INTRODUCTION
In recent years the global demand for food has had an exponential growth, and aquaculture has turned out an important ally for the production of animal protein. The development of new production processes and cultivation of new species has been postulated as alternatives to fill the increasing demand of fish and shellfish (Rise et al., 2019; FAO, 2020).
The genus Piaractus (Serrasalmidae) comprises fishes native to South America with three species: P. brachypomus (pirapatinga), P. mesopotamicus (pacú) and P. orinoquensis (cachama blanca) (ITIS, 2021). Piaractus mesopotamicus (Holmber, 1887) are naturally found in lakes and rivers with vegetation, provided that water temperature is between 20-30°C, dissolved oxygen concentration is 7-8 mg/L, and pH ranging from 7-8 (Soncini & Glass, 1997; dos Santos et al., 2020). Pacú is also of great importance in aquaculture and recreational fishing due to its robustness, ease of handling and adaptation to artificial feeding, as well as, its high growth rates, efficient food conversion and high fertility rates (Bacchetta et al., 2019). Piaractus spp. have been cultivated under varied aquacultural conditions, i.e.: in polyculture especially with carps (Kumar et al., 2018), in saline or brackish waters, pacú has tolerated up to 4ppt NaCl (Jomori et al., 2012). Assays at different water temperatures have shown that below 24°C, there is a delay in muscle growth in pacú juveniles (de Paula et al., 2014). In addition, pacú produced in aquaculture systems has great acceptance among consumers due to its pleasant taste (Pavon et al., 2018). Barrero et al. (2012), did not find significant differences in sensory appreciations (smell, flavor, texture and color), nor in bromatological parameters (protein, moisture and lipid contents), between P. brachypomus grown in recirculating aquaculture systems (RAS) and those obtained in extensive fish farms.
The advantages of RAS over conventional fish farms are: diminishing of water requirements and growing space, higher crop density (Piedrahita, 2003); production independent of seasonal variations, and reduction of environmental impacts, together with a better management of biosecurity measures (Aslam et al., 2019). However, the intensification of aquaculture practices increase among others, the concentration of ammonia (NH3) in water, being a metabolic residue of major concern as it represents 70 to 90% of the total nitrogen input into aquaculture systems. NH3 concentrations larger than 0.0125 mg/L, can deteriorate gills’ structures (Timmons et al., 2009), also it may accumulate in tissues and blood plasma causing morphological, physiological and behavioural alterations on most aquaculture species, affecting the immune system, growth and production (Medeiros et al., 2016).
Currently, the use of antibiotics in aquaculture has been banned due environmental and health issues, being a sustainable alternative the use of probiotics, prebiotics, and symbiotics (Lee et al., 2019). Probiotics are microbial supplements with either therapeutic, prophylactic, growth-promoting, stress tolerance-enhancing, and reproductive-improvement effects (Hoseinifar et al., 2016; Martínez-Cruz et al., 2012).
Prebiotics are no digestible substrate that are metabolized by specific health-promoting microorganism, therefore, probiotic microorganism use these compounds as a source of carbon for their developmet. A combination of pro-and prebiotics is referred to as a symbiotic product. It provide a competitive advantage over endogenous populations (Merrifield et al., 2010 Mugwanya et al., 2022). This is done to increase the survival and implantation of probiotics in the host’s gastrointestinal tract. So far, symbiotic studies have been conducted in salmonids, soft-shell turtles, shrimps and lobsters (Ringo et al., 2010; Hoseinifar et al., 2016; Lee et al., 2019). Regarding the intestinal bacterial communities present in pacú; Castañeda-Monsalve et al. (2019) reported the presence of Fusobacteria, Spirochaetes, Firmicutes and Proteobacteria in P. brachypomus; while Rossi et al. (2020) found Bacteroidetes, Firmicutes and Fusobacteria in P. mesopotamicus. However, the effect of symbiotics on Piaractus intestinal bacterial communities has not yet been studied.
The objective of this study was to compare the effect of the administration mode of a commercial symbiotic on the growth of pacú (P. mesopotamicus), on the bacterial communities present in the intestinal microbiota and in the water of a RAS system.
MATERIAL AND METHODS
Experimental design and fish culturing conditions. The 87-day experiment consisted of a block design, using symbiotic administration mode as the only factor. After acclimatization, 234 P. mesopotamicus specimens (5.74 ± 0.51cm total length, 4.630 ± 0.369cm standard length, 2.607 ± 0.218cm height and 3.53 ± 0.89g weight) were randomly distributed in 9 aquariums/tanks of 70 L each, that were grouped into three vertical RAS. Therefore 26 organisms were placed in each aquarium, accounting for 78 fishes/treatment (n=78), at a density of 1 fish/2.7 L of water (Fig. 1a).
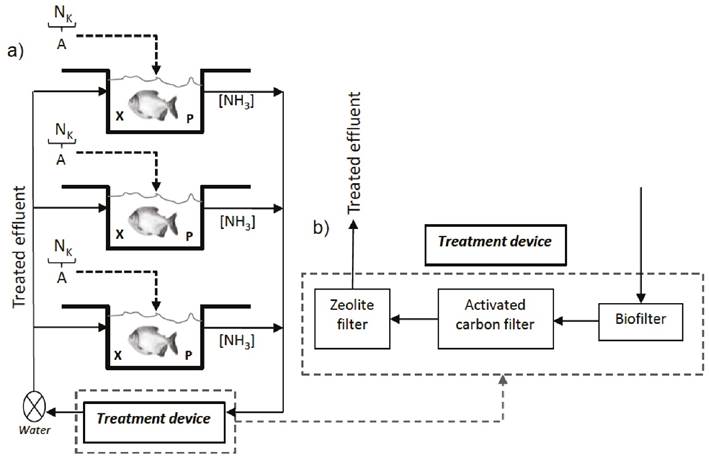
Figure 1 Scheme of the vertical Recirculating aquaculture system (RAS) used in the assays. Each system contained 78 P. mesopotamicus distributed in three 70 L-tanks (26 fishes/tank) The treatments included two ways of symbiotic administration, and a control non amended with symbiotic. Registered parameters included: A - amount of food (g); NK - Kjeldahl nitrogen in the food (gN/gfood); X - recorded weight of fish (g); P - pacú protein content (%); [NH3] - ammonia concentration (mg/L). Modified from Badiola et al. (2018); Timmons et al. (2009).
