Introduction
The Agave genus, situated within the Agavoideae subfamily (Asparagaceae, Asparagales), comprises a diverse collection of 265 species. Remarkably, 153 of these species are native to Mexico, with 150 of them being exclusive to this region (Vázquez-García et al., 2022). This geographical concentration establishes Mexico as the epicenter of both the origin and diversification of these plants (García-Mendoza & Galván-V., 1995). This genus holds immense significance in ethnobotanical, ecological, and economic contexts, as these plants fulfill various roles such as serving as living fences, sources of food, materials for fibers, construction, medicines, cosmetics, fodder, and the production of alcoholic beverages like bacanora, pulque, tequila, mescal, and others (Bermúdez-Bazán et al., 2021; Vázquez-Delfin et al., 2022).
However, the over-exploitation of specific Agave species, coupled with uncontrolled plant extraction, presents a substantial danger to their survival (Aguirre-Dugua & Eguiarte, 2013). This risk is intensified because these plants are harvested before reaching the flowering stage, thereby preventing sexual reproduction (Monja-Mio et al., 2021). In response to this pressing need for a substantial number of individuals from selected genotypes, in vitro culture techniques, particularly somatic embryogenesis has been developed as a promising vegetative propagation process, offering an alternative for the conservation of Agave angustifolia (Reyes-Díaz et al., 2017). Also, preservation methods such as cryopreservation and encapsulation have been utilized to conserve somatic embryos long-term (Arzate-Fernández et al., 2016).
Somatic embryogenesis (SE) plays a vital role in the mass propagation and genetic transformation of various plant species (Ramírez-Mosqueda, 2022). In this process, the transition to developmental totipotency in cells is initiated by temporarily exposing them to genes responsible for maintaining the meristematic state regulated by endogenous hormone levels like response to exogenous plant growth regulators (PGR) and controlling different stages of plant morphogenesis during SE (Loyola-Vargas & Ochoa-Alejo, 2016). A widely accepted model suggests that the conditions inducing somatic embryogenesis led to the dedifferentiation of somatic plant cells, followed by the acquisition of developmental totipotency. At this totipotent stage, cells can respond to specific developmental signals guiding them toward embryogenesis. Understanding the genetic control of totipotency is pivotal for advancing plant biotechnology and enhancing our knowledge of plant physiology (Schmidt et al., 1997).
Thus, the identification and characterization of genes associated with somatic embryogenesis offer the potential to assess the embryogenic capacity of somatic cells at early developmental stages and provide insights into the molecular regulation of this process (De Oliveira Santos et al., 2005; Liu et al., 2018; Ramasamy et al., 2022).
Receptor-Like Kinases (RLKs) with Leucine-Rich Repeats (LRRs) within the family have pivotal roles in various aspects of cellular signaling in plant development. This includes their involvement in disease resistance, microsporogenesis, vascular tissue differentiation, embryo pattern formation, control of cellular death, and self-incompatibility (Fisher & Turner, 2007; Hu et al., 2005). RLKs function by facilitating the transmission of external signals and information from neighboring cells, thereby triggering specific responses. In case of somatic embryogenesis process, the responses of cultured cells are contingent on the cell type and the composition of the culture medium (Baudino et al., 2001; Ma et al., 2012; Nodine et al., 2007; Ramasamy et al., 2022; D.-Z. Zhao et al., 2002).
The expression of flavin monooxygenase-encoding genes is stimulated during the establishment of cell or tissue polarity and embryo development in the presence of synthetic auxins like 2,4-dichlorophenoxyacetic acid (2,4-D) in the culture medium (Ramírez-Mosqueda, 2022). These enzymes play a crucial role in auxin biosynthesis, resulting in an increase in endogenous indoleacetic acid (IAA) levels, a key step in meristem formation and, by extension, embryo development (Wójcik et al., 2020). It has been observed that cells originating from provascular cells promote most somatic embryos, with their embryogenic commitment confirmed through the expression of a Receptor-Like Kinase known as Somatic Embryogenesis Receptor-Like Kinase (SERK). This gene expression serves as a recognized marker of embryogenic competence (Koehler et al., 2020; Maulidiya et al., 2020; D.-Z. Zhao et al., 2002).
What distinguishes SERK from other RLKs is the presence of a Serine-Proline-Proline motif (SPP) situated between five LRRs and the transmembrane (TM) domain (Cueva et al., 2012; De Oliveira Santos et al., 2005; Hecht et al., 2001; Santos et al., 2009).
SERK genes are well-known for their pivotal role in signal transduction during embryogenic cell development, with their expression mainly occurring in the early stages of embryogenesis (Hecht et al., 2001). This expression gradually diminishes as development progresses. In Daucus carota and Arabidopsis thaliana, for instance, SERK genes are only expressed in embryogenic structures, not in non-embryogenic cultures (Hecht et al., 2001; Salaj et al., 2008; Schmidt et al., 1997).
