ECOSISTEMAS COSTEROS TROPICALES
The tropical zone is located between 24° north and south latitudes, delimited by the Tropics of Cancer and Capricorn, respectively. It is one of the largest areas on the planet, while the subtropical region is located between the tropical and temperate zones, between 24°and 40° north and south latitudes. These regions are characterized by fewer temperature fluctuations and constant rainfall, mainly near the equator. The tropics account for 40% of the planet´s total surface area and harbor a greater diversity than temperate and cold latitudes, which is distributed in terrestrial, marine and coastal ecosystems (Willing et al., 2003).
Tropical coastal areas have diverse ecosystems, including coastal lagoons, estuaries, and mangroves. These ecosystems are extremely complex, they are transition zones between freshwater terrestrial drainage and the coastal marine zone; this mixture of water with different salinity levels creates brackish conditions (Pérez-Ruzafa et al., 2011). Despite of this common characteristic, each one has specific properties that distinguish them from each other.
Estuaries are more common on the coasts of temperate climates, where freshwater inflow is sufficient to keep the sea mouth open (Harris, 2008). The main axis of an estuary is perpendicular to the coastline; morphologically, it is funnel-shaped and river inflow flows directly into the ocean up to the effective limit of tidal influence, diluting seawater and forming a longitudinal salinity gradient (Perillo, 1995). In addition to the salinity gradient, other chemical gradients are usually present, including a decrease in the content of organic matter and nitrogenous compounds (mainly ammonium and nitrates) from the river input area to the sea mouth, while oxygen content increases in the same direction (Webster et al., 2015).
Coastal lagoons are generally shallow bodies (most are less than 5 m deep), temporarily or permanently open to the sea, characterized by the presence of a sandy barrier that separates the lagoon from the ocean and connection to the sea is maintained through the mouth or tidal channels (De Wit et al., 2001). The main axis of the lagoon is parallel to the coast and most lagoons are connected to a freshwater continental basin through river inputs, some permanent and some temporary. In tropical latitudes, these inputs can fluctuate considerably, with minimum volumes during the dry season and maximum volumes during the rainy season, due to increased precipitation. In contrast to estuaries, coastal lagoons are common in tropical and subtropical coasts, where precipitation patterns are highly seasonal, resulting in significant fluctuations in river discharge and associated hydrological gradients, which are usually more complex than those in estuaries and which influence on ecosystem functioning (Barnes, 2001; Harris, 2008).
Mangrove forests are coastal wetlands constituted by woody trees and affected by tidal and freshwater influences. They are ecosystems adjacent to coastal lagoons and estuaries. Mangrove roots are adapted to flooded and anoxic soils that favor the development of anaerobic metabolisms, including methanogenesis (Taketai et al., 2010). Mangrove forests support an important food web based on detritus, they are ecologically important, protect the coastline and act as sediment and nutrients traps (Holguin et al., 2001).
Coastal lagoons, estuaries and mangroves are areas of high biodiversity and productivity due to high nutrient content, which are ecologically viable for fisheries; provide different ecosystem services such as nutrient retention, flood control and sediment stabilization, as well as social benefits (Knoppers, 1994; Cloern et al., 2014; Pérez-Ruzafa et al., 2019). Despite their importance, estuarine ecosystems are among the most vulnerable environments to climate change and anthropogenic activities (urbanization, industrialization, tourism, agriculture, livestock, fishing) that introduce various pollutants and lead to eutrophication issues and oxygen deficits (Esteves et al., 2008; Howarth et al., 2011).
In estuarine-coastal lagoon and mangrove ecosystems, biodiversity research has been centered on macroorganisms and less attention has been paid on microorganisms, particularly on the group of prokaryotes and much less on archaeal groups. The goal of this contribution was synthesized, as far as possible, the knowledge of the Archaea Domain, their diversity, metabolism, and distribution in tropical and subtropical coastal ecosystems.
METHODS
A descriptive review of the information available in Academic Google and Scopus databases was carried out. In the first phase of the search, general keywords were used: archaea+tropical coastal zones, archaea+subtropical coastal zones, archaea+tropical or subtropical coastal lagoons, archaea+tropical or subtropical estuaries, archaea+mangroves. In the second phase, the name of the Superphyla was included as keyword and other specific keywords were used.
From the available information, articles that were published from 2010 to date were selected, however, some articles with a publication time greater than ten years (2000-2010) were considered due they contained information on the archaea identified in coastal ecosystems, and which mainly included the group of methanogens related to the objective of this work. In some cases, secondary references from these contributions were consulted to clarify concepts or to be precise in questions do not solve yet.
As a result of the review, 68 articles were selected, 14 included characteristics of archaea and perspectives related to their evolution, diversity, and ecology. 20 publications contained information on the distribution of archaea in tropical estuarine environments and their relationship with environmental characteristics. Finally, 34 articles included joint information on diversity, distribution, and ecological processes (participation in biogeochemical cycles).
ARCHAEA. CHARACTERISTICS AND CLASSIFICATION
Estuarine ecosystems and mangroves are important reservoirs of organic matter of both autochthonous origin (produced by the ecosystem itself) and allochthonous origin (derived from terrestrial runoff and entering through river inflow, as well as from adjacent vegetation). This organic matter, along with nutrients, favor the development of a complex microbiota, bacteria, and archaea, that contribute to maintain the health of the ecosystem (Danovaro & Pusceddu, 2007).
