INTRODUCTION
Heavy metals are released to the environment as a consequence of human activities such as smelting, mining, solid waste disposal, wastewater contaminated with metals, and utilization of insecticides that contain metals and metalloids, among others (Azabou et al. 2007). When heavy metals are released into the environment, they may cause serious and sometimes permanent damage to the biota (Micera and and Dessi 1988). Metals in aquatic environments may be present in several forms such as soluble forms, colloids, suspended material, and sedimentary phases (Peng et al. 2009). The removal of heavy metals present in different effluents can be carried out efficiently through sulfate reduction, which is a biological process that enables the reduction of sulfate to sulfide using sulfate-reducing bacteria (SRB). SRB are anaerobic bacteria that can thrive in different environments under low redox potential (˂ 200 mV). They possess the capability of reducing sulfate while oxidizing organic matter (Postage 1984, Muyzer and Stams 2008).
It has been observed (Mokone et al. 2012) in batch assays, that the origin of sulfide (either biological or chemical) does not significantly affect the efficiency of ZnS precipitation. Thus, an undesirable product such as sulfide may be used for the precipitation of heavy metals or as an electron donor in another biological process such as denitrification or in microbial fuel cells (MFC) as an electron donor to the anode electrode (Yang et al. 2016, Dai et al. 2020). The removal and recovery of heavy metals by sulfate-reducing microorganisms are possible under various system configurations that have been designed to remove the sulfide metals; among them, an up-flow anaerobic sludge blanket (UASB) reactor, which is an anaerobic digestor that allows high feeding rates and formation of conglomerate biomass (granules), which is widely used in wastewater treatment (Pererva et al. 2020). However, most studies have focused on sulfide generation and metal precipitation within a single bioreactor (one stage). For example, Hsu et al. (2010) evaluated simultaneously sulfate reduction and precipitation of copper in batch cultures using sulfate-reducing bacteria at a concentration between 19 and 235 mg VSS/L immobilized in polyvinyl alcohol (PVA) at a concentration range of copper of 10-100 mg/L, finding the maximum sulfate reduction rate at a concentration of copper of 51.5 mg/L and 138.5 mg VSS/L of sulfate-reducing bacteria immobilized with PVA. Meanwhile, Álvarez et al. (2007) used sulfide for the precipitation of metals from an effluent collected in a mine and obtained removal efficiencies of 100 and 94 % for copper and zinc, respectively. Also, Karri et al. (2006) reported the removal of relatively high concentrations of Cu2+ (200 mg/L). However, the utilization of one-stage systems has some disadvantages, such as the difficulty in treating influents with low pH and high concentrations of heavy metals due to the direct interaction between the SRB and heavy metals (Kumar et al. 2021). It has been observed that highly metal-tolerant bacteria are completely inhibited when exposed to high concentrations of copper (Teitzel and Parsek 2003). In this sense, the evaluation and design of systems that avoid direct contact of sulfur-producing microorganisms with heavy metals is desirable. In this order of ideas, it is possible to couple the production of sulfide by SRB in the first stage, followed by the chemical precipitation of heavy metals in the second stage (two-stage system). For example, in a study of two-stage reactors in which copper precipitation was evaluated, sulfate reduction was carried out in the first reactor, followed by a second reactor where the precipitation of the metal was conducted in a range of copper concentrations between 15 and 600 mg/L, giving as a result a complete Cu2+ removal; however, the production of sulfide was not quantified (Bilgin and Jaffé 2019). The authors mentioned that one of the advantages of this type of system is the elimination of toxic effects on microorganisms caused by metal concentrations. Some additional advantages of the two-stage systems are that the concentration of metals needed to be recovered could be higher than the inhibitory concentration to microorganisms and that the recovery of metal precipitates is easier. In addition, the hydraulic retention time (HRT) is another factor that can affect the performance of sulfate reduction. Polo et al. (2006) reported that sulfide concentration decreased when lower HRTs were tested.
On the other hand, information on systems in which precipitation of heavy metals is carried out with biogenic sulfide produced by sludge generated from marine sediments is scarce. The microorganisms present in marine environments could be more susceptible to adapt to adverse environments due to the large variations in pH, temperature, etc., that occur in those environments (Dash et al. 2013). Work on sulfate reduction with different compositions of the microbial community has also been conducted with lake and river sediments exposed to acid mine drainage and the combination of them with other active sulfidogenic acidic bacteria. Some of the bacteria that have been used in combination with the contaminated acidic sediments are Desulfosporosinus M1 and Db acidavidus strain (Ňancucheo and Johnson 2012). However, the direct use of sediments in bioreactors has not been attempted even though marine environments are a richer pool of microorganisms that can be strengthened under appropriate conditions to almost any useful condition for which an application can be found in environmental biotechnology. Indeed, several recent reports provide evidence of the biotechnological applications that may be developed by using river or marine sediments as inoculum (Sánchez-Andrea et al. 2012, Doyle and Marsili 2015, Xia et al. 2015).