Three treatments were established: i) symbiotic in powder mixed with the food (MP), administered at 1g symbiotic powder/kg food; ii) activated symbiotic (AP); 1ml of the activated product was added to each tank during feeding; iii) control treatment (CT), with no symbiotic added. Activation of the product for AP treatment is described in “Symbiotic” subsection. The concentration of administered symbiotic was the same in the MP and AP treatments because the product was used according to the producer’s instructions. The fishes were fed three times a day considering 3% of the biomass per day. The ration was adjusted weekly according to weight increases. The food was trout minipellet Silver Cup-El Pedregal (45% crude protein and 16% fat), and water temperature was maintained at 25 ± 2 °C, with 300W heater (Doplhin®). When needed, calcium carbonate (CaCO3) was added to maintain the pH at alkaline values. The volume of water lost by evaporation was replenished daily. Every third day, the remaining food and accumulated faeces were collected, using a siphon and a net with a mesh size of 0.3 mm.
Biological material. Four hundred P. mesopotamicus juveniles (30 ± 1 days old, 4.35 ± 0.73 cm mean total length, 3.44 ± 0.626 cm mean standard length, 1.66 ± 0.381 cm mean height and 1.66 ± 0.90 g mean weight), were cultivated in recirculating aquaculture systems located at a greenhouse facility (19°18´32.8” N 99°06´17.0” W). The fishes were acclimatized for 20 days in an 80 L aquarium, with water parameters set at: 24 ± 1°C temperature, dissolved oxygen (DO) 6.86 ± 0.05 mg/L, and pH 8.5 ± 0.5. The fishes were fed daily with commercial flake food (Wardley, minimum 38% crude protein and 4% fat), at a 3% proportion of their body biomass, fed was divided into two servings. During the first 10 days of acclimatization Sulcoll (Collins Veterinary Division, Guadalajara, Mex), a sulpha-derived antibiotic was administered daily at 17 µL/L water.
Symbiotic. The commercial symbiotic Aqua-BOOSTER® (Smart Microbials, INC.) was used in the assay. It is a powder-formulated product containing viable non-disclosed microorganisms, among others Saccharomyces cerevisiae, and prebiotic oligosaccharides (mannans and b-glucanes). According to the manufacturer’s instructions, this product is focused on improving the health, performance of aquaculture organisms in culture, and can be administered directly into the water as a powder, or after being activated by inoculating 0.5% (w/vol) of the powder, in 1% (w/vol) molasses in water, and incubating for 12 h at room temperature.
RAS. Water biofiltration was carried out in a three-phase cascade system, formed by 3 containers with 50 L each, operating in continuous flow at 2.5 L/min (Fig. 1b). The first phase contained 3cm-diameter plastic spheres aimed to generate nitrifying microbial biofilm, production of the biofilm was carried out during the fish acclimation; the second and third phases used a filtration system based on activated carbon and zeolite, respectively.
Growth analysis. Morphometric data were obtained weekly, by randomly selecting ten fishes from each (30 fishes per treatment). Total length (Lt), standard length (Lp) and height (H) were obtained with a vernier Scala 222b (Metromex), and the weight was determined in an Ohaus Scout STX balance. These morphometric data were used to calculate the following morphophysiological parameters:
Specific growth rate constant (SGR), is obtained by integrating the growth equation dW/dt = μW and plotting ln(Wt/Wo) as a function of culture time, where: Wo and Wt - Average weight of the fishes at time 0 and time t (Lugert et al., 2016).
Food conversion ratio, FCR = ΣF/(Wt-Wo); where: Σf - food administered during cultivation (Usaid-Harvest, 2011).
Protein efficiency ratio, PER = (Wt-Wo)/[N]food; where [N]food - nitrogen concentration of the food (Rojas et al., 2014).
Crop density, CD = Wt/VT. Maximum mass of aquaculture product that can be maintained within the fish farm (Timmons et al., 2009), where: VT - total water volume in the RAS.
Fulton condition factor, K = 100 (W/L3); where: W - fish weight and L - Total fish length (Leyton F. et al., 2015).
Length-weight relationship, WL = αLβ; where: α - intercept, L - standard length and β - ratio coefficient between length and weight (Rennie & Verdon, 2008). The experimental data was used to graphically represent each of the three treatments. Subsequently, the images were overlaid to facilitate comparison in a single figure.
Hypothesis of isometric growth, tb: A Student’s t test (p <0.05), tb = (b-3)/Sb; where b - coefficient of proportion between weight and length, Sb is the slope standard error (Ibáñez, 2015).