Archaea are microscopic, single-celled, prokaryotic organisms originated approximately 2.6-2.8 G years ago. One of the accepted phylogenetic hypotheses mentions that Archaea, together with Eukarya, originated from a last common ancestor, possibly hyperthermophilic, more recent than LUCA (Last Universal Common Ancestor), for which these two domains are considered sister lineages (Woese et al., 1990; Gribaldo & Brochier-Armanet, 2006). Their name derives from the Greek “ἀρχαῖα”, which means “ancient things”, since they have so far been one of the oldest molecular structures ever studied. Archaea have unique characteristics that differentiate them from the Bacteria Domain, such as the lack of peptidoglycans in their cell wall, making them resistant to lysozymes and penicillin; they also have a cell membrane composed of a monolayer of lipids with ether bonds, instead of ester bonds (present in bacteria and eukaryotes), which give them greater thermal resistance. Their DNA replication mechanism is like Eukarya Domain, with several RNA polymerases as well as characteristic tRNAs and rRNAs (Woese et al., 1978).
Study of the Archaea Domain was initially limited because most of these microorganisms cannot be cultured in the laboratory and were considered a unique group in the microbial biosphere, since the first ones were identified in extremophilic environments (hyperthermophile, hypersaline or acidophilic). However, technological advances of culture-independent techniques, have made it possible to detect them in a wide variety of moderate environments, making clear that archaea are widely distributed throughout the biosphere and that they possess great metabolic versatility (Bhattacharyya et al., 2015).
Archaea was established as the third Domain in the phylogenetic tree of life in 1977, based on the work of Woese and Fox, who analyzed 16S rRNA sequences. Initially, this domain was divided into two major phyla: Crenarchaeota (hyperthermophiles) and Euryarchaeota (mesophiles, methanogens, and halophiles); since then, the taxonomy and phylogeny of Archaea has changed significantly, largely due to the use of culture-independent molecular techniques, which have advanced significantly in the last ten years, mainly with the application of metagenomics.
The recent development of whole metagenome shotgun from environmental communities, has contributed to the discovery of new genes, enzymes, and metabolic pathways, providing insight into the structure and function of different communities of the Archaea domain in different habitats (Hernández De Lira et al., 2014; Cortés-López et al., 2020). The analysis of metagenomic data from many archaeal lineages in environmental samples, in addition to the analysis of transcriptomes and the development of different computational tools, (bioinformatics) has changed the classification of the Archaea Domain, leading to proposals for new clades at the level of phyla, classes, and orders, as well as the designation of new names for them (Adam et al., 2017; Baker et al., 2020). The current taxonomic classification of archaea recognizes three Superphylum: Asgard, DPANN and TACK, and the Phyllum Euryarchaeota. The names of Superphylum DPANN and TACK are formed using the initials of each of the phyla that were initially included; however, in Baker’s et al. (2020) more phyla have been added to each one (Table S1). The recognized superphyla have been described based on ribosomal protein phylogenies as well as on the review of concatenated protein phylogenies (Seitz et al., 2016; Baker et al., 2020).
Superphylum Asgard (Phyla Lokiarchaeota, Thorarchaeota, Odinarchaeota, and Heimdallarchaeota). Asgard was recently described from the analysis of genomes available in public databases (NCBI, MG-RAST, GenBank), the phyla that are part of this group have been reported mainly in the sediment from aquatic environments (MacLeod et al, 2019). Asgard is apparently monophyletic and is considered key to understanding the origin of the Eukarya Domain (Seitz et al., 2019; Cai et al., 2020), as several of its members have genes encoding numerous eukaryotic signature proteins (ESPs) such as those related to cytoskeleton formation; they also have mitochondrial and plastid sequences present in eukaryotes (Petitjean et al., 2015; Baker et al., 2020) as well as some similarities to the genetic structure of eukaryotes (introns, histones, and RNA polymerases).
Superphylum DPANN (Phyla Diapherotrites, Parvarchaeota, Aenigmarchaeota, Nanoarchaeota, Nanohaloarchaeota, Altiarchaea, Pacearchaeota, Woesearchaeota). DPANN was proposed by Rinke et al. (2013), includes ultra-small archaea (0.1-0.6 μm) with reduced genomes (490 kb-1.2 Mb) (Baker et al., 2010; Adam et al., 2017) and limited metabolic capacity because they lack several essential genes that are necessary for most biosynthetic pathways. It has been suggested that they depend on the metabolism of other archaea, with some of them considered symbionts of the Thermoplasmatales (Baker et al., 2010) or parasitic ectosymbionts of the Thermoprotei class (genus Ignicoccus) (Huber et al., 2002; Rinke et al., 2013).
Superphylum TACK (Phyla Thaumarchaeota, Aigarchaeota, Crenarchaeota, and Korarchaeota) was proposed by Guy & Ettema (2011). The genetic diversity of this phylum found in environmental samples from uncultured communities revealed the existence of mesophilic groups in addition to the commonly reported acidophilic and thermophilic groups, such as Crenarchaeota. Geoarchaeota was later proposed as a phylum in the Superphylum TACK (Baker et al., 2020).
Phylum Euryarchaeota, is not classified as a Superphylum, it consists mainly of methanogenic and halophilic archaea, as well as different kinds of archaea from extremophilic environments. The methanogen group has been one of the most studied.
DISTRIBUTION AND DIVERSITY OF ARCHAEA IN TROPICAL AND SUBTROPICAL COASTAL ECOSYSTEMS
Archaea were thought to be a minor microbial component of coastal zones; however, recent studies have shown that they account for a significant component of these ecosystems. Archaea from different phyla have been found in different coastal ecosystems, nevertheless, their function and distribution in tropical coastal ecosystems are poorly understood (Cadena et al. 2019).