While the role of SERK genes in many plant species has been extensively studied, the molecular aspects of somatic embryogenesis in the Agave genus remain unexplored, and genes associated with this process have not been yet identified. This study represents the first characterization of a SERK gene from A. angustifolia, referred to as AaSERK. The results of this study may offer insights to enhance the regeneration rate of somatic embryogenesis and improve the genetic transformation process.
Materials and methods
Establishment of embryogenic culture
Aseptic, fully developed zygotic embryos (Figure 1a) were meticulously dissected from seeds of Agave angustifolia and served as the initial explants for inducing callus formation, as shown in Figure 1a. The cultures of embryogenic callus (Figure 1b) were initiated on a medium containing one-quarter strength of Murashige and Skoog (MS) nutrients (Murashige & Skoog, 1962). This medium was supplemented with 3.0 mg L−1 of 2,4-dichlorophenoxyacetic acid (2,4-D) and 1.0 mg L−1 of 6-benzyladenine (BA) as plant growth regulators (Reyes-Díaz et al., 2017). The cultures were then incubated under conditions featuring 16 hours of light and 8 hours of darkness, utilizing cool white light at an intensity of 60 μmol m−2 s−1, at a temperature of 25 ± 2 °C. Remarkably, mature somatic embryos (Figure 1d) were observed after a duration of 120 days from the initiation of the culture.
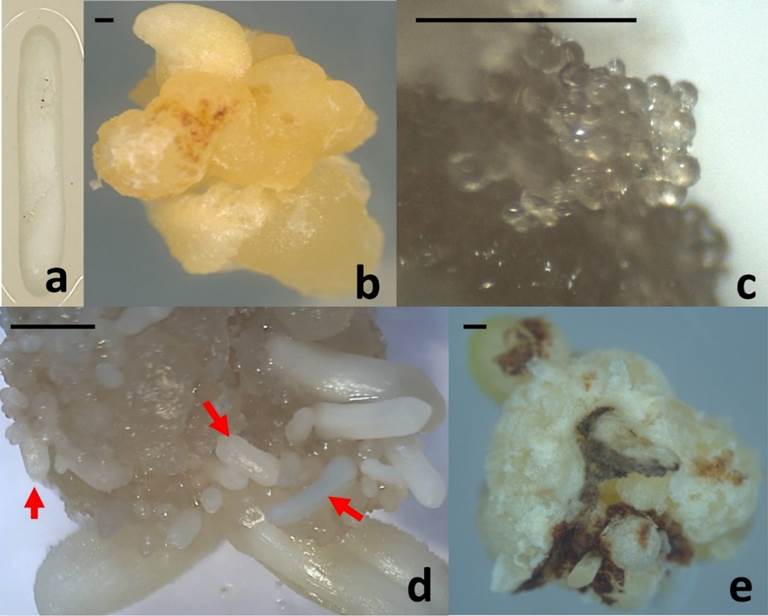
Figure 1 Agave angustifolia somatic embryogenesis process: (a) Initial explant. (b) Embryogenic callus. (c) Embryogenic callus with globular somatic embryos (60 days after the induction) used for SERK gene isolation. (d) Mature somatic embryos (arrows) (120 days after the induction). (e) Non-embryogenic callus. Bar = 5mm
Cloning and sequencing of Agave angustifolia SERK (AaSERK) gene
Genomic DNA was extracted from 60-day-old embryogenic callus with globular somatic embryos (Figure 1c) and from callus without embryogenic response (Figure 1e) using the modified CTAB method (Aboul-Maaty & Oraby, 2019). The DNA was subsequently dissolved in TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 7.0). The quality and concentration of the extracted DNA were assessed through agarose gel electrophoresis and bio photometry (Eppendorf AG, Germany), respectively.
To amplify a fragment of Agave angustifolia SERK (AaSERK), degenerate primers were designed based on consensus SERK sequences from Zea mayz, Oryza sativa, Daucus carota, Arabidopsis thaliana, and Cyrtochilum loxense stored in the NCBI database (www.ncbi.nlm.nih.gov/). These primers were crafted from conserved regions and possessed the following sequences: forward primer 5´-NTGGTGAGGTGGCGGAGG-3´ and reverse primer 5´-TGTHACRTGGGTRTNCTTCTARTCCAT-3´. Polymerase Chain Reaction (PCR) was conducted using these primers. The PCR reaction was prepared with a final volume of 10 µl, consisting of 10 ng of genomic DNA, 6.3 µl of Taq DNA polymerase buffer, 5 mM dNTPs, 15 mM MgCl2, 1 U of MyTaq DNA Polymerase (Bioline, USA), and 0.4 µM of each forward and reverse primer (Sigma, USA). The thermal amplification parameters for the PCR reaction included an initial denaturation at 95 °C for 5 min, followed by 35 cycles of amplification (95 °C for 30 s, 47.5 °C for 30 s, 72 °C for 2 min), and a final extension step at 72 °C for 10 min. The amplifications were conducted in a thermal cycler (Multigene Optimax, Labnet, USA). Subsequently, the PCR products were subjected to 1% (w/v) agarose gel electrophoresis and visualized using a gel documentation system (UVP Transilluminator 95-0403-01, UK).