It has been observed that sulfidogenic sludge generated from marine sediments is a consortium of SRB composed of complete oxidizers that can oxidize acetate and incomplete oxidizers that can utilize propionate and butyrate; the consortium can also operate at room temperature (García-Solares et al. 2014). This is an advantage to minimize operational costs due to heating and, at the same time to remove acetate, which tends to accumulate in sulfidogenic reactors when the consortium is constituted mainly of SRB that are incomplete oxidizers. Most of the sulfate-reducing reactors reported to date are evaluated with lactate, ethanol, or glycerol (Kumar et al. 2021). It is important to count with consortia that can utilize lower chain volatile fatty acids as substrate. To date, the study of sulfate reduction coupled with the precipitation of metals in relationship to different electron donors continues to be a cause of concern, as demonstrated by the recent study of the effect of lactate, sucrose, and glycerol on the removal of sulfate and the precipitation of metals (Costa et al. 2021). Nevertheless, the accumulation of acetate prevails in sulfidogenic consortia (SRB) composed of incomplete oxidizers and may hinder the sulfate reduction process when lactate or ethanol are utilized as electron donors (Sahinkaya et al. 2009).
On the other hand, it has been reported that the initial pH did not affect metal removal when H2S was enough for precipitation, being the sulfide concentration an important factor that affects this process (Cao et al. 2009).
The aim of this study was to evaluate the production of sulfide and metal precipitation at room temperature in a two-stage system, in which the first stage is a UASB reactor inoculated with sulfidogenic sludge generated from hydrothermal sediments utilizing acetate and a mixture of acetate-butyrate as electron donors (a mixture of volatile fatty acids that is a frequent residual of higher organic compounds). The second stage of the system consisted of a second reactor (crystallizer) in which the metal precipitation was carried out. For this purpose, the UASB reactor was operated at different hydraulic retention times (HRT) and different sulfate loading rates at a chemical oxygen demand (COD)/SO4 -2 ratio of 0.9. The possible application of sulfide on the precipitation of copper, zinc, and aluminum was also evaluated by coupling the UASB to a crystallizer in which the sulfide was recovered and mixed with the metals.
MATERIALS AND METHODS
Hydrothermal vent sediments
The hydrothermal vent marine sediments were collected from Nayarit, Mexico (20º 44’ 12” N, 105º 28’ 641” W) and the handling was conducted as indicated in Guerrero-Barajas and García-Peña (2010).
Culture medium
The culture medium utilized in the entire experiment was as follows (in g/L): K2HPO4, 1.2; KH2PO4, 1.6; CaCl2·2H2O, 0.02; MgCl·6H2O, 0.166; NaCl, 2; NH4Cl, 0.56; yeast extract, 0.04; vitamins solution, 5 mL/L, and trace metals solution, 2 mL/L. The trace metals solution composition was as follows (in g/L): H3BO3, 0.05; FeSO4·7H2O, 2.8; ZnSO4·7H2O, 0.106; MnSO4·7H2O, 0.70; (NH4)6Mo7O24·4H2O, 0.05; AlK (SO4)·12H2O, 0.175; Na3Co (NO2)6 3.4; NiSO4·6H2O, 0.026; CuSO4·5H2O, 0.175; EDTA, 1, and resazurin, 0.2. The vitamins solution composition was as follows (in g/L): biotin, 0.02; dehydrated folic acid, 0.02; pantothenic acid, 0.05; nicotinamide, 0.05; p-aminobenzoate, 0.05; thiamine, 0.05; lipoic acid, 0.05, and piridoxine, 0.1. The sources of sulfate and carbon were Na2SO4, and acetate or a mixture of acetate and butyrate, respectively.
Enrichment of the sediments under sulfate-reducing conditions
The sediments were enriched in batch assays under sulfate-reducing conditions prior to the inoculation of the reactor. The enrichment process consisted of inoculation of a reactor (1 L flask) with 300 g of wet sediment (1.12 g VSS/L). The reactor was maintained in the dark at room temperature for 13 d with manual shaking once a day. After each batch, fresh culture medium was added. A total of four successive batches were conducted. The first and second batches were carried out with a COD/SO4 -2 ratio of 0.67. The source of sulfate was Na2SO4 at a concentration of 1.47 g/L (1.0 g/L SO4 -2) and acetate (0.627 g/L) was used as the carbon and energy source. The cultures were supplemented with mineral medium. In the third and fourth batches the COD/SO4 -2 ratio of 0.67 was maintained, but the source of carbon and energy was a mixture of acetate and butyrate at a COD ratio of 2:1. After the fourth batch the enrichment was used as inoculum for the reactor, at this point the efficiency on the removal of sulfate was approximately of 60%.