Table 1 shows the groups in the Archaea Domain that have been identified in estuaries, coastal lagoons, and mangroves areas from tropical and subtropical latitudes, mainly in Brazil, China, and India, with few studies in Australia, Thailand, and Mexico. Particularly in Mexico, research on archaea has focused on the study of methanogens and methane production.
Table 1 Diversity of the Archaea Domain reported from tropical and subtropical coastal ecosystems.
| Ecosystem | Location | Identified groups of Archaea | Reference |
|---|---|---|---|
| Tropical estuary (surface water) | Guanabara Bay, Brazil | Euryarchaeota (Thermoplasmatales, Methanosarcinales, Methanococcoides, Methanoplanus petrolearia (Olliver et al., 1998) Göker et al. 2015) | Vieira et al., 2007 |
| Crenarchaeota, Candidaytus Nitrosopumilus maritimus | |||
| Subtropical estuary (sediment) | Estuary of Pearl River, China | Lokiarchaeota (DSAG), Bathyarchaeota (MCG), Thorarchaeota (MBG-B), Euryarchaeota (Methanomicrobiales, Methanosarcinales, Halobacteriales), Hadasarchaea (SAGMEG) | Jiang et al., 2011 |
| Tropical estuary (surface and bottom water) | Cochin estuary, India | Crenarchaeota | Vipindas et al., 2015 |
| Tropical estuary (sediment) | Mandovi estuary, India | Euryarchaeota (Methanomicrobiota, Methanococci, Methanopyri, Halobacteria) | Khandeparker et al., 2017 |
| Crenarchaeota, Thermoprotei, Methanosarcinales,Thermoplasmatales | |||
| Subtropical estuary (sediment) | Estuary of Pearl River, China | Thaumarchaeota, Bathyarchaeota, Methanomicrobia, Parvarchaeota, Aenigmarchaeota | Liu et al., 2014, Liu X. et al., 2018 |
| Coastal lagoon | Celestún Lagoon, Yucatan, Mexico | Methanosaetaceae, Verstraetearchaeota | Cadena et al., 2019 |
| Burnett River Estuary (pore and surface water) | Australia | Methanobacteriales, Methanocellales, Methanococcales, Methanomassiliicoccales, Methanomicrobiales | Euler et al, 2020 |
| Methanosarcinales | |||
| Mangrove (sediment) | Dar-es-Salaam, Tanzania | Methanococcoides strain | Lyimo et al., 2009 |
| Mangrove (sediment) | Dar-es-Salaam, Tanzania | Euryarchaeota (MBG-D, Methanosarcinales, Methanomicrobiales, Methanobacteriales, halophilic cluster, | Lyimo et al., 2009 |
| Crenarchaeota (MBG-B, Crenarchaea) | |||
| Mangrove (sediment) | Sao Paulo, Brasil | Methanopyrus kandlery (Kurr et al., 1992) | Taketai et al, 2010 |
| Género Methanococcus, Methanosarcina cluster | |||
| Mangrove | Buri, Thailand | Candidatus Nitrosopumilus | Yasawong et al., 2013 |
| Euryarchaeota (Methanomicrobiales, Methanosarcinales, Thermoplasmatales) | |||
| Mangrove (water and sediment) | Parnaioca river, Brazil | Thaumarchaeota, Crenarchaeota, Euryarchaeota | Silveira et al., 2013 |
| Mangrove (sediment) | Chol Buri mangrove, Thailand | Thaumarchaeota (Candidatus Nitrosopumilus maritimus), Euryarchaeota (Methanobacteriales, Methanosarcinales, Methanomicrobiales, Thermoplasmatales, Candidatus Nitrososphera) | Yasawong et al., 2013 |
| Mangrove (sediment) | Sundarbans, India | Euryarchaeota (Thermoplasmatales, Marine Group II, Halobacteria, Methanomicrobia, Methanobacteria) | Batthacharyya et al., 2015 |
| Thaumarchaeota (Marine Group I) | |||
| Mangrove (sediment) | Southeastern China | Euryarchaeota,Thaumarchaeota, Lokiarchaeota, Bathyarchaeota, Nitrospirae | Zhang et al., 2019 |
| Mangrove (sediment) | Southeastern China | Woesearchaeota, Thaumarchaeota, Bathyarchaeota, Euryarchaetoa, Asgard, Aenigmarchaeota, Altiarchaeota | Zhang et al., 2021 |
In addition to the identified phyla, a significant number of new genomes from uncultured and unclassified lineages have also been reported in these ecosystems (Adam et al., 2017; X. Liu et al., 2018; Baker et al., 2020).
From an ecological point of view, communities have a series of characteristics, including biodiversity, abundance, and distribution, which together determine their organization. The organization of the community depends on different factors, such as its response to changes in physicochemical conditions (environmental gradients), and biological relationships (such as competition, predation, parasitism, symbiosis, or mutualism); as well as the performance of the community itself (null model).
Most studies of the archaea in tropical estuarine ecosystems, as well as in mangrove forests, have established that their organization seems to be closely related with their tolerance and response to the physicochemical gradients existing in these environments. Inputs of organic matter and nutrients, as well as temperature, salinity, pH, and oxygen content, are the environmental variables that have been commonly associated with the structure of archaea (Purdy et al., 2002; Hugoni et al., 2015; Zou et al., 2017; Zhang et al., 2021).
