong 2020). Adsorption has been widely used as a low-cost, high efficiency, simple and sludge-free method for treating heavy metal contamination in water. However, a low-cost and highly efficient As adsorbent is currently needed for the real application of this technique (Hao et al. 2018, Cao et al. 2021). Various materials have been used for the removal of As from polluted water and waste waters. Because of high specific surface area, high adsorption capacity, strong affinity and low cost, iron-based adsorbents (e.g. iron oxide and zero-valent iron (ZVI)) are usually chosen to removal As from water (Hao et al. 2018, Adlnasab et al. 2019, Uddin and Jeong 2020).
Magnetite (Fe3O4) and hematite (α-Fe2O3) are the major derivatives of iron oxide which facilitate the As remediation. These iron oxides have been utilized to remove As from water (Siddiqui et al. 2019). Iron oxide nanoparticles possess attractive properties such as super-paramagnetism at room temperature, which is an efficient and convenient method to separate As-adsorbed particles (Wong et al. 2017). Also, zero valent iron (ZVI) or metallic iron (Feº) is a cost-effective agent for removal of inorganic and organic pollutants (Siddiqui et al. 2019). Nano-ZVI can be distributed or dispersed in the subsurface of water which carries the contaminants (Siddiqui et al., 2019). Many studies have suggested that nano-ZVI chemistry allows the safe, effective, and economic reduction of As from water (Tanboonchuy et al. 2011, Li et al. 2014, Adio et al. 2017, Li et al. 2017).
Adsorption of arsenic by various adsorbents is a complex process involving different mechanisms and controlled by different variables. Factors such as solution pH, contact time, nature of adsorbent, initial metal ion concentration, particle size of adsorbent, and presence of competing contaminant ions can influence As removal from water. Optimizing these factors might give excellent outcomes for As treatment (Wong et al. 2017, Uddin and Jeong 2020). Over the years, conventional optimization in which a process parameter is varied while other parameters are kept constant is expensive and time-consuming. Nevertheless, a more robust technique to optimize several process parameters has been provided by Taguchi (Idris et al. 2020, Egbosiuba et al. 2021). Taguchi optimization method is highly effective in optimizing multifactor with a minimum number of experiments, thereby reducing cost and improving quality (Maazinejad et al. 2020, Egbosiuba et al. 2021). Also, the Taguchi optimization method is one such method that has several advantages over the conventional “one-at-a-time” because it provides us with more information from fewer experiment runs (Yadav et al. 2015). Despite the several applications of iron particles in environmental sciences and As adsorption, optimization of the adsorption processes is less known. Also, little information was found about the removal efficiency of iron-based materials with different particle size. Therefore, the objective of this study was to compare the adsorption capacity of iron-based particles and investigate the optimum conditions of some factors such as contact time, initial As concentration, pH and adsorbent dose, type, and size for the maximum adsorption of As from the aqueous solution using the Taguchi method. Furthermore, Langmuir and Freundlich adsorption isotherms were studied to explain the As adsorption mechanism.