Water quality assessment. Water temperature, pH and dissolved oxygen concentration were monitored on a daily basis (YSI digital oximeter). The NH3 concentrations were quantified weekly with a modified Nessler method according to the Hanna C99 photometer and its reagents kit (Hanna Instruments Mexico). The ammonia removal capacity of the biofilter (ϒNH3) was calculated with a modified mass balance as proposed by Badiola et al. (2018), using the following equation:
ϒNH3 = W°N - VT(μX/Y) - V(dN/dt), where: W°N = nitrogen consumption by fish; VT(dX/Y) = ammonia nitrogen accumulation in fish; and V(dN/dt) = ammonia nitrogen accumulation in the tanks.
Statistical analysis. All values in tables are expressed as means ± SD. Normality of the data was assessed with the Shapiro-Wilk test, and the Levene test was used to assess equality of variances. Data meeting these premises were analysed by one-way ANOVA (P <0.05), and further post hoc Tukey test for multiple comparisons, by using SAS JMP Software version 10. While data that didn’t meet the premises, in despite of mathematical transformations, were subject to Tukey’s nonparametric analysis (Montgomery, 2004).
DNA extraction and PCR amplification. Metagenomic DNA was extracted from the suspended bacteria in water (W), and from the symbiotic product, either as powder or activated, and from the fish gut content (FGC) from each treatment using the Wizard kit from Promega Inc., with modifications according to Aguirre-Garrido et al. (2016). A fragment of the 16S rRNA gene comprising the V6-V8 regions was amplified using the universal primers rL1401 (5´- GCGTGTGTACAAGACCC -3´) and f968 GC (5´-GCCCGGGGCGCGCCCCGGGCGGGGCGGGGGCACGGGGGAACGCGAAGAACCTTACC- 3´, the underlined sequence corresponds to the GC-clamp), following the protocols described by Felske et al. (1996). The polymerase chain reaction (PCR) was carried out according to Aguirre-Garrido et al. (2016), and the amplification products were examined in agarose gel electrophoresis (1% agarose in TAE buffer) under previously described protocols (Ramírez-Saad et al., 2004).
DGGE analyses. Metagenomic DNA was obtained from i) the fish gut contents (FGC), ii) the suspended bacteria in water (W) and in the symbiotic. The analysis was based on PCR-DGGE profiles of the V6-V8 region of the 16S rRNA. Obtained amplicons were electrophoresed in a DCode System (BioRad Labs, Hercules, CA, USA). PCR conditions and DGGE general methodology were performed according to Felske et al. (1996). The denaturing gels were silver-stained and preserved (Sanguinetti et al., 1994). Predominant DGGE bands from each profile were excised, purified, and reamplified (Ramírez-Saad et al., 2004). Image analysis of DGGE profiles was performed with Syngene software (Synoptics Ltd., UK), using Pearson matrix for pairwise similarity and UPGMA as clustering method. Furthermore, band surface and mean pixel intensities of every band within each pattern were integrated, these values were converted to percentage of relative abundance and used as surrogate abundance measure for each sequenced band within its respective profile.
Sequencing and bioinformatic analysis. Reamplified products from DGGE were sequenced (Macrogen, Seoul, Korea). Obtained sequences (<400 nt) were identified with the16S-based ID tool of the EZBioCloud (Yoon et al., 2017). Furthermore, the taxonomic identification of sequences and their respective surrogated abundances were introduced in the PICRUSt2 (Douglas et al., 2020) pipeline (https://www.github.com/picrust/picrust2).
Accession numbers. The sequences obtained in this study were deposited in GenBank databases under the accession numbers: MN845131 to MN845138.
RESULTS
Growth and morphophysiological parameters. The growth parameters of pacú were scored weekly (Table 1). ANOVA comparisons of weight, total length, standard length and height values (followed by post hoc Tukey test; p < 0.05) showed significantly higher values in the four parameters when using MP as compared to CT and AP treatments. AP and CT didn’t show significant differences among them in any of the measured growth parameters. Also a significantly higher SGR (p <0.05) was obtained when using MP, in relation to CT and AP. No mortality occurred during the experiment in any of the treatments.