The influence of chemical gradients (mainly salinity and oxygen) existing in estuaries and coastal lagoons, apparently it could depend on the physiological versatility of the different phyla, since some archaea groups proliferate at low salinities in subtropical estuaries, while others require higher salinity (Xie et al., 2014); the same situation occurs with oxygen, according to the review carried out by Zou et al. (2020). The structure of the archaeal community may also be the result of their adaptation to anthropogenic pollution, having reported a high archaeaplankton diversity (termoplasmatales and methanogenic groups) in the polluted region of Guanabara Bay (Brazil) while in pristine areas the diversity was lower (Vieira et al., 2007). A greater diversity of archaea, mainly from the group of methanogens, was reported in mangrove sediments contaminated with hydrocarbons and heavy metals (Yasawong et al., 2013; Hu et al., 2016).
It has been determined that there are differences in the diversity of archaea between the water column and the sediment, with the lowest diversity in the former. The phyla Euryarchaeota and Thaumarchaeota (lineage Nitrosopumilales), one of the most abundant chemoautotrophs in the picoplankton, predominate in the water column, showing that light is an important variable related to their abundance and distribution. Apparently, the abundance of Thaumarchaeota decreases as the amount of light increases in the water column (Battacharyya et al., 2015; Li et al., 2015; Zou et al., 2020).
As a result of the accumulation of organic matter and its aerobic degradation, oxygen is depleted within the first few centimeters of estuarine sediments, creating anoxic conditions (Böttcher et al., 2000) and microniches favorable to the development of a greater number of archaeal species. As a result, a stratification of the archaeal community has been observed, in some cases diversity is less in the upper strata, which increases at greater depth. Bathyarchaeota, Lokiarchaeota and Woesearchaeota are predominant in the surface layer, reporting that these groups are more abundant in oxygen-limited zones (Castelle et al., 2015). Thorarchaeota and Halobacteriales were reported in the transition zone between sulfate reduction and methanogenesis, while Methanomicrobiales, Methanosarcinales and ANME-2 were found at greater depth (Jiang et al., 2011).
Distinctive phyla Bathyarchaeota, Thaumarchaeota, Woesearchaeota and Euryarchaeota have also been found in mangrove sediments. Zhang et al. (2021) determined that the diversity of the archaeal community was greater in the first 10 cm of the sediment and decreased at greater depth, reporting a stratification of the identified phyla: Woesearchaeota (the most diverse) and Thaumarchaeota were predominant from 0 to 10 cm; in contrast, Bathyarchaeota (more abundant), Euryarchaeota and Asgard archaea were predominant in the strata of 10-30 cm depth. Aenigmarchaeota and Altiarchaeota were detected as rare taxa.
It seems that the dynamics of Archaea communities in these sediments also depend on environmental factors, including temperature, salinity, pH and the concentration of ammonium, nitrate, and organic carbon, that influence each of the Archaea phyla differently. Thaumarchaeota was negatively related to salinity, pH, and total carbon concentration, and positively related to the amount of nitrite (Zhang et al., 2019). Euryarchaeota, the most abundant group and whose sequences, for the most part, are related to methanogens, has been directly related to organic matter content and negative oxidation-reduction potentials (-150 mV) (Lyimo et al., 2002; Taketai et al., 2010) and its abundance is influenced by temperature (23-35°C), and pH (6.5-8.3) (Lyimo et al., 2009; Yasawong et al., 2013; Euler et al., 2020). Other studies have shown a higher diversity of methanogens in the upper layer of the sediment compared to the deep layer, being related with high levels of nutrients, while it decreases with high concentrations of heavy metals (Jing et al., 2016).
In Indian mangroves, Thermoplasmatales was the most abundant euryarchaeal group (18.75-23.3%), followed by Halobacteriales (Bhattacharyya et al., 2015). Thermoplasmatales is an order with acidophilic members that can grow both with or without oxygen and were predominant in surface sediments, relating their presence to high concentrations of organic matter and hydrocarbon pollution; most of the sequences were affiliated to the genus Halogranum. In contrast, methanogens of the classes Methanomicrobia and Methanobacteria grew in pristine conditions.
An important aspect of community ecology is the latitudinal distribution of biodiversity, and it has been shown that biodiversity is high in tropical regions in almost all taxonomic groups. X. Liu et al. (2018) analyzed 4,000 16S rRNA gene sequences from 24 estuaries of different latitudes, seven of them located in tropical and subtropical areas of Brazil, India, and China (estuaries of the Cunha, Zuari, Urucu, Jiulong, Mandovi, Santos-Sao Vicente and Pearl rivers), and determined that Archaea diversity seems to follow the same global distribution pattern as other taxonomic groups, i.e., their diversity is great in estuaries of low latitudes and decreases towards middle and high latitudes. This same study reported that the phylum Thaumarchaeota was the predominant group (approximately 40% of the total), followed by Bathyarchaeota and Euryarchaeota, both accounting for 25% each; Woesearchaeota accounted for 6%, and the rest of the phyla made up the remaining 4%. The few temperature fluctuations in tropical areas, as well as a big influence of anthropogenic activities in coastal areas, mainly the discharge of domestic and industrial wastewater introducing terrestrial microbial populations, and high concentrations of ammonium and organic matter are factors that explain the diversity of archaea reported in tropical latitudes (Vieira et al., 2007).