MATERIALS AND METHODS
Synthesis of iron oxides and ZVI nanomaterials
All the chemicals used were of analytical grade and obtained from Merck Company. Hematite, magnetite, and ZVI nanomaterials were synthesized using hydrothermal (Trpkov et al. 2018), co-precipitation (Hedayati et al. 2017), and green methods (Wang et al. 2014), respectively. Hematite particles were synthesized by mixing 0.01 mol/L of FeCl2.4H2O and 0.02 mol/L of Fe (NO3)3 and 10 mL of ammonia solution at room temperature (Trpkov et al. 2018). The mixture was then transferred to a 120 mL galvanized stainless-steel autoclave and reacted at 200 ºC for 2, 4, and 6 hours to control different size of nanoparticles. After the reaction, the autoclave was cooled and the dark precipitates were collected by centrifugation and washed three times with distilled water and ethanol. Finally, the precipitate was dried at 70 ºC for 12 hours. For the synthesis of magnetite particles, 0.01 mol FeCl2.4H2O and 0.02 mol FeCl3.9H2O were dissolved in 100 mL of water and then a mixture of 10 mL of 25 % ammonia were slowly added to the solution under nitrogen atmosphere. The quality of hematite particles was controlled by adjusting the reaction temperature from 60 to 150 ºC. The final precipitate was centrifuged and washed with distilled water and air dried (Hedayati et al. 2017). To synthesize the ZVI particles, eucalyptus leaves were used. It is probably that the eucalyptus leaf extract is a mixture of various naturally derived compounds with different reducing properties (Wang et al. 2014). Eucalyptus leaves were obtained from a local farm in Rafsanjan, Kerman province, Iran. Deionized (DI) water was used in all experiments. First, leaves were thoroughly washed with distilled water and then dried at room temperature. The plant extract was prepared by adding 15 g of dried leaves in 250 mL of distilled water for 1 hour at 80 ºC. The extract was then filtered in a vacuum and stored at 4 ºC. To prepare the ZVI nanomaterials with different sizes, the eucalyptus extract was stirred with 0 0.1 mol/L Fe (SO4).2H2O in a ratio of 1:1, 1:2, and 1:3 at room temperature for 30 min. The precipitates were then collected in a vacuum and washed three times with ethanol. Finally, the ZVI nanomaterials particles were dried under a vacuum for 12 hours at 50 ºC and stored in a nitrogen atmosphere (Wang et al. 2014).
Characterization of iron-based particles
The morphological characteristics of iron-based particles were analyzed by field emission scanning electron microscopy (FESEM, ZEISS, Sigma VP) coupled with an energy dispersive X-ray (EDX). The Powder X-ray diffraction (XRD) data were collected on a Bruker D8 Advance X-ray diffractometer (Cu Ka X-radiation at 40 kV and 40 mA).
Adsorption studies
The As adsorption optimization process was performed using the standard method proposed by Taguchi to achieve maximum adsorption of As ions. The Taguchi method utilizes an orthogonal array (OA) for experimental design and applies the signal-to-noise ratio (S/N) for quality evaluation (Huang et al. 2009, Paul et al. 2014, Zolgharnein and Rastgordani 2018). There are several types of S/N, such as the smaller is better, the larger is better and the nominal is better. In this paper, the larger is better S/N was determined because the purpose of this work was to achieve a high adsorption percentage. Thus, the eq. 1 presented by Taguchi for S/N calculation the larger-the better criteria was:
Where y i is the experimental response (As adsorption percentage), and n is the repetition number of the experiment. The suggested design with 27 experiments conducted for the selected factors with their corresponding levels is presented in table I. Six controllable factors were considered to design the experiment, including contact time, pH, adsorbent size, adsorbent type, initial concentration, and adsorbent dose, each at three different levels. These factors’ levels were selected based on previous works and preliminary experiments.
TABLE I CONTROLLABLE FACTORS AND THEIR LEVELS IN THE TAGUCHI METHOD.
| Factor | Level | ||
| 1 | 2 | 3 | |
| (X1) Contact time (h) | 0.5 | 2 | 4 |
| (X2) pH | 6 | 7 | 9 |
| (X3) Adsorbent size* | * | ** | *** |
| (X4) Adsorbent type | Hematite | Magnetite | ZVI |
| (X5) Initial As concentration (µg/L) | 500 | 1000 | 3000 |
| (X6) Adsorbent dose (g) | 0.01 | 0.06 | 0.1 |
*: average particle size > 80 nm, ** average particle size 30-80 nm, *** average particle size < 30 nm.
Table II presents the required experiments to optimize the batch adsorption system designed using the Taguchi method. In each run, the As adsorption capacity by the iron-based particles was investigated using the background electrolyte of 0.01 mol/L NaNO3 at room temperature (25 ºC). For this purpose, 0.05 g of each iron-based particle (with three different sizes and at desired pH values) was added to polyethylene containers containing 10 mL of arsenic solution with an initial concentration of 500-3000 µg/L. Samples were then shaken at the speed of 150 rpm for 24 h. Then the supernatant was separated from the solid by centrifugation at 20 000 g for 20 min. The concentration of As was then analyzed by graphite furnace atomic absorption spectrophotometry (SavantAA, GBC). All experiments were repeated three times.