Implementation and monitoring of the UASB reactor for production of sulfide
A UASB reactor (1.03 L ID = 6.4 cm) was set to be operated continuously under sulfate-reducing conditions (Fig. 1 ). Biogas was measured in a gas displacement column filled with a saturated NaCl solution to prevent biogas dissolution in the liquid. The UASB reactor was inoculated with 300 g (1.2 g VSS/L) of enriched sediments and operated at room temperature. The reactor was initially fed with acetate (0.562 g/L) and butyrate (0.162 g/L) as carbon and energy source at a COD ratio of 2:1 and supplemented with mineral medium. The reactor was operated for more than 200 days at room temperature with an initial pH of 6.2 ± 0.3 and a COD/SO4 -2 ratio of 0.9. The source of sulfate was Na2SO4 at an initial concentration of 1 g SO4 2-/L. The operation period consisted of six stages (Table I). These stages were divided into two groups: in the first group (stages I-III) the HRT was evaluated and in the second group (stages IV-VI) the sulfate loading rate was studied. During the first three stages, the HRT was increased from 1.4 to 3 d. From the third stage on, the HRT was maintained at 3 d, whereas in stages IV and V the concentration of sulfate was increased in the feeding. The sulfate concentration in stage V was twice the concentration of sulfate used in stage III. Stage VI was conducted under the same conditions than stage IV, to maintaining a high sulfate removal efficiency.
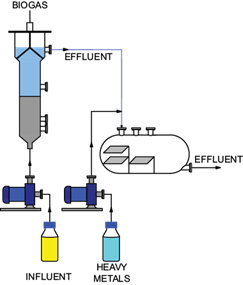
Fig. 1 Schematic diagram of the sulfidogenic an up-flow anaerobic sludge blanket reactor coupled to a crystallizer.
TABLE I OPERATION STAGES OF THE UP-FLOW ANAEROBIC SLUDGE BLANKET SULFIDOGENIC REACTOR.
| Stage | HRT (d) | SO4 -2 (mg/L/d) | COD (mg/L/d) |
| I | 1.4 | 713.3 | 642.0 |
| II | 2.5 | 400.0 | 360.0 |
| III | 3.0 | 332.8 | 300.0 |
| IV | 3.0 | 500.0 | 450.0 |
| V | 3.0 | 665.7 | 599.1 |
| VI | 3.0 | 500.0 | 450.0 |
HRT: hydraulic retention time; COD: chemical oxygen demand.
Heavy metals precipitation in the crystallizer with sulfide from the UASB reactor (two-stage system)
The coupling of the UASB and the crystallizer was carried out in the half of the third UASB reactor stage of operation, when high sulfate removal and high production of sulfide were reached. At this time, the experiments for the precipitation of metals were carried out. The crystallizer was continuously fed with a CuCl2•2H2O solution whose concentration in copper was 75 to 150 mg Cu2+/L (Fig. 1). In terms of loading rates, this interval of Cu2+ concentrations corresponds to 25 to 50 mg/L/d. The copper solution was received in the crystallizer along with the effluent from the UASB reactor and the metal precipitation proceeded. Additionally to copper, during stage VI Zn2+ and Al3+ were also evaluated. On day 150, the Cu2+ was replaced by Zn2+ (100 mg/L). On day 166, the Zn2+ was replaced by Al3+ (100 mg/L). Finally, a mixture of Cu2+, Zn2+, and Al3+ (at a concentration of 50 mg/L each) was added to the crystallizer.
Analytical methods
Sulfate was quantified by a turbidimetric method at 420 nm according to standard methods (APHA 1998), which consist of the precipitation of the sulfate ion with barium chloride. The dissolved sulfide (HS-) was determined spectrophotometrically according to the methylene blue method reported by Trüper and Schlegel (1964) with several modifications; 200 µL of the sample were added to a 25 mL volumetric flask containing 5 mL of a zinc acetate solution (2%), plus 2.5 mL of p-phenylenediamine sulfate solution (0.2% in 20% H2SO4) and 0.125 mL of FeNH4(SO4)2 solution (10% in 2% H2SO4). The flask was filled up to 25 mL with distilled water and then shaken. Finally, the absorbance of the samples was read at 670 nm. The undissociated sulfide (HS-) was calculated from the equilibrium diagram pH vs. sulfide species provided by Lewis (2010). H2S was calculated following Henry’s Law with the corresponding partitioning coefficient (García-Solares et al. 2014). COD and VSS were determined according to standard methods (APHA 1998).