Table 1 Growth scores of P. mesopotamicus (Holmber, 1887) cultured 87 days in a Recirculating Aquaculture System (RAS). The treatments were: CT) Control, AP) Activated symbiotic, MP) Mixed symbiotic. The obtained growth parameters: W) weight, Lt) Total length, Lp) Standard length, H) Height, were used to calculate SGR) Specific Growth Rate constant. Values not connected with the same symbol or letter are significantly different (P<0.05)
| Days of culture | SGR | |||||||||||
| 0 | 28 | 35 | 42 | 50 | 56 | 63 | 70 | 77 | 87 | (day-1) | ||
| CT | W | 3.5±0.7 | 5.5±1.4 | 6.2±1.8 | 8.7±2.4 | 14.0±2.2 | 13.4±3.4 | 15.7±3.6 | 15.7±4.3 | 19.3±4.9 | 24.9ˆ±7.4 | |
| Lt | 5.8±0.4 | 6.6±0.6 | 7.2±0.7 | 8.0±0.7 | 8.8±0.4 | 9.1±0.8 | 9.7±0.7 | 9.6±0.9 | 10.4±0.9 | 11.0δ±1.0 | 0.0253a | |
| Lp | 4.6±0.3 | 5.3±0.5 | 5.7±0.5 | 6.3±0.5 | 7.22±0.4 | 7.3±0.6 | 7.7±0.6 | 7.8±0.6 | 8.2±0.6 | 8.7°±0.8 | ±0.008 | |
| H | 2.6±0.2 | 3.0±0.3 | 3.2±0.3 | 3.6±0.4 | 4.2±0.3 | 4.1±0.4 | 4.4±0.4 | 4.4±0.4 | 4.7±0.5 | 5.1*±0.6 | ||
| AP | W | 3.5±0.7 | 5.6±1.0 | 6.6±1.3 | 9.1±2.0 | 13.9±2.3 | 13.1±2.8 | 14.8±3.3 | 17.7±4.0 | 21.2±4.6 | 26.3ˆ±6.6 | |
| Lt | 5.7±0.4 | 6.7±0.5 | 7.1±0.6 | 8.0±05 | 9.1±0.5 | 9.1±0.8 | 9.6±0.8 | 10.2±0.9 | 10.7±0.9 | 11.1δ±1.0 | 0.0258a | |
| Lp | 4.6±0.3 | 5.3±0.4 | 5.8±0.4 | 6.4±0.5 | 7.2±0.4 | 7.3±0.6 | 7.6±0.6 | 8.0±0.6 | 8.6±0.6 | 8.8°±0.8 | ±0.007 | |
| H | 2.6±0.2 | 3.1±0.3 | 3.3±0.2 | 3.7±0.3 | 4.2±0.3 | 4.2±0.4 | 4.2±0.4 | 4.5±0.4 | 4.7±0.4 | 5.1*±0.5 | ||
| MP | W | 3.5±0.7 | 4.9±1.4 | 7.2±1.8 | 10.0±2.5 | 14.9±3.4 | 17.5±4.6 | 17.5±4.4 | 20.8±4.7 | 24.5±5.5 | 31.5×±6.7 | |
| Lt | 5.8±0.4 | 6.78±0.5 | 7.5±0.6 | 8.3±0.5 | 9.4±0.6 | 9.6±0.8 | 10.1±0.7 | 10.7±0.7 | 10.7±0.7 | 11.8~±0.7 | 0.0321b | |
| Lp | 4.6±0.3 | 5.48±0.5 | 6.0±0.5 | 6.6±0.5 | 7.3±0.4 | 7.6±0.7 | 8.0±0.6 | 8.5±0.7 | 8.9±0.6 | 9.6ф±0.7 | ±0.008 | |
| H | 2.6±0.2 | 3.24±0.4 | 3.4±0.3 | 3.8±0.3 | 4.4±0.3 | 4.6±0.5 | 4.5±0.4 | 4.8±0.4 | 5.0±0.4 | 5.4ɤ±0.3 | ||
On their side, PER values with both symbiotic treatments were significantly higher than the control. While after 87 days of culture, the obtained values for food conversion ratio (FCR), crop density (CD) and Fulton factor (K) did not present significant differences between treatments. However, in the latter factor values greater than 3.0 were obtained, indicating that the growth of the animals was not food limited (Leyton F. et al., 2015). Likewise, the values obtained of tb, determined isometric growth in all conditions (Table 2). The weight/length ratios (b) in pacú were close to 3.0 g/cm in all treatments so fishes deep their shape as they grow, however, a statistical significant difference (p <0.05) was obtained when using MP, as seen in Figure 2.
Table 2 Morphophysiological growth parameters of P. mesopotamicus (Holmber, 1887) after 87 days culture in a RAS. FCR - Protein efficiency ratio, CD - Crop density, K - Fulton condition factor, tb - Hypothesis of isometric growth. Values not connected with the same letter are significantly different (p<0.05).
| FCR (gfood/gfish) | PER | CC | K | tb | |
| (gfish/gprot.) | (kgfish /m3) | ||||
| Control | 1.67a ±0.39 | 1.80a ±0.78 | 5.68a ±1.03 | 3.59a ±0.36 | 0.848 |
| Activated symbiotic | 1.60a ±0.55 | 2.31b ±0.86 | 6.00a ±1.29 | 3.67a ±0.29 | 0.851 |
| Mixed symbiotic | 1.18a ±0.36 | 2.57b ±0.76 | 7.20a ±0.43 | 3.56a ±0.31 | 0.744 |
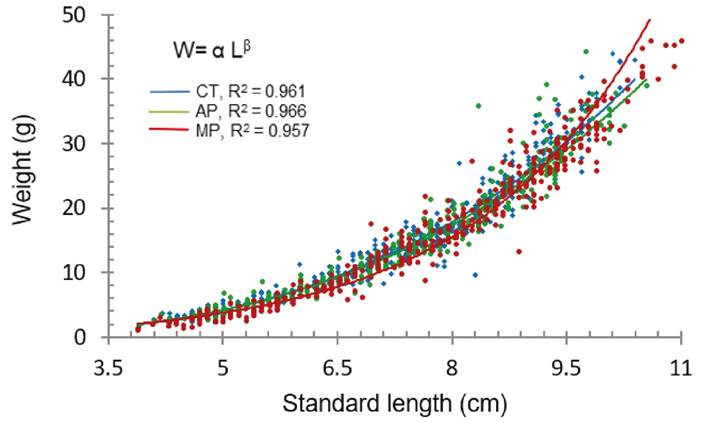
Figure 2 P. mesopotamicus length-weight relationship (W= a Lb) at different symbiotic administration modes. a - intercept on X axis, b -ratio coefficient between length and weight, R2 - determination coefficient; Control (CT), a = 0.029, b =3.08a; Activated symbiotic (AP), a= 0.033, b =3.0247; Mixed symbiotic (MP), a = 0.026, b =3.1374b. Levels not connected with the same letter are significantly different (p<0.05).
Water quality. The average values of temperature, pH and DO did not show significant differences in any of the two experimental treatments and the control (Table 3). Although the ammonia nitrogen removal capacity of the biofilters (ϒNH₃) did not present significant differences, the ammonia concentration at the end of the culture was significantly higher with MP.