TABLE II L27 ORTHOGONAL ARRAY (LEVEL OF SIX DIFFERENT FACTORS; CONTACT TIME (X1), pH (X2), ADSORBENT SIZE (X3), ADSORBENT TYPE (X4), INITIAL ARSENIC CONCENTRATIONS (X5), ADSORBENT DOSE (X6) AND EXPECTED RESULTS.
| Run | X1 | X2 | X3 | X4 | X5 | X6 | qe (µg/g) | S/N ratio |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 71.1 | 37.04 |
| 2 | 1 | 1 | 1 | 1 | 2 | 2 | 290.45 | 49.26 |
| 3 | 1 | 1 | 1 | 1 | 3 | 3 | 744 | 57.43 |
| 4 | 2 | 1 | 2 | 2 | 1 | 1 | 28.5 | 29.10 |
| 5 | 2 | 1 | 2 | 2 | 2 | 2 | 202.6 | 46.13 |
| 6 | 2 | 1 | 2 | 2 | 3 | 3 | 736 | 57.34 |
| 7 | 3 | 1 | 3 | 3 | 1 | 1 | 332.1 | 50.43 |
| 8 | 3 | 1 | 3 | 3 | 2 | 2 | 1112.5 | 60.93 |
| 9 | 3 | 1 | 3 | 3 | 3 | 3 | 1365.5 | 62.71 |
| 10 | 1 | 2 | 2 | 3 | 1 | 1 | 17.9 | 25.06 |
| 11 | 1 | 2 | 2 | 3 | 2 | 2 | 152 | 43.64 |
| 12 | 1 | 2 | 2 | 3 | 3 | 3 | 671 | 56.53 |
| 13 | 2 | 2 | 3 | 1 | 1 | 1 | 61.7 | 35.81 |
| 14 | 2 | 2 | 3 | 1 | 2 | 2 | 408 | 52.21 |
| 15 | 2 | 2 | 3 | 2 | 3 | 3 | 1225.5 | 61.77 |
| 16 | 3 | 2 | 1 | 2 | 1 | 1 | 88.4 | 38.93 |
| 17 | 3 | 2 | 1 | 2 | 2 | 2 | 323 | 50.18 |
| 18 | 3 | 2 | 1 | 2 | 3 | 3 | 2616.5 | 68.35 |
| 19 | 1 | 3 | 3 | 2 | 1 | 1 | 125.8 | 41.99 |
| 20 | 1 | 3 | 3 | 2 | 2 | 2 | 1073 | 60.61 |
| 21 | 1 | 3 | 3 | 2 | 3 | 3 | 1548.5 | 63.80 |
| 22 | 2 | 3 | 1 | 3 | 1 | 1 | 227.3 | 47.13 |
| 23 | 2 | 3 | 1 | 3 | 2 | 2 | 1080 | 60.67 |
| 24 | 2 | 3 | 1 | 3 | 3 | 3 | 1660 | 64.40 |
| 25 | 3 | 3 | 2 | 1 | 1 | 1 | 71.47 | 37.08 |
| 26 | 3 | 3 | 2 | 1 | 2 | 2 | 1098.5 | 60.82 |
| 27 | 3 | 3 | 2 | 1 | 3 | 3 | 1195 | 61.55 |
Adsorption isotherms of As onto the adsorbents were determined in batch experiments. Ten milliliters of 0.01 mol/L NaNO3 solution with different concentration ranges of As (100-6800 µg/L) were added into centrifuge tubes containing 0.05 g adsorbent. Blank samples (without adsorbent) were also considered for each concentration of As. The pH values of the suspensions were adjusted to 7.0 by adding 100-300 μL of 0.03 mol/L NaOH or HNO3 solution. The suspensions were shaken for 4 h with a rotary shaker at a speed of 150 rpm. The equilibrium solutions were separated by centrifugation for 20 min at 20 000 g, and As concentration was measured using graphite furnace atomic absorption spectrophotometry (SavantAA, GBC). All experiments were repeated three times. The amount of As adsorbed per unit mass of the adsorbent was evaluated, using the eq. 2:
where C i and C e are the initial and equilibrium concentrations of As (µg/L) in solutions, respectively. q e (µg/g) is adsorption capacity, V (L) is the volume of solution, and m (g) is the mass of adsorbent.