The sample was placed in the oven at 105 ºC during 1 h to obtain total suspended solids (TSS); afterwards, it was placed in the muffle at 550 ºC during 1 h to obtain fixed suspended solids (FSS). VSS was obtained by the difference between TSS and FSS. Copper, zinc, and aluminum were determined using a mass spectrometer with inductively coupled plasma (Perkin Elmer), with argon as carrier gas with a flow of 16 L/min. Prior to analysis, samples were passed through a 0.45 μm filter and then they were acidified with nitric acid, hydrochloric acid, and hydrogen peroxide in a 2.5: 1.2: 0.6 proportion, respectively. Also, the acidified solid samples were heated until dissolving the precipitate. Metal sulfide precipitates were characterized by scanning X-ray diffraction (XRD). Previous to this analysis, samples were dried at 105 ºC. Gas samples (200 µL) for CH4 analysis were taken every other day and analyzed using a GOW Mac Seri 580 chromatograph under the following conditions: temperatures for the column, detector and injector were 25, 120, and 75 ºC, respectively; the nitrogen flow rate was 30 mL/min, using a silica gel column (60/80; 18” × 1/8” × 0.085”, Alltech).
RESULTS AND DISCUSSION
Development of sulfate reduction in the enriched sediments
The results obtained from the batch assays for the obtainment of a sulfidogenic sludge are shown in table II. Sulfate removal efficiencies (Esulfate) increased as the batch assays progressed; however, when acetate was used as electron donor Esulfate and COD removal efficiencies (ECOD) were low; conversely, when a mixture of acetate and butyrate was used, both efficiencies increased up to values of 60 and 80%, respectively. These results agree with previous studies carried out with sulfidogenic sludge generated from hydrothermal vent sediments (González-Paz et al. 2020) in which acclimation of the sludge to acetate as the only carbon source was conducted (sulfate reduction ~ 70% and COD removal 70-80% with acetate/butyrate mixtures). This suggests a similarity in the behavior of the microbial community developed in the sludge under these conditions.
TABLE II SULFATE AND CHEMICAL OXYGEN DEMAND (COD) REMOVAL (%) OBTAINED IN THE SULFATE REDUCING BATCH CULTURES.
| Original sediment | First subculture | Second subculture | Third subculture | |
| Electron donor | Acetate | Acetate + butyrate | ||
| Sulfate removal (%) | 24.17 | 14.5 | 53.4 | 60.2 |
| COD removal (%) | 1.7 | 27.9 | 62.5 | 80.4 |
SRB may be divided into two groups. The first one presents the capability of degrading completely the organic matter to CO2, and the second one of oxidizing organic matter to acetate (Muyzer and Stams 2008). In sulfidogenic sludge developed with these sediments, we had previously observed bacteria of the genera Desulfovibrio, Desulfomicrobium, and Desulfotomaculum when the source of carbon was a mixture of butyrate, propionate, and acetate (Guerrero-Barajas et al. 2014). It has been observed that species of the genera Desulfovibrio and Desulfomicrobium do not oxidize acetate (Liamleam and Annachhatre 2007); therefore, the addition of butyrate as source of carbon along with acetate favored the growth of these species and Esulfate and ECOD increased when butyrate was included as electron donor. Furthermore, it has been reported that the identification of sequences related to members of the families Desulfobulbaceae and Desulfobacteriaceae in sediments could suggest the presence of bacteria capable of directly coupling butyrate oxidation (as electron donor) to sulfate reduction (Struchtemeyer et al. 2011). This direct coupling is associated to much faster growth rates of bacteria compared to those observed as a result of synthrophic metabolism in microorganisms (Oude-Elferink et al. 1994). Desulfovibrio belongs to the Desulfobulbaceae family; thus, it is possible to infer that the sediment utilized in the present work may contain microorganisms that degrade butyrate directly, which could explain the improvement observed in the process when butyrate and acetate are combined in contrast to the utilization of acetate only, since this process is tightly linked to the performance of microorganisms present in the sediment. Therefore, this sludge allows the consumption of acetate/butyrate, which are frequent residuals in the sulfate reduction process when lactate, sucrose or glycerol are utilized as electron donors (Costa et al. 2021).