Table 3 Water quality parameters (average values ± SD) of the RAS, after 87-day culturing of P. mesopotamicus (Holmber, 1887). T - Temperature; DO - Dissolved oxygen concentration; NH3 Ammonia water concentration; ϒNH3 - Ammonia removal capacity of the biofilter. Values not connected with the same letter are significantly different (P<0.05).
| T | DO | pHϯ | NH3* | NH3** | ϒNH3 | |
| (°C) | (mg/L) | (mg/L) | (mg/L) | (gNdía-1) | ||
| Control | 22.4a±1.9 | 6.1a ±0.9 | 8.3a ±0.7 | 0.19ª ±0.06 | 0.43ª ±0.01 | 51.7ª ±16.8 |
| Activated symbiotic | 22.4a±1.9 | 6.2a ±1.1 | 8.3a ±0.7 | 0.20ª ±0.12 | 0.44ª ±0.07 | 42.6ª ±13.5 |
| Mixed symbiotic | 23.0a±2.0 | 7.7a ±1.4 | 8.4a ±0.7 | 0.19a ±0.03 | 0.62b ±0.03 | 38.01ª ± 7.4 |
* Initial values (0 day-culturing, n=9) **Final values (87-day culturing, n=9)
Analysis of microbial communities. Initial similarity clustering analysis pointed out that the DGGE patterns revealed that DGGE profiles clustered according to samples origin; fish gut content (FGC) and water from the tanks (W), regardless of the treatment. The FGC samples showed more homogeneous profiles forming a coherent cluster with 58% similarity (Fig. 3a). Eight DGGE bands considered representative of the different treatments were selected, for re-amplification, sequencing and identification (Fig. 3b, numbers 1-8). Six sequences were identified at the genus level, while the other two were identified at the species level (Table 4). Although, bands 1 and 7 migrated to different position, they were identified as Microbacterium aerolatum, sequence comparison pointed several differences as they may be originating from different operons. The profiles of activated symbiotic and mixed symbiotic (Fig. 3b) showed some differences that could be related to activation in molasses than enriched certain populations, yielding different prominent bands. From the FGC profiles (Fig. 3b), the band identified as Asaccharospora irregularis (band 5) remained throughout the culture in the three experimental conditions (CT, AP and MP), and Turicibacter sanguinis (band 6), only in the FGC of CT fishes. While from the bands of the bacterial community in the water, Prosthecobacter sp (band 3) and Limnohabitans planktonicus (band 8) disappeared after 50 days. Regarding bands 1 and 7, both corresponded to Microbacterium aerolatum and they were found in the symbiotic powder, and in 3 experimental conditions at 50 days, but only prevailed in the CT tanks at the end of the culture. Bacillus horti (band 4), was detected in the MP water along the culture, but was not present in the FGC of any treatment.
Table 4 Phylogenetic identification and percentage of sequence similarity in the V6-V8 region of the gen 16S rRNA obtained from the excised DGGE bands (see Figure 4a). Labeling is as follows: first two letters are treatments; AP- activated symbiotic, MP- mixed symbiotic, CT- control. Letters after de hyphen correspond to sample origin; W- water in the RAS tank, FGC- fish gut content, and the numbers to the sampling time; 0, 50 and 87 days of P. mesopotamicus (Holmber, 1887) culturing. APP-Aquabooster activated product and MPP-Aquabooster mixed product.
| DGGE band number (treatment-sample origin) | Accesion number | Phylum | Closest relative | Similarity (%) |
| 1 (APP) | MN845131 | Actinobacteria | Microbacterium aerolatum (BJUW01000027) | 99.71 |
| 2 (MP-W87) | MN845132 | Proteobacteria | Variovorax sp (BCUT01000013) | 92.95 |
| 3 (AP-W50) | MN845133 | Verrucomicrobiae | Prosthecobacter sp (AB305640) | 95.02 |
| 4 (MPP) | MN845134 | Firmicutes | Bacillus horti (D87035) | 98.33 |
| 5 (MP-FGC50) | MN845135 | Firmicutes | Asaccharospora irregularisT (X73447) | 98.53 |
| 6 (CT-FGC0) | MN845136 | Firmicutes | Turicibacter sanguinisT (AF349724) | 100 |
| 7 (CT-W87) | MN845137 | Actinobacteria | Microbacterium aerolatum (BJUW01000027) | 98.58 |
| 8 (CT-W50) | MN845138 | Proteobacteria | Limnohabitans planktonicusT (LFYT01000006) | 99.72 |
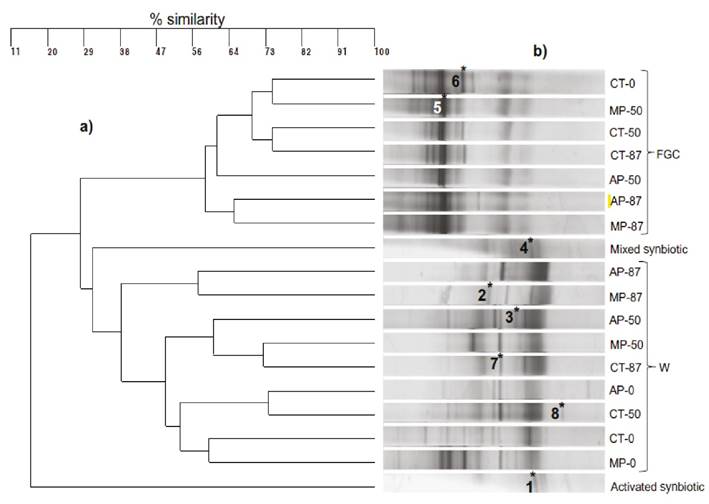
Figure 3 a) DGGE banding pattern of amplicons of the V6-V8 regions of the 16S rRNA from the experimental treatments CT, AP and MP, samples were obtained from the bacterial communities in the fish gut content (FGC) and in the suspended bacteria in the water (W) culture at days 0, 50 and 87. b) UPGMA dendrogram based on the DGGE profiles. * denotes the bands selected for excision and sequencing, and the 1-8 numbers the identification of each band.