ARCHAEA AND BIOGEOCHEMICAL CYCLES
The complexity of estuarine ecosystems favors the presence of specific ecological niches for the resident microbiota that participates in several biogeochemical cycles (Euler et al., 2020). Mainly on their potential metabolism, inferred from the genetic information of the different Archaea phyla, as well as the information obtained experimentally using culture-dependent techniques, it has been established that archaea, along with bacteria, play an active role in the cycles of matter and energy, both in the water column and in the sediment, and the coexistence of archaea with other microbial groups could give rise to different ecological relationships in the microbial web (Offre et al., 2013; Zou et al., 2020). The main cycles in which archaea could play a key role in coastal ecosystems are the carbon, nitrogen, and sulfur cycles.
1. Carbon cycle
Estuarine ecosystems receive organic matter of allochthonous and autochthonous origin, this organic matter is the key component of the biogeochemical carbon cycle and most of the Archaea phyla identified in coastal ecosystems of tropical and subtropical latitudes have the potential to participate in this cycle, either with heterotrophic or autotrophic metabolism, under aerobic or anaerobic conditions (Fig. 1).
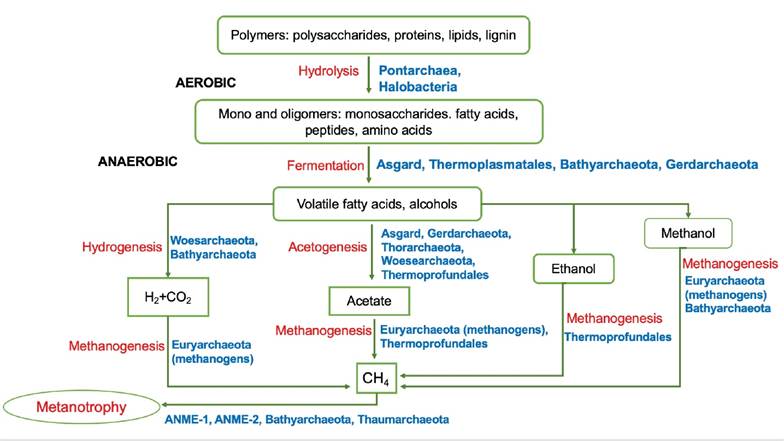
Figure 1 Schematic representation of the participation of archaea during the degradation of organic carbon. The name of the group of archaea that participates in the metabolic pathway is indicated in blue. The name of the main metabolic process is indicated in red: the decomposition of polymers is carried out with water as one of the reagents (Hydrolysis), the monomers obtained are oxidized in the absence of oxygen during Fermentation, releasing different products such as CO2, hydrogen (Hydrogenesis), acetate (acetogenesis) and alcohols (ethanol, methanol). These products will be used by different groups of archaea to form methane (Methanogenesis). Methane represents the substrate in Methanotrophy.
Most of the archaea of Superphylum Asgard could be obligate anaerobes and heterotrophs, capable of degrading different organic compounds, including amino acids, peptides and fatty acids produced by the hydrolytic activity of other microorganisms. Thorarchaeota, identified in the sulfatereduction-methanogenesis transition zone in mangrove sediments, could grow from both organic and inorganic carbon compounds (mixotrophy), and would be able to fix CO2 through autotrophic acetogenesis (Y. Liu et al., 2018; MacLeod et al., 2019). Additionally, Heimdallarchaeota would be capable of phototrophic metabolism at low oxygen conditions.
Asgard archaea also present the genes of the Wood-Ljungdahl pathway, meaning that these microorganisms can use H2 as an electron donor and CO2 as an acceptor in the reductive acetyl-coenzime A (acetyl-CoA) pathway during biosynthesis (MacLeod et al., 2019). Superphyllum Asgard could also be important in the degradation of toxic organic compounds, Lokiarchaeota is potentially capable of metabolizing halogenated organic compounds, with hydrogen-dependent growth (Sousa et al., 2016; Adam et al., 2017; Manoharan et al., 2019). Helarchaeota could degrade alkane hydrocarbons (ethane and butane) under the anaerobic conditions reported in estuarine sediments (Seitz et al., 2019); while Thorarchaeota, Heimdallarchaeota and Lokiarchaeota, by presenting malonyl-CoA and benzoyl-CoA pathways, have the potential to utilize aliphatic and aromatic hydrocarbons (Firrincieli et al., 2021).
In the Superphylum DPANN, Woesearchaeota has been identified mainly in sediments, and its genomic information suggests that it could play a role in acetogenesis and hydrogenesis; and the group could have a syntrophic relationship with methanogens (X. Liu et al., 2018).
Phylum Euryarchaeota is abundant both in the water column and in sediments. The members of this phylum can develop with or without oxygen and may play a central role in the sedimentary carbon cycle (Zou et al., 2020). In aerobic conditions, groups MG-II and MG-III, which were proposed to be grouped as Pontarchaea (Adam et al., 2017), are capable of degrading carbohydrates, lipids and proteins and could be related to the degradation of high molecular weight organic compounds (Rinke et al., 2019; Tully, 2019), while Halobacteria are most active in the degradation of organic matter in hypersaline conditions and its presence in mangrove sediments is correlated with high amounts of organic matter produced during algal blooms, Halobacteria can grow aerobically as well as anaerobically (Bhattacharyya et al., 2015; Webster et al., 2015).