The removal percentage of As was calculated for each experiment by eq. 3:
The equilibrium adsorption data were analyzed with the utilization of the Freundlich and Langmuir equilibrium isotherms. Eqs. 4 and 5 represent non-linear form of Freundlich and Langmuir isotherms, respectively (Hamidpour et al. 2018).
Where C e is the equilibrium concentration of the As (µg/L), qe denotes the amount of the As adsorbed per unit mass of the adsorbent at equilibrium (µg/g), q max (µg/g), and b (L/µg) are the Langmuir constants related to the monolayer uptake capacity and energy of adsorption, respectively (Zhou et al. 2020). The K f (L/g) and 1/n (-) are the Freundlich constants.
The parameters of adsorption isotherms were determined by the non-linear curve fitting analysis method using the DataFit statistical software.
RESULTS
Characterization
XRD analysis
The characterization by X-ray diffraction (XRD) results are displayed in figure 1. The successful synthesis of iron-based nanoparticles was proved by XRD analysis. All diffraction peaks in figure 1 can be indexed to the hematite and magnetite crystalline phase, which are in accordance with the data reported in the literature (Compeán-Jasso et al. 2008, Cuong et al. 2014). The XRD patterns of the synthesized ZVI nanoparticles had a broad peak, indicating the production of an amorphous structure (Ebrahiminezhad et al. 2017, Kheshtzar et al. 2019). Fabrication of amorphous ZVI was also reported by using leaf extract of different plants and food industry wastes (Wang et al. 2014, Ebrahiminezhad et al. 2017).
FESEM analysis
The morphology of the synthesized iron-based nanoparticles was evaluated using FESEM (Fig. 2). In figure 2a, the well-developed hematite spheres are seen. Some representative images in figure 2 indicated that the hierarchically assembled hematite sphere was a multi-scale organized nanoarchitecture. Also, the FESEM image in figure 2b shows that the prepared magnetite sphere possesses dense nanostructures on its surface, constructing a hierarchical dandelion-like structure. Although it is difficult to identify an individual nanostructure from the FESEM image, it can be found that the synthesized magnetite nanostructures were aligned on the surface of the particles. Figure 2c shows a FESEM image of ZVI nanomaterials. In the FESEM image of ZVI, the formation of some irregular shapes due to aggregation and fusion of the particles was observed.
Taguchi method
Analysis of signal/noise ratio
In this study, six controllable factors were chosen as input variables and each factor was evaluated at three levels according to an L27 orthogonal array designed by the Taguchi method (Table I). The adsorption capacity (q e ) of As was chosen as a response in the Taguchi L27 array. Since in the case of As ions adsorption “Larger is better’’ is the desired case, the optimum level of the controllable factors was the level that corresponds to the highest S/N ratio according to eq. 1. The experimental results for As ions adsorption and the S/N ratio values are reported in table II. The objective of applying the Taguchi method is to determine the optimum conditions at which qe is maximized (Yadav et al. 2015, Googerdchian et al. 2018).
According to the S/N ratio, the optimum levels of the factors for obtaining the maximum response variable (removal percentage of As) are shown in figure 3. It is observed that initial As concentration had a remarkable effect on the adsorption process (Fig. 3e). An increase of the As initial concentration accelerates the diffusion of As from the solution onto the adsorbents due to the increasing driving force at higher ion concentration (Hamidpour et al. 2018).
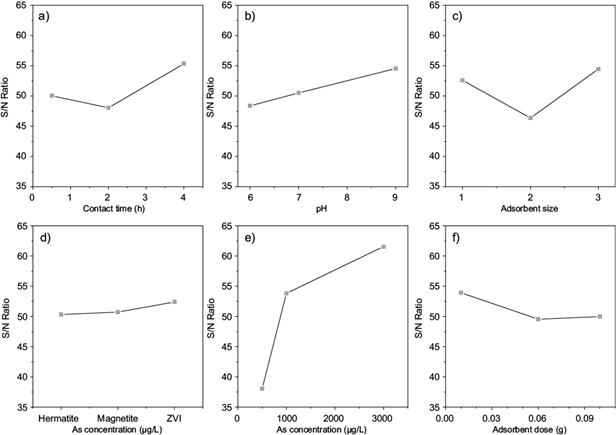
Fig. 3 The main effect of each factor by signal-to-noise (S/N) ratio for different factors: a) Contact time, b) pH, c) Adsorbent size, d) Adsorbent type, e) Initial As concentration and f) Adsorbent dose. Signal-to-noise ratio: The larger-The better.