Hydrogen sulfide production at different HRT in the UASB reactor
The UASB reactor was operated under continuous regime with a COD/SO4 -2 ratio of 0.9 and the operation period was divided into several stages. The stoichiometric COD/SO4 -2 ratio is 0.67, which is 0.67 mol of COD required to reduce 1 mol of sulfate. Thus, COD was in excess to sustain bacterial growth. During the first three stages, the HRT was varied. The sulfate loading rates and sulfide total production in the UASB reactor are shown in figure 2, where it can be seen that sulfate consumption increased when HRT was increased from 1.4 to 3 d (stage I to III). When the HRT was 1.4 d (stage I), the sulfate loading rate in the influent was of 241.8 ± 12.0 mg of SO4 -2-S/L/d and the production of total sulfide obtained was of 45.5 mg/L/d (Table III). This stage was maintained during 14 d. The removal efficiency of sulfate was lower than 30% with a yield of HS- (YHS-) of 0.63 ± 0.2. In the second stage, the HRT was increased to 2.5 d to optimize and increase the reduction of sulfate. These operating conditions were maintained for 7 d. In this period the sulfate loading rate was of 128.9 ± 5.8 mg/L/d and the total sulfide obtained was of 53.3 S mg/L/d, with a YHS- of 0.77 ± 0.15.
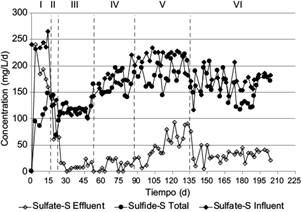
Fig. 2 Sulfate (SO4 -2) consumption at different hydraulic retention times (HTR) and sulfate loading rates in the up-flow anaerobic sludge blanket reactor. HRT stage I = 1.4 d; stage II = 2.5 d; stages III, IV, V, and VI = 3 d.
TABLE III MASS BALANCE OF SULFUR SPECIES AT A CHEMICAL OXYGEN DEMAND (COD)/SULFATE (SO4 -2) RATIO OF 0.9.
| Stage | HRT (d) | SO4 -2 -S influent (mg/Ld) | SO4 -2 -S effluent (mg/Ld) | HS--S (mg/Ld) | H2S aq (mg/Ld) | H2S gas (mg/Ld) |
| I | 1.4 | 241.8 ± 12 | 169.8 ± 70.6 | 31.8 ± 3.5 | 13.15 ± 1.36 | 0.55 ± 0.06 |
| II | 2.5 | 128.9 ± 5.8 | 60.5 ± 4.5 | 40.6 ± 9.7 | 12.19 ± 7.24 | 0.51 ± 0.30 |
| III | 3.0 | 110.3 ± 4.9 | 12.5 ± 7.2 | 104.3 ± 13.5 | 17.73 ± 6.31 | 0.73 ± 0.274 |
| IV | 3.0 | 165.8 ± 14.2 | 11.3 ± 10.1 | 125.6 ± 0.8 | 13.52 ± 2.39 | 0.56 ± 0.01 |
| V | 3.0 | 219.2 ± 9.3 | 47.2 ± 24.4 | 105.62 ± 25.1 | 63.66 ± 37.51 | 2.67 ± 1.5 |
| VI | 3.0 | 169.2 ± 18.3 | 32.9 ± 17.1 | 78.1 ± 24.7 | 75.73 ± 23.95 | 3.18 ± 1.01 |
HS-: dissolved sulfide (dissociated sulfide); H2S: aqueous sulfide (undissociated sulfide).
In the third stage the HRT was 3 d, and the reactor was operated for 27 d with a sulfate loading rate of 110.32 ± 4.9 mg SO4 -2-S/L/d with a YHS- of 1.2 ± 0.08. In the case of a YHS- >1, this may indicate that part of the sulfate present in the sediment was solubilized in the medium becoming bioavailable to microorganisms. It has been suggested that in sediments, sulfate may be associated to carbonates, such as for example calcites (Rennie and Turchyn 2014). The sulfate removal efficiency increased to 84% from the third day of operation on. The production rate of total sulfide was of 122.7 ± 13.2 mg of S/L/d. It can be said that the reactor at this point was operating at steady state and the COD was in excess to support growth.