Metatranscriptomic analysis suggests that Marine Benthic Group D (MBG-D, or Thermoprofundales) under anoxic conditions may be mixotrophic, having genes for autotrophic pathways, as well as to produce acetate and ethanol via fermentation, though it may also be involved in acetate utilization (Lazar et al., 2017; Zou et al., 2020). It has been suggested, based on a co-occurrence analysis, that the order Thermoprofundales could potentially interact with the phyla Lokiarchaeota and Hadesarchaeota (Zhou et al., 2019). Thermoplasmatales could degrade long-chain fatty acids and reduce sulfite.
One of the most studied groups in the phylum Euryarchaeota, related to the anaerobic carbon cycle, is the methanogens. Organic matter in brackish ecosystems is mineralized mainly in the sediment through anaerobic processes, and methanogenic archaea (MA), together with sulfate-reducing bacteria (SRB), play a key role in the later stages of anaerobic mineralization (Holguin et al., 2001; Yasawong et al., 2013). Due to the salinity gradient present in estuaries and coastal lagoons, sulfate reduction is predominant in marine zones, while methanogenesis is predominant in freshwater (Fukui et al., 1997); in this area, the highest abundance of MA has been recorded, as well as the highest production and emission of methane (Lyimo et al., 2002; Torres-Alvarado et al., 2013, 2016). The key factor that regulates this difference is the sulfate concentration (Takii & Fukui, 1991; Purdy et al., 2001, 2002).
MA can use different substrates, such as acetate and methylated compounds, as a source of carbon, or they can grow autotrophically from H2/CO2 (Thauer et al., 2008). Methanobacteriales, the most abundant order (57.7% of the total methanogenic community), with clones affiliated to the genera Methanobacterium, Methanothermobacter, Methanobrevibacter and Methanoculleus, can use H2/CO2 to produce CH4. Methanosarcinales with clones related to the genera Methanolobus, Methanomethylovorans, and Methanococcoides, can utilize methylated compounds, as well as Methanosaeta sp., an acetate obligate. Methanomicrobiales the least abundant, accounting for 11.5% of the total community, with most of the sequences affiliated with the genus Methanoculleus, is a methylotrophic methanogen (Yasawong et al., 2013).
Acetate and H2/CO2 are also important substrates for SRB, resulting in competition between SRB and MA for available hydrogen and acetate, sulfate reduction in brackish ecosystems is thermodynamically favored because it produces more energy per mole of hydrogen (∆G° of 98.8 kJ/mol) or acetate (∆G° of -43.8 kJ/mol). In comparison, methane formation produces a ∆G° of -74.8 kJ/mol and -19.9 kJ/mol with hydrogen and acetate, respectively (Howarth, 1993; Canfield et al., 2005). The coexistence of SRB and MA in estuarine and mangrove sediments is possible due the latter use non-competitive substrates such as methanol or mono-, di- or trimethylamines; this would explain why methylotrophic methanogens are the major component in these sediments (Lyimo et al., 2002). In addition, pectin is released in the early stages of tree lignin degradation and quickly hydrolyzed to produce methanol in mangrove sediments, which favors the growth of methylotrophic methanogens. It has also been reported that the species Methanosarcina semesiae Lyimo can utilize dimethyl sulfide (Lyimo et al., 2000).
Methanogenesis results in the formation of methane (CH4) and CO2, which are important gases involved in the greenhouse effect related to global climate change (Karl &Tilbrook, 1994). Estuaries, coastal lagoons, and mangroves are the main marine environments that emit methane to the atmosphere (Kreuzwieser et al., 2003).
Methane can also be used as an energy source by methanotrophic or methane-oxidizing bacteria (MOB) and by nitrifying bacteria in the oxic-anoxic interface of the sediments, both microbial groups are strict aerobes (Higgins et al., 1981; Lidstrom, 2001) and can consume approximately 90% of the methane produced (Carini et al., 2003).
Methane can also be oxidized under anaerobic conditions by some methanogenic archaea, which can use it as a source of reduced carbon (Blair & Aller, 1995). Anaerobic methane oxidation is carried out by at least two phylogenetically distinct groups of archaea, ANME-1 and ANME-2, that generally form consortia with SRB, mainly of the class Deltaproteobacteria, and with denitrifying or nitrate-reducing bacteria, the consortium metabolism involves a syntrophic relationship based on interspecific electron transfer. The archaea seem to oxidize methane, and the resulting compounds are utilized by the bacterial groups (Orphan et al., 2001; Valenzuela et al., 2017). The phyla Batyarchaeota and Thaumarchaeota could also utilize methane (Valenzuela et al., 2017; Baker et al., 2020). Methane oxidation via aerobic and anaerobic processes is biogeochemically important because it helps reduce methane emission into the atmosphere.
Several of the phyla included in Superphylum TACK are heterotrophs, while others have a chemolithoautotrophic metabolism (Baker et al., 2020). Species of the phylum Bathyarchaeota could be key microorganisms in the carbon cycle in estuarine sediments due to the abundance of organic matter, and based on their genomics, it has been suggested that they could be involved in the degradation of carbohydrates, fatty acids and proteins; some representatives of this group apparently grow under both aerobic and anaerobic conditions (Adam et al., 2017); the latter are characteristic of estuarine sediments where such archaea could play an important role in fermentation processes, mainly in acetogenesis from H2+CO2, as well as in the methane cycle, since they can be involved in methyl-dependent methanogenesis or in dissimilatory oxidation of methane. It has been suggested this phylum could also play a role in the degradation of recalcitrant organic compounds such as lignin, abundant in mangrove sediments. Some representatives of this group could have bacteriochlorophyll, suggesting they could carry out photosynthesis (Jiang et al., 2011; Kubo et al., 2012; Evans et al., 2015; Lazar et al., 2016; Yu et al., 2018; Zhou et al., 2018; Baker et al., 2020; Zou et al., 2020). The abundance of Bathyarchaeota is positively related with high concentrations of nutrients and organic matter in the sediments, and to salinity, existing freshwater, and saline groups (Zou et al., 2020).