Figure 3b shows the effect of solution pH on As uptake. As expected, an increase in the pH leads to an increase in As removal percentage. In the process of As removal from aqueous solutions, the pH value of the solution is a significant factor since it affects both the As speciation and the surface charge of the adsorbents due to protonating or deprotonating of surfaces of the adsorbents (Bilici-Baskan and Hadimlioglu 2020). The species of As are negatively charged where the solution pH value is more than 2.2. Arsenic species were attracted to the positively charged surfaces of iron-based adsorbents by the effect of electrostatic forces. Bilici-Baskan and Hadimlioglu (2020) reported similar findings for the adsorption of As onto graphene oxide-iron modified clinoptilolite composites. According to figure 3, the optimum condition was found to be adsorbent dose = 0.01 g/L, initial As concentration = 3000 µg/L, pH = 9, contact time = 4 h, adsorbent type = ZVI, and adsorbent size < 30 nm in the studied range for different parameters.
Figure 4 graphically depicts the contribution of each parameter to As adsorption. The percent contributions (ρ) of initial As concentration, adsorbent size, contact time, pH adsorbent dose, and adsorbent type, were 74.5 %, 9.4 %, 7.4 %, 5.1 %, 3 % and 0.06 %, respectively. The initial As concentration had the greatest ρ. However, the adsorbent type had the lowest ρ.
Response tables related to the calculated S/N ratios are shown in table III. Also, the significance of controllable factors might be determined by the rank of S/N ratio given in table III (the symbol “∆” shows the difference between the maximum and minimum S/N ratios). The more significant factor shows a larger range and should be utilized first (Zolgharnein and Rastgordani 2018). It was evident from the ranks obtained for each factor that initial As concentrations and size of adsorbent were the most influential factors (rank 1 and 2) for the responses of qe. The rank was initial As concentration (23.48) > size of adsorbent (8.11) > contact time (7.29) > pH (6.18) > adsorbent dosage (4.38) > type of adsorbent (2.06) in descending order (Table III).
TABLE III RESPONSE TABLE FOR SIGNAL TO NOISE RATIOS (S/N); LARGER IS BETTER.
| Level | Factors | |||||
| Contact time | pH | Adsorbent size | Adsorbent type | Initial As concentration | Adsorbent dose | |
| 1 | 50.04 | 48.37 | 52.6 | 50.33 | 38.06 | 53.92 |
| 2 | 48.05 | 50.51 | 46.36 | 50.72 | 53.83 | 49.54 |
| 3 | 55.34 | 54.55 | 54.47 | 52.39 | 61.54 | 49.97 |
| Δmax-min | 7.29 | 6.18 | 8.11 | 2.06 | 23.48 | 4.38 |
| Rank | 3 | 4 | 2 | 6 | 1 | 5 |
Adsorption isotherms
Adsorption isotherms exhibit As distribution between the adsorbent and the bulk solution at equilibrium conditions. The two well-known isotherm equations (Langmuir and Freundlich) were employed for explaining and modeling the experimental data in the systems. The results and their corresponding plots are shown in table IV and figure 5, respectively. The results of As adsorption was found to fit well with the Freundlich isotherm model due to the larger values of coefficient of determination (R2) and lower error values of SSE (Zolgharnein and Rastgordani 2018) and adsorption constants evaluated from the isotherms are listed in table IV. It follows, therefore, that the adsorption is not limited to a monolayer and that As molecules migrate to heterogeneous surfaces on the iron-based particles following the Freundlich isotherm theory. The Freundlich model describes the multilayer adsorption on heterogeneous adsorbent surfaces with non-identical sites (Adlnasab et al. 2019). The values of n between 1 and 10 (i.e., 1/n less than 1) imply favorable adsorption, and the value of 1/n between 0 and 1 shows the degree of non-linearity between solution concentration and adsorption. If the value of 1/n equals 1, the adsorption is linear. A higher value of Kf reveals a higher adsorption capacity than a lower value (Maji et al. 2018).