For PICRUSt analysis, the presence and relative abundance of each sequenced band in the different DGGE profiles allowed to predict the functional potential of the respective bacterial communities on the basis of marker gene sequencing (Douglas et al., 2020). In this regard, some DGGE profiles; i.e. MP-0-W, CT-0-W and CT-50-W (Fig. 3b) shared 4 out of the 8 sequenced (identified) bands, while other profiles only shared 3, 2 or 1, except for AP-87-FGC that did not presented any shared band. Under this approach, around 90% the predicted genes for the different profiles were grouped under 21 KEGG (Kyoto Encyclopaedia of Genes and Genomes) pathways. The pathways related to membrane transport and carbohydrates metabolism were the most represented in the majority of the community metabolic profiles (Supplementary Table 1). Based on these prediction values, several differences in gene numbers were detected among the different profiles, however, these differences were more related to the number of identified bands within the profiles, rather than to the administration of symbiotics.
Supplementary Table 1 Metabolic functional predictions derived from PICRUSy of the experimental treatments control (CT), activated synbiotic (AP) and mixed synbiotic (MP) MP in the bacterial communities in the fish gut content and in the suspended bacteria in the wáter culture at days 0, 50 and 87. APP-Aquabooster activated product and MPP-Aquabooster mixed product. Values correspond to number of predicted genes in each
| KEGG_Pathways; metabolic routes | Water Treatments | Fish gut content treatments | APP | MPP | ||||||||||||||||
| CT 0 | CT50 | CT87 | AP0 | AP50 | AP87 | MP0 | MP50 | MP87 | CT0 | CT50 | CT87 | AP50 | AP87 | MP50 | MP87 | |||||
| Metabolism; Amino Acid Metabolism | 87 | 97 | 63 | 63 | 67 | 0 | 97 | 62 | 87 | 65 | 65 | 65 | 43 | 43 | 43 | 42 | 94 | 53 | ||
| Metabolism; Biosynthesis of Other Secondary Metabolites | 7 | 7 | 5 | 6 | 7 | 0 | 7 | 6 | 7 | 7 | 7 | 7 | 6 | 6 | 5 | 5 | 6 | 5 | ||
| Metabolism; Carbohydrate Metabolism | 112 | 111 | 97 | 107 | 109 | 0 | 114 | 98 | 97 | 97 | 97 | 97 | 98 | 98 | 98 | 97 | 98 | 97 | ||
| Celular Processes; Cell Motility | 51 | 43 | 13 | 34 | 45 | 0 | 61 | 34 | 38 | 43 | 36 | 36 | 30 | 30 | 14 | 30 | 28 | 30 | ||
| Metabolism; Energy Metabolism | 64 | 78 | 50 | 67 | 54 | 0 | 96 | 62 | 74 | 54 | 68 | 68 | 53 | 53 | 38 | 54 | 47 | 34 | ||
| Metabolism; Enzyme Families | 32 | 43 | 24 | 34 | 24 | 0 | 48 | 26 | 32 | 27 | 32 | 32 | 27 | 27 | 26 | 24 | 26 | 20 | ||
| Genetic Information Processing; Folding, Sorting and Degradation | 46 | 47 | 23 | 27 | 23 | 0 | 46 | 27 | 40 | 24 | 28 | 28 | 26 | 26 | 25 | 23 | 27 | 25 | ||
| Unclassified, Genetic Información Processing | 32 | 32 | 21 | 26 | 20 | 0 | 34 | 23 | 32 | 22 | 26 | 26 | 20 | 20 | 19 | 20 | 34 | 18 | ||
| Metabolism; Glycan Biosynthesis and Metabolism | 38 | 38 | 21 | 24 | 21 | 0 | 37 | 26 | 30 | 25 | 24 | 24 | 21 | 21 | 22 | 21 | 30 | 23 | ||
| Metabolism; Lipid Metabolism | 79 | 82 | 47 | 54 | 41 | 0 | 83 | 48 | 45 | 42 | 46 | 45 | 43 | 43 | 38 | 41 | 47 | 41 | ||
| Environmental Information Processing; Membrane Transport | 199 | 218 | 158 | 168 | 197 | 0 | 218 | 167 | 197 | 149 | 149 | 149 | 163 | 163 | 86 | 97 | 97 | 94 | ||
| Unclassified; Metabolism | 57 | 57 | 32 | 45 | 43 | 0 | 63 | 45 | 45 | 34 | 43 | 43 | 34 | 34 | 33 | 33 | 34 | 31 | ||
| Metabolism; Metabolism of | 56 | 56 | 47 | 48 | 26 | 0 | 54 | 50 | 52 | 27 | 47 | 47 | 26 | 26 | 25 | 26 | 45 | 30 | ||
| Metabolism; Metabolism of Terpenoids and Polyketides | 43 | 39 | 16 | 22 | 16 | 0 | 43 | 23 | 41 | 17 | 23 | 23 | 16 | 16 | 16 | 16 | 20 | 16 | ||
| Metabolism; Nucleotide Metabolism | 48 | 56 | 32 | 35 | 34 | 0 | 52 | 35 | 47 | 39 | 31 | 31 | 34 | 34 | 32 | 34 | 39 | 24 | ||
| Unclassified; Poorly Characterized | 63 | 67 | 36 | 43 | 49 | 0 | 68 | 48 | 64 | 26 | 43 | 43 | 39 | 39 | 36 | 49 | 23 | 30 | ||
| Genetic Information Processing; Replication and Repair | 93 | 95 | 84 | 97 | 93 | 0 | 98 | 92 | 94 | 87 | 95 | 95 | 73 | 73 | 70 | 93 | 82 | 85 | ||
| Environmental Information Processing; Signal Transduction | 30 | 32 | 20 | 20 | 17 | 0 | 32 | 20 | 32 | 23 | 19 | 19 | 18 | 18 | 15 | 17 | 24 | 17 | ||
| Genetic Information Processing; Transcription | 37 | 39 | 32 | 36 | 32 | 0 | 47 | 36 | 38 | 28 | 33 | 33 | 31 | 31 | 20 | 32 | 28 | 19 | ||
| Genetic Information Processing; Translation | 77 | 78 | 41 | 64 | 54 | 0 | 74 | 63 | 68 | 68 | 54 | 54 | 57 | 57 | 50 | 54 | 41 | 53 | ||
| Metabolism; Xenobiotics Biodegradation an Metabolism | 71 | 60 | 37 | 45 | 39 | 0 | 72 | 52 | 53 | 40 | 41 | 41 | 39 | 39 | 32 | 39 | 62 | 30 | ||