These results indicate that the ECOD and ESO4 -2 increased when the HRT was increased. The ESO4 -2 increased up to three-fold when the HRT increased from 1.4 to 3 d (Table IV). In this regard, Mukwevho et al. (2020) observed that when HRT decreased from 7 to 2 d, sulfate reduction diminished more than 50%; however, when they added more substrates to the process, the sulfate reduction was recovered although it was slightly lower than that obtained with the highest HRT. The utilization of low HRT has also been associated to a decrease in the removal efficiencies of COD (Wei et al. 2018). On the other hand, the utilization of a low HRT has been associated to a washing of the biomass in the reactor (Sipma et al. 2007); thus, under the operation conditions utilized in the present work, it is possible that the higher HRT favored the steady presence of microorganisms in the reactor. Additionally, when HRT was increased sulfate loading rates decreased, therefore the increase in efficiencies could be due to the combined effect of both factors. However, low sulfate consumption and small sulfide production were observed at HRT of 1.4 d, even when loading rates were higher than those at 3 d, hence this behavior could not be associated to a possible inhibition of the process by the sulfide produced, which could explain the low efficiency obtained. Additionally, sufficient reducing power was supplied for sulfate reduction, so the observed behavior was mainly associated to the effect of HRT. The concentration of dissolved sulfide increased up to 312.9 mg/L (OLR = 104.3 mg/L/d) at HRT of 3 d. Polo et al. (2006) used lactate as source of electrons and obtained a sulfide concentration of 190 mg/L at HRT of 20 h. Moreover, Kaksonen et al. (2004) observed that, for values of HRT below 12 h, acetate was accumulated in the reactor, whereas in the present work a sulfide concentration of 368.1 mg/L at HRT of 3 d was obtained. Thus, the HRT is an important parameter that can be used to control the production of sulfide and the removal efficiencies of the substrates.
TABLE IV REMOVAL EFFICIENCIES (E) OF SULFATE (SO4 -2), CHEMICAL OXYGEN DEMAND (COD) AND HEAVY METALS.
| Stage | ESO4 -2 (%) | ECOD (%) | ECu (%) | EZn (%) | EAl (%) |
| I | 29.4 ± 11.9 | 92.3 ± 3.9 | ______________ | _______________ | ______________ |
| II | 53.1 ± 2.3 | 44.7 ± 4.5 | ______________ | _______________ | ______________ |
| III | 88.6 ± 6.6 | 86.5 ± 2.5 | 98.3 ± 1.7 | _______________ | ______________ |
| IV | 93.5 ± 5.6 | 84.6 ± 9.1 | 98.5 ± 1.2 | _______________ | ______________ |
| V | 78.6 ± 10.6 | 86.1 ± 4.8 | 99.1 ± 0.5 | _______________ | ______________ |
| Vla | 80.8 ± 9.1 | 77.3 ± 10 | 99.2 ± 0.3 | 99.2 ± 0.5 | 98.6 ± 0.3 |
| VIb (mixture) | 80.8 ± 9.1 | 89.4 ± 5.1 | 82.7 ± 16.8 | 86.5 ± 11.5 | 81.3 ± 12.3 |
aThe addition of each metal was evaluated separately; bthe addition of a mixture of the three metals was evaluated.
ECOD,: removal efficiency of the chemical oxygen demand; ESO4-2: removal efficiency of sulfate; ECu: removal efficiency of copper; EZn: removal efficiency of zinc; EAl: removal efficiency of aluminum.
Hydrogen sulfide production at increased loading rates in the UASB reactor
Once the reactor reached steady state and was physiologically stable, it became robust enough to tolerate increments in the concentration of sulfate. To increase the production of total sulfide, the HRT was maintained at 3 d and the concentration of the substrates was increased in the influent maintaining the ratio of COD/SO4 2- = 0.9. Thus, in the fourth stage the concentration of sulfate was increased to 1.5 g/L. The COD/SO4 2- ration has been proved to exert an effect on the sulfide production. Cunha et al. (2019) observed that the maximum production of dissolved sulfide was obtained at a ratio of COD/SO4 2- = 0.8, being higher in 18% than the obtained at the stoichiometric ratio (COD/SO4 2- = 0.67). The sulfate loading rate in the influent was 165.8 ± 14.2 mg of SO4 -2-S/L/d, while the total sulfide production was of 139.6 ± 24.4 mg S/L/d and the YHS- was 0.9 ± 0.1. In the fifth stage the reactor operated 44 d at a concentration of 2.0 g SO4 -2/ L. During this time the sulfate loading rate was 219.2 ± 8.7 mg of SO4 -2-S/L/d and the production of total sulfide was of 171.9 ± 37.7 mg of S/L/d and the YHS- was 1.02 ± 0.2. The implemented strategy made possible that sulfate loading rates in the fifth stage were similar to those maintained in the first stage, but with high sulfate removal efficiencies and high total sulfide production. However, from day 115 on, the efficiencies of sulfate consumption decreased, reaching 60% on day 129; therefore, it was decided to decrease the concentration of the sulfate fed to 1.5 g/L in the sixth and final stage. In this regard, it has been reported that a long period of exposure to sulfide may cause failure of the process, even though it is not yet clear which is the inhibitory concentration of sulfide; however, some concentrations have been mentioned, for example 110 mg S/L (Jing et al. 2013, Lu et al. 2016). Nevertheless, the inhibitory sulfide concentration may depend on the operation conditions and characteristics of the inoculum, among other reasons. In the sixth and final stage the sulfate loading rate was of 169.2 ± 18.2 mg of SO4 -2-S/L/d and the production of total sulfide was of 157.01 ± 49.6 mg S/L/d, with a YHS- of 1.16 ± 0.3. The sulfate removal efficiency was recovered on day 7 of operation, and it remained at ~ 80% during this last stage. In this sense, it has been reported that sulfate reduction is optimal to a relatively low concentration of sulfate (i.e., 0.5 g SO4 -2/L), though a significant inhibition by higher concentrations has not been observed (Isa et al. 1986).