TABLE IV PARAMETERS OF THE ISOTHERM MODELS.
| Iron particle | Langmuir isotherm model | ||
| b (L/µg) | qm (µg/g) | R2 | |
| Magnetite1 | 0.0010 | 1199.5 | 0.85 |
| Magnetite2 | 0.0009 | 1990 | 0.95 |
| Magnetite3 | 0.0055 | 2000 | 0.97 |
| NZVI1 | 0.027 | 1490 | 0.50 |
| NZVI2 | 0.0230 | 1900 | 0.84 |
| NZVI3 | 0.0050 | 1989 | 0.84 |
| Hematite1 | 0.0020 | 1990 | 0.87 |
| Hematite2 | 0.0029 | 2398 | 0.80 |
| Hematite3 | 0.0045 | 1499 | 0.66 |
| Iron particle | Freundlich isotherm model | ||
| Kf (L/g) | n | R2 | |
| Magnetite1 | 2.86×10-6 | 0.371 | 0.980 |
| Magnetite2 | 0.273 | 0.826 | 0.996 |
| Magnetite3 | 11.276 | 1.168 | 0.974 |
| Zero-valent iron1 (ZVI1) | 2.02×10-9 | 0.141 | 0.887 |
| Zero-valent iron2 (ZVI2) | 8.941 | 0.754 | 0.889 |
| Zero-valent iron3 (ZVI3) | 0.811 | 0.715 | 0.964 |
| Hematite1 | 0.018 | 0.568 | 0.993 |
| Hematite2 | 15.618 | 1.329 | 0.771 |
| Hematite3 | 0.051 | 0.560 | 0.759 |
The investigated adsorption conditions and adsorption capacities in the present study were compared with the previous studies over the literature as presented in table V. The values are reported in the form of monolayer adsorption capacity. High adsorption capacities of some adsorbents may be due to the high As concentrations taken in performing the batch experiments. The experimental data of the present study show that iron-based adsorbents exhibited a reasonable adsorption capacity for As and, therefore, may help remove As from polluted waters. In general, iron-based adsorbents have a high affinity for As species. The formation of inner-sphere surface complexes is the primary mechanism for the strong interactions between As species and the functional groups on the adsorbent surfaces. (Bilici-Baskan and Hadimlioglu 2020, Feng et al. 2012).
TABLE V ADSORPTION CAPACITIES OF DIFFERENT IRON COMPOUNDS FOR ARSENIC.
| Adsorbents | pH | Initial concentration (mg/L) | Adsorption capacity (mg/g) | References |
| α-Fe2O3 | 3-10 | 1 | 0.2 | Tang et al. 2011 |
| Fe3O4 nanoparticles | 5 | 70 | 16.56 | Feng et al. 2012 |
| Zero-valent iron | 7 | 0.2 | 1.8-2 | Biterna et al. 2010 |
| Zero-valent iron | 7 | _ | 3.5 | Ye et al. 2017 |
| Zero-valent iron | 6 | 2-100 | 1.7 | Wu et al. 2017 |
| Magnetite | 6-9 | 0.1-6.8 | 2.0 | This study |
| Hematite | 6-9 | 0.1-6.8 | 2.4 | This study |
| Zero-valent iron | 6-9 | 0.1-6.8 | 1.9 | This study |
CONCLUSION
This study evaluated the feasibility of iron-based adsorbents (hematite, magnetite, and ZVI) for As adsorption using the Taguchi method to determine the optimum removal condition through 27 trials. The optimum condition was found to be adsorbent dose = 0.01 g/L, initial As concentration = 3000 µg/L, pH = 9, contact time = 4 h, adsorbent type = ZVI, and adsorbent size < 30 nm in the studied range for different parameters. It is also concluded that the initial As concentration is the most critical parameter in the adsorption process. The As adsorption on the adsorbents conforms well to the Freundlich isotherm compared to the Langmuir model. This research revealed that iron-based adsorbents used in this study show high adsorption behavior for removing arsenic from water.











 nueva página del texto (beta)
nueva página del texto (beta)