DISCUSSION
Growth and morphophysiological parameters. Determining the gradual increase in length or weight of cultivated organisms is a basic parameter in aquaculture. In this study, dynamics of weight increase in pacú presented an adaptation phase of 28 days in the three experimental conditions. Normally, fishes in their early stages of development grow a larger proportion in length, than in the other growth parameters (Nash et al., 2006). Coincidentally, Mourad et al. (2018), also found an initial delay phase of 28 days in P. mesopotamicus juveniles. According to Jomori et al., 2003, the adaptation phase delay could be shortened to a 6 to 9 day-period under intensive culturing of pacú larvae based on live food.
The statistically significant differences in size, weight, and growth rate of MP treatment as compared to CT and AP do not imply a random variability or small fluctuations, but a treatment-related variation in the fish growth parameters. Therefore, it is likely that with the use of larger tanks, such a biomass quantity will be produced with MP that will allow for greater gains for producer compared to the other experimental conditions. Guidoli et al., 2018 reported similar values in the growth rate in culture of P. mesopotamicus larvae, by administering a mixture of probiotic bacteria. Considering the way that activated symbiotic was administered, by adding the fermented preparation directly into the water tanks (AP treatment). Under this condition, the symbiotic has been highly diluted in the water tanks (3ml AP/day in 50L water tanks), and the fishes did not take full advantage of it. While the administration of the symbiotic in powder mixed with food (MP treatment), allowed the fishes to consume it more efficiently. The water and in the FGC of the AP treatment were analyzed during culturing, in such low numbers that sensitivity of DGGE profiling was not enough to detect their presence. However, our results showed that amending with symbiotic as in MP treatment, significantly improved the development and growth of the fishes. Furthermore, our results exceeded those of Inoue et al. (2019), that doubled the initial weight of P. mesopotamicus in RAS at 50 days, while in our trial the biomass increased three-fold in that time and became six-fold in 90 days. Although in this study the type of food used was the same in all treatments, the administration of symbiotic mixed with the food (MP) allowed a significantly higher SGR in relation to AP and CT treatments. Our SGR values with MP treatment contrast with those reported by Bicudo et al. (2010), between 0.0228 and 0.0264 days-1 in P. mesopotamicus, depending on the protein concentration in the diet.
Regarding the food conversion ratio (FCR), the values (1.18 - 1.67g food/g fish) obtained in the three experimental conditions indicated that the efficiency of P. mesopotamicus to use the food was practically the same, which may be due to the quality, nutritional composition and digestibility of the used feed. According to Bicudo et al. (2010), a low FCR value may indicate that the activity of fish proteolytic enzymes increase the availability of amino acids necessary for fish metabolism. Similarly, Gomez-Penaranda et al. (2016), reported a FCR of 1.28 in P. brachypomus, in contrast, Klein et al. (2014), observed mean FCR values of 1.7 when the protein content of food was 18.5%. The FCR values can vary from 1.2 to 4.0 depending on the aquaculture species, development stage, culture conditions, as well as the quality and frequency of the rations (Hickling, 1966), while the FCR values for P. mesopotamicus may range from 1.06 to 2.89 (Bicudo et al., 2010).
Both symbiotic treatments produced statistically significant increases in protein efficiency ratio (PER) as compared to the CT treatment. Considering that protein is the most expensive part of the food (Rojas et al., 2014), the use of symbiotic would allow a more profitable conversion ratio (gfish/gprot), that could contribute to the fish health and formation of body mass. The significantly higher levels of PER in MP and AP compared to the control could be attributed to the symbiotic effect that was exerted favoring the production of digestive enzymes and the absorption of dietary proteins, reflected in enhanced body growth. Similar studies with pacú reported a PER of 2.4 gfish/gprot when mixing soybean and wheat flour (Machado-Neto et al., 2016), and 2.5 gfish/gprot when food was supplemented with 19.6 g of digestible lysine/kg of food (Abimorad et al., 2010), however, this amino acid is more expensive than the probiotics.