Some other parameters have been studied to increase the production of sulfide. Velasco et al. (2008) evaluated the effect of the COD/sulfate ratio and found that the maximum dissolved sulfide concentration achieved was of 470 mg S/L at a COD/SO4 -2 ratio of 2.5. In the present work the maximum dissolved sulfide concentration was 376.8 mg S/L obtained in stage IV at an initial sulfate concentration of 1500 mg SO4 -2/L and HRT of 3 d.
The formation of methane was not observed in this work. One possible explanation is that the production of dissolved sulfide from the second stage was > 100 mg S/L, and the inhibition of methanogenic activity has been reported from 90 mg S/L (Koster et al. 1986). Additionally, a ratio of COD/SO4 -2 ˂ 2 has been related to an improvement in sulfidogenesis that would suppress methanogenesis due to the competition for electrons and inhibition of methanogens by sulfide (Lu et al. 2016). Another possible explanation is that the mineral medium does not contain cobalt, a metal that has been reported to greatly stimulate methanogenesis cobalt greatly stimulated methanogenesis (Florencio et al. 1993, González-Gil et al. 1999).
Evaluation of heavy metals precipitation using sulfide from the UASB reactor (two-stage system)
In the third stage, the crystallizer was coupled to the UASB reactor to receive its effluent; then, the feeding of copper solution started under continuous mode. The performance of the process during the metal removal is shown in figure 3, where it can be seen that the process was evaluated at loading rates of 25, 33, and 50 mg Cu2+/L/d. High removal efficiencies were obtained in all cases regardless of the increase in the loading rates (Tables III and IV). In the fourth stage, concentrations of copper of 25 and 33 mg Cu2+/L/d were evaluated, whereas in the fifth stage copper concentrations were of 33 and 50 mg Cu2+/L. The sixth stage was divided further into several stages. In the first stage Cu2+ (50 mg/L/d) was fed and subsequently replaced with Zn at a loading rate of 33.3 mg Zn2+/L/d for two weeks. Afterwards, Al3+ was fed at a concentration of 33.3 mg/L/d for a week. Finally, the crystallizer was fed with a solution of Cu2+, Zn2+, and Al3+ at a loading rate of 16.7 mg/L/d each. Table IV shows the removal efficiencies of metals at the outlet of the crystallizer. It can be observed that when the metals were fed separately the removal efficiencies obtained were higher than 98% and the final concentration was < 1 mg/L or below detection limits. These results are consistent with different works in which sulfide has been used to obtain high removal efficiencies for metals (Álvarez et al. 2007, Velasco et al. 2008). For example, Najib et al. (2017) reported removal percentages of 99.99 and 96.87 for Ni and Zn, respectively.
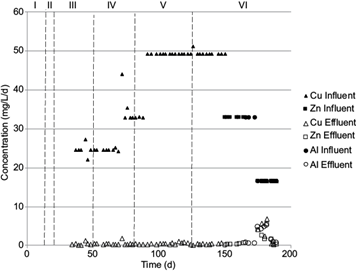
Fig. 3 Heavy metals removal at different hydraulic retention times and sulfate loading rates in the crystallizer.
The stoichiometric equations for the formation of metal sulfide are:
Based on equations 1, 2, and 3 it is possible to estimate the theoretical amount of HS- required to precipitate the metal. For example, to precipitate 150 mg/L of Cu2+ a concentration of dissolved sulfide of 78.1 mg S/L is needed. In the present work, from the third stage on, the average concentration of dissolved sulfide generated in the UASB reactor was of 312.9 mg S/L; therefore, sufficient reducing power for the precipitation of Cu2+ was generated.