A second important phylum is Gerdarchaeota, with members apparently facultative, capable of utilizing organic carbon compounds under aerobic or anaerobic conditions; in anoxic sediments, they could be capable of carrying out a fermentation process, producing acetate (acetogenesis), though they could also grow lithoautotrophically with H2 and CO2 (Cai et al., 2020).
2. Nitrogen cycle
The nitrogen cycle involves compounds with different oxidation-reduction states. The cycle between the different forms of nitrogen is the base for several microbial metabolisms, both aerobic and anaerobic. Results of metagenomic and metatranscriptomic studies suggest that different phyla of archaea could be participating mainly in the fixation of molecular nitrogen into ammonium, as well as in the nitrification, denitrification, and disassimilatory reduction of nitrate (Fig. 2).
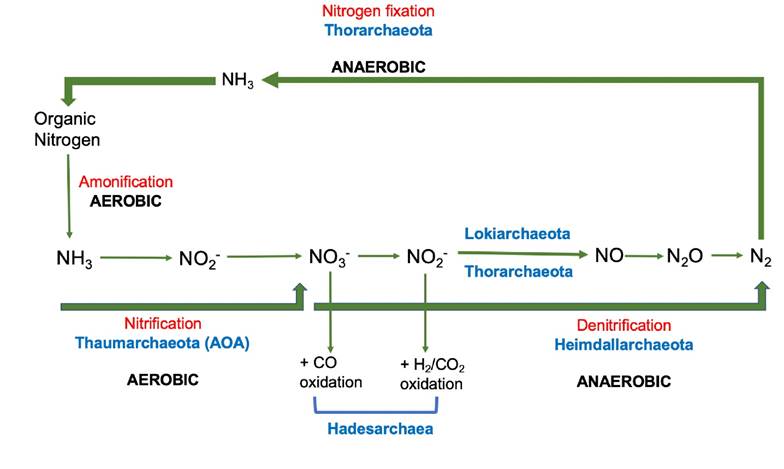
Figure 2 Schematic representation of the participation of archaea in nitrogen cycle. The name of the group of archaea that participates in the metabolic pathway is indicated in blue. The name of the main metabolic process is indicated in red: the decomposition of organic nitrogen involves aerobic and anaerobic processes. In the presence of oxygen, organic N is transformed into ammonium (ammonification), which is subsequently converted into nitrates, releasing nitrites as an intermediate product (nitrification). Under anaerobic conditions, nitrates are transformed into molecular nitrogen. This compound is assimilated in the absence of oxygen to form organic molecules.
Nitrification is an aerobic metabolism, where ammonium (NH3) is transformed to nitrates (NO3-) via nitrites (NO2-) involving ammonium oxidizing archaea (AOA) and ammonium oxidizing bacteria (AOB) (Abell et al., 2010). This superphylum TACK is one of the most important in the nitrogen cycle, mainly Thaumarchaeota, an AOA that participate in the first phase of nitrification; among the AOA, the Nitrosopumilales group (Nitrosopumilus, Nitrosoarchaeum, and Cenarchaeum genera) is dominant in water column, and Nitrososphaerales in sediments (Bhattacharyya et al., 2015; Adam et al., 2017). In estuaries of subtropical zones, AOA are important oxidizing ammonia, having quantified 2.27x105 genes/g sediment in the Tobarí Bay (Beman, 2014), while in a tropical estuary of India (Cochin estuary), AOA were more abundant at the surface (0.56-6.3 x 103 cells/ml) of the water column compared to the bottom (0.32-2.9 x 103 cells/ml), and the greatest diversity was determined in the mesohaline region (Vipindas et al., 2015). The presence of AOA was related to temperature and oxygen concentration, it has been established that nitrification in estuarine ecosystems in tropical areas decreases with low oxygen concentrations and the presence of H2S (Corredor et al., 1999); however, it seems that some AOA could carry out autotrophic ammonia oxidation at low oxygen concentrations such as those found in the first strata of the sediment (Silveira et al., 2013; Yasawong et al., 2013).
These archaea involved in the nitrification process could be sharing a niche in the water column with nitrifying bacteria (Nitrosomonas sp.). A relatively high nitrification rate by AOA was reported in the Cochin estuary before the monsoon season, however with the influence of the monsoon and limnetic characteristics, nitrification by bacteria was more important (Vipindas et al., 2015). An important aspect of AOA is that their presence is favored in polluted, eutrophic estuaries because they have genes associated with the transport and regulation of heavy metals (Zou et al., 2019).
Nitrification contributes to the reduction of nitrogen from anthropogenic activities and, being an oxygen-consuming process, it could participate in the formation of hypoxic and anoxic conditions in coastal ecosystems (Caffrey et al., 2007). Nitrification is a key process in the nitrogen cycle, because is coupled with anaerobic processes that oxidize ammonia, including denitrification, and dissimilatory nitrate reduction. During denitrification, nitrate and nitrite are reduced to molecular nitrogen, producing nitrous oxide (N2O) as an intermediate product. This metabolism is carried out under anaerobic conditions by denitrifying bacteria and archaea mainly in estuarine sediments (Francis et al., 2007).