After 87 days of culture our CD values ranged from 5.68 to 7.2kg fish/m3, these values are higher than the RAS-reared pacú (control treatment; 5.5kg fish/m3) obtained by Inoue et al. (2019). However, their fishes subjected to sustained swimming showed up to 51% growth increase, after 50 days rearing. While there are no significant differences in CD among treatments, our results showed logarithmic trends after the initial 28-day delay phase. In this regard, MP exhibits higher exponential growth (SGR) compared to AP and CT, suggesting that a longer cultivation time may be required to manifest even higher CD values. This is supported by Poleo et al. (2011), that reported a CD of 12 kg fish/m3 after 192 days of culture with P. brachypomus. Our results showed logarithmic trends after the initial 28-day delay phase, making possible to obtain larger CD values with a longer culturing time.
The weight-length relationship and the K factor are relevant parameters for understanding the life cycle of a fish population, allowing individuals’ growth estimations and determining their degree of robustness, respectively (Khanipour et al., 2020; Leyton et al., 2015). The K factor value can vary between 0.1 and 4.0, depending on fish maturity and the spawning periods (Barriga et al., 2002; Ruiz & Marchant, 2006). In this study, K values greater than 3.0 were observed without significant differences between treatments, similar results were obtained by Bacchetta et al. (2019) also with P. mesopotamicus. Pointing to a tendency in pacú towards an isometric growth. However, other aspects of their performance may differ, as noted by Leyton et al., 2015. So far, this is the first report regarding the length-weight relationship during the cultivation of P. mesopotamicus.
Water quality. The pH, temperature and OD values obtained throughout the assay remained in the correct physiological range for P. mesopotamicus culturing (Soncini & Glass, 1997; dos Santos et al., 2020). Ammonia concentration is one of the most critical water quality parameters for the growth of aquatic animals. Its apparent toxicity is extremely variable and does not depend solely on its mean or maximum concentration in water (Timmons et al., 2009; Quaresma et al., 2020; Aissaoui et al., 2017). Although desirable levels of ammonia for the farming of tropical fish species should be less than 0.025 mg/L, P. mesopotamicus has proven highly tolerant to different ammonia concentrations; Barbieri & Bondioli (2015), reported LC50 = 0.023mg NH3/L, in 96h; Nitz et al. (2019), found a higher value: LC50 = 0.5mg NH3/L during 10 days. While Abreu de et al. (2012), reported LC50 values up to 3.0mg NH3/L during 24 h, however, although this exposure to ammonia caused an elevation in total hemoglobin, and blood glucose increased to 2.0 mg/L after the trial period. Our results report pacú highest tolerance to ammonia concentration (average 0.62 mg NH3/L) in the MP treatment, and also the longest with 87 days. Generally, those fishes like pacú, growing in temperate waters are more tolerant to ammonia, than those from cold or salty waters (Timmons et al., 2009). It does not seem to be a correlation between the use of symbiotics and the ammonia removal capacity in the biofilter. Instead, the biofilm was primarily composed of nitrifying bacteria developing during the fish acclimation process, prior to the assay. The observed increase in the final values of ammonia may be associated with the high biomass accumulated throughout the culture (reflected in the loading capacity), producing a larger amount of this compound that could not be efficiently removed by the filtration system. The ¡NH3 values of these systems could be increased if the adaptation of the nitrifying biofilm in the biofilter is extended, as recommended by Wang et al., 2018; or even more, by inoculating the biofilter with selected nitrifying bacteria to have a mature biofilm at the beginning of the assays. Although the NH3 values in MP treatment were at least twice those of the other treatments, there were no detectable negative effects on the growth parameters of the fishes in the MP treatment.
Analysis of bacterial communities. Studies on the FGC of P. mesopotamicus have reported the presence of Bacteroidetes, Firmicutes and Fusobacteria (Rossi et al., 2020), as well as Fusobacteria, Spirochaetes, Firmicutes and Proteobacteria (Castañeda-Monsalve et al., 2019). Our results partially coincide with those, since Firmicutes were detected in the FGC of the fishes throughout the culture, standing out Asaccharospora irregularis (Firmicutes/Clostridiales) detected in all the experimental conditions, and Turicibacter sanguinis (Firmicutes) mainly in the control treatment; while another Firmicutes (Bacillus horti), was detected in MP treatment. The habitat of Bacillus is very wide and its application in aquaculture is widely documented (Mendoza et al., 2019; Soltani et al., 2019; Thurlow et al., 2019). Microbacterium aerolatum (Actinobacteria) isolated from marine environments has been detected in the activated symbiotic (Fig. 3a, bands 1 and 7), a probiotic potential has been suggested for this lactic acid bacteria (Orla-Jensen, 1943).
PICRUSt analysis requires both; identity and quantification of OTUs as provided by massive sequencing. We have implemented a surrogated quantification of the populations (bands) within our DGGE profiles, however, only few bands were identified, and our results are focused on those bands. Although, in this sense the approach is limited, it provided information on specific and prominent bacterial populations present in the water and the FGC under the experimental conditions.
CONCLUSION
This is the first report on the size-weight relationship of pacú at different times during a 87 days rearing, showing isometric growth. The administration of symbiotic mixed with the feed during the cultivation of P. mesopotamicus, significantly promotes the growth of the fishes in recirculating aquaculture systems. Furthermore, the use of activated symbiotic may have improved the capacity of the biofilter to partly remove high ammonia concentrations. In the mixed and activated symbiotic treatments were detected Bacillus horti and Microbacterium aerolatum, respectively, both species have the potential to be used as probiotics in aquaculture.











 nueva página del texto (beta)
nueva página del texto (beta)