However, when the mixture of metals was used efficiencies decreased to 17% with respect to the individual feeding of metals, despite having sufficient reducing power. This may be due to the possible interaction between the metallic sulfides or ions. In this sense, Gómez (2020) evaluated the formation of sulfides with cadmium and zinc ions, finding a possible competition between metal ions since cadmium showed preference in the formation of sulfides over zinc; this could be related to the value of the solubility product and the ionic radius, which are greater in the case of cadmium. The ionic radius of Zn2+, Cu2+, and Al3+ are 0.74 Å (Minchola and Angelats-Silva 2019), 0.73 Å (Lee et al. 2018), and 0.53 Å (Ríos-León et al. 2017), respectively. Therefore, the highest preference for the formation of metal sulfides would be zinc, followed by copper and lastly aluminum, which coincides with the removal efficiencies obtained. Moreover, some researchers have evaluated selective precipitation of metals at low pH values to improve metal recovery (Sahinkaya et al. 2009); however, this strategy generates effluents that must be neutralized before being discharged, implying an additional process.
Experimentally, the formation of a black or grey-green precipitate indicates the formation of metal precipitates. Precipitates obtained in this work were analyzed by XRD. The following compounds were identified as possible components of the precipitates: CuS (Fig. 4), Cu2S, and Cu6S6. This information corroborates the formation of metal sulfide that was recovered by sedimentation. The precipitates formed with Zn, Al, and the mixture of metals are being analyzed.
The metals recovery was estimated below 57%. This can be explained by the formation of very small particles of difficult sedimentation that can only be recovered by filtration, which were washed out of the crystallizer. This result agrees with the findings of Mokone et al. (2012), who obtained a copper recovery < 40% due to the generation of highly small copper particles and reported a particle size of 0.01 μm. A recent review carried out by Kumar et al. (2021) indicates that nanoparticles generated during the formation of metal sulfides could have several biotechnological applications that need to be studied, therefore their recovery is necessary. The utilization of a two-stage system allows a precipitation carried out chemically in the absence of microorganisms, which could facilitate the long-term operation and the recovery of metal sulfides, allowing an environmentally friendly process.
A survey conducted on the precipitation of metals in two-stage systems in which acetate or butyrate are utilized as electron donors to sustain sulfate showed scarce results. For example, Sahinkaya et al. (2009) precipitated Cu2+ and Zn2+ with biogenic sulfide from streams containing metals concentrations in the range of 50-100 mg/L, achieving a 100% removal of Cu2+ and an 84-98% removal of Zn2+. These authors obtained the sulfide in a sulfate reducing bioreactor from an initial sulfate concentration of 2000 mg/L, achieving a sulfate removal of 65% and generating 320 mg/L of sulfide at 35 ºC. They reached a COD removal of 85% in the sulfate reducing process, which was sustained with lactate and ethanol. In the present work, the Cu2+ concentration was in a range of 75-100 mg/L and its removal was in a range of 82.7-99.2%, whereas sulfate reduction was carried out in a range of 78.6-93.5% at room temperature, along with a COD removal of 77.3-89.4% with acetate-butyrate as electron donor. The sulfide concentration obtained was 312.9-376.8 mg/L. This confirms that the system proposed in the present work is feasible and allows the utilization of low-chain volatile fatty acids to promote sulfate reduction as a source of sulfide to recover metals in a separate unit.
CONCLUSIONS
A two-stage system to remove sulfate, COD and recovery of metal precipitates was developed using a sulfidogenic sludge generated from hydrothermal vent sediments in a UASB coupled to a crystallizer. The sludge derived from these sediments presented the metabolic capability to produce and tolerate high concentrations of sulfide. The increase of HRT from 1.4 to 3 d in the sulfidogenic reactor promoted a more efficient reduction of sulfate; therefore, an increase in the production of sulfide utilizing acetate/butyrate as electron donors at room temperature (18-21 ºC). The sulfide produced in the first stage (UASB reactor) was used for the precipitation of Cu2+ in the second stage (crystallizer), in which different precipitates were obtained. An increase in the sulfate loading rate resulted in a maximum concentration of sulfide. The maximum concentration of sulfide obtained was of 376.8 at 500 mg SO4 -2 mg S/L. The removal efficiencies of copper, zinc, and aluminum were higher than 98% when the metals were fed separately. However, when the mixture of metals was added the efficiencies decreased by 17% with respect to the individual feeding of metals. This behavior could be due to competition of the metal ions for sulfide. The XRD analysis indicated the formation of crystal structures mainly composed by CuS, Cu2S, and Cu6S6 when copper was used. It can be concluded that the precipitation of metals using sulfide produced by sludge obtained from sediments in a two-stage system, is a promising strategy in bioremediation and treatment of different effluents containing metals.











 nueva página del texto (beta)
nueva página del texto (beta)