It has been suggested that Asgard in estuarine sediments have the potential to participate in denitrification and dissimilatory nitrate reduction, having been reported that Heimdallarchaeota possesses the genes for the enzymes nitrate reductase and nitrite reductase, while the presence of nitrite reductase has also been established in Lokiarchaeota and Thorarchaeota (Seitz et al., 2019). In the Phylum Euryarchaea, Hadesarchaea (SAGME) seems to be heterotrophic and presents genetic information for carbon monoxide oxidation, coupling it to nitrate reduction; and it can couple H2/CO2 oxidation to nitrite reduction (Lazar et al., 2017). The activity of different phyla during denitrification could also include the production of nitrous oxide (N2O), a greenhouse gas (Santoro et al., 2011).
The information for nitrogenase has only been reported in the Phylum Thorarchaeota, proposing that this group could carry out an assimilation of molecular nitrogen into ammonium (MacLeod et al., 2019).
3. Sulfur cycle
In the sulfur cycle, there are oxidation and reduction reactions that transform sulfur from its most oxidized form (sulfate) to its most reduce form (sulfide); these transformations are regulated by different microorganisms, bacteria, and archaea. Archaea can use many sulfur compounds as electron donors or acceptors during sulfur metabolism (Offre et al., 2013). Major processes are found in archaea include sulfur oxidation, anaerobic sulfate or sulfite reduction and assimilatory metabolism (Fig. 3).
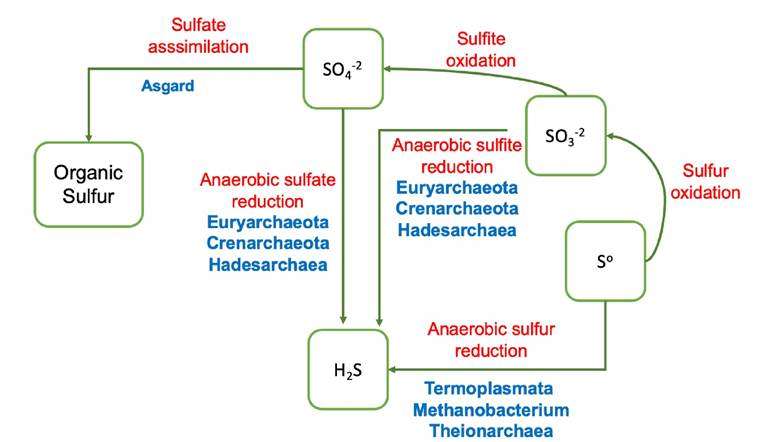
Figure 3 Schematic representation of the participation of archaea in sulfur cycle. The name of the group of archaea that participates in the metabolic pathway is indicated in blue. The name of the main metabolic process is indicated in red: sulfates represent the central compound of the sulfur cycle, in the absence of oxygen, they can be used as electron acceptors during sulfate reduction, producing sulfide (sulfidogenesis). The reduction of elemental sulfur and sulfites also generate sulfide. Sulfates can also be transformed into organic compounds through an assimilation process.
Thermoplasmata can carry out anaerobic reduction of elemental sulfur using H2 as electron donor (Liu et al., 2012); the ability to produce H2S from elemental sulfur, has also been observed in methanogenic archaea such as Methanobacterium (Offre et al., 2013). The ability to use sulfate or sulfite during anaerobic respiration (sulfate reduction/sulfite reduction), producing sulfide, has been demonstrated in some members belong to Euryarchaeota and Crenarchaeota. Hadesarchaea has also the potential to carry out sulfidogenesis (Lazar et al., 2017).
It is suggested that all the phyla of the superphylum Asgard possessing the genetic information for the sulfate adenylyl transferase and phospho-adenosine-sulfate-reductase, could have the capacity to carry out an assimilatory sulfate reduction, reducing sulfate and incorporate it as a sulfur source for biosynthesis of proteins (MacLeod et al., 2019). Thorarchaeota, have elemental sulfur and thiosulfate reduction genes suggesting they participate in intermediate sulfur cycling (Seitz et al., 2016). Theionarchaea could be associated with the reduction of sulfur compounds (Lazar et al., 2017).
CONCLUSIONS
In the last ten years knowledge of the Archaea domain has increased, metagenomic and metatranscriptomic studies are providing important information on metabolic pathways and physiology of these microorganisms in natural ecosystems. Metagenomics and, in general, “omics” technologies, represent an opportunity in studies of prokaryotic diversity and ecology, and for maximum exploitation of the data generated by these techniques, it is extremely important to have bioinformatics and computational biology tools.
From the development and application of independent cultivation techniques, it is a fact that in coastal ecosystems the presence, distribution and diversity of Archaea is evident; however, it is necessary to carry out a greater number of studies in tropical coastal lagoons and estuaries to have an extensive inventory of the biodiversity of the Archaea Domain. The discovery of new environmental sequences has been changing our understanding of their diversity, distribution, and metabolic functions, and it is clear there are still more archaeal species to be discovered, as well as their role in ecosystems.
An important aspect to consider in future research is the study of possible ecological relationships between archaea and the other important group of prokaryotes, bacteria. Ecological relationships influence the organization of communities and metabolic pathways in biochemical cycles, as well as its relationship with another group of important microorganisms in these ecosystems, bacteria.











 nueva página del texto (beta)
nueva página del texto (beta)