The AaSERK fragment, amplified from genomic DNA, underwent purification using the GenElute™ PCR Clean-up Kit (Sigma, USA) following the manufacturer's instructions. The purified fragment was then sequenced twice, once from each direction using the forward and reverse primers, at both the Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria (SENASICA) and the Universidad Nacional Autónoma de México (UNAM). BLASTn and BLASTx tools were employed to compare the nucleotide and translated sequences, respectively. Sequences exhibiting the most significant homology in both nucleotide and amino acid sequences were selected for multiple sequence alignments using Mega 6.0, then conserved domains were identified.
For the analysis of the relationship between the AaSERK protein and known SERK protein sequences from six phylogenetically related species (selected based on identity percentage), a consensus and unrooted phylogenetic tree was constructed using the UPGMA method within the Mega program (version 6.06).
Results
In this investigation, a fragment of the Agave angustifolia Somatic Embryogenesis Receptor-Like Kinase (AaSERK) gene was successfully amplified from DNA obtained from embryogenic calli. It should be noted that its amplification was not possible in non-embryogenic callus (Figure 2).
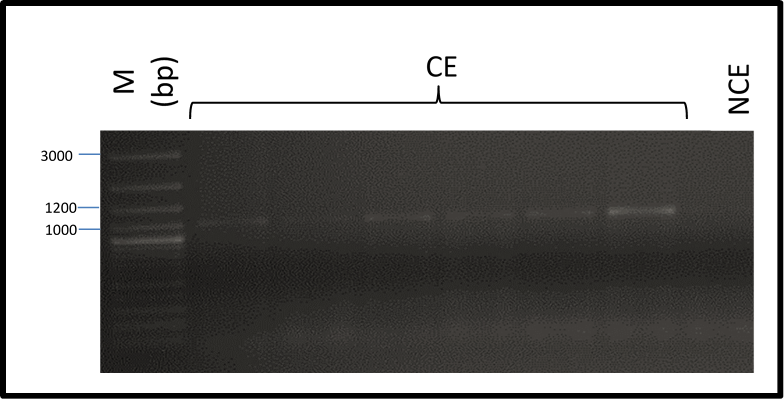
Figure 2 Amplification of Agave angustifolia Somatic Embryogenesis Receptor-Like Kinase gene from DNA of embryogenic (CE) and non-embryogenic calli (NCE): molecular weight marker (M), base pairs (bp).
Sequencing of the fragment obtained indicates that AaSERK encompassed 968 nucleotides, which encodes a protein of 327 amino acids. This nucleotide sequence has been formally submitted to GenBank under the accession number KX247683.1 (available at https://www.ncbi.nlm.nih.gov/nuccore/1134518460). Furthermore, the amino acid sequence of AaSERK obtained in our study, with the accession number APX18295.1 (accessible at www.ncbi.nlm.gov/protein/1134518461), exhibited a noteworthy degree of identity with other SERK proteins, exceeding 80%. The highest similarity was observed with ClSERK from Cyrtochilum loxense (97% similarity, 619 amino acids, CBV98085.1), DcSERK from Dendrobium catenatum (97% similarity, 633 amino acids, AKN89445.1), CmSERK from Cattleya maxima (89% similarity, 357 amino acids, CCD32850.1), AcSERK from Ananas comosus (85% similarity, 629 amino acids, AEC46975.1), CnSERK from Cocos nucifera (82% similarity, 629 amino acids, AAV58833.2), and PdSERK from Phoenix dactylifera (82% similarity, 621 amino acids, XP_008780820.1).
The confirmation of AaSERK identity was corroborated by the presence of characteristic domains commonly observed in SERK proteins from other species (as depicted in Figure 3a). Multiple reports suggest that the length of SERK proteins typically is considered within the range of 250 to 1650 amino acids. What unites these proteins is the presence of a distinctive domain known as SPP, which is notably absent in other Leucine-Rich Repeat Receptor-Like Kinases (LRR-RLKs) (De Oliveira Santos et al., 2005; Hecht et al., 2001; Koehler et al., 2020; Salaj et al., 2008; Santos et al., 2009; Schmidt et al., 1997). In this research, the SPP domain was successfully identified, which exhibited a high degree of identity in comparison to other SERK proteins found in phylogenetically related species to Agave angustifolia (as seen in Fig. 3b). Lastly, the transmembrane and kinase domains were found to be highly conserved due to structural constraints imposed by their catalytic requirements (Schwessinger & Rathjen, 2015).
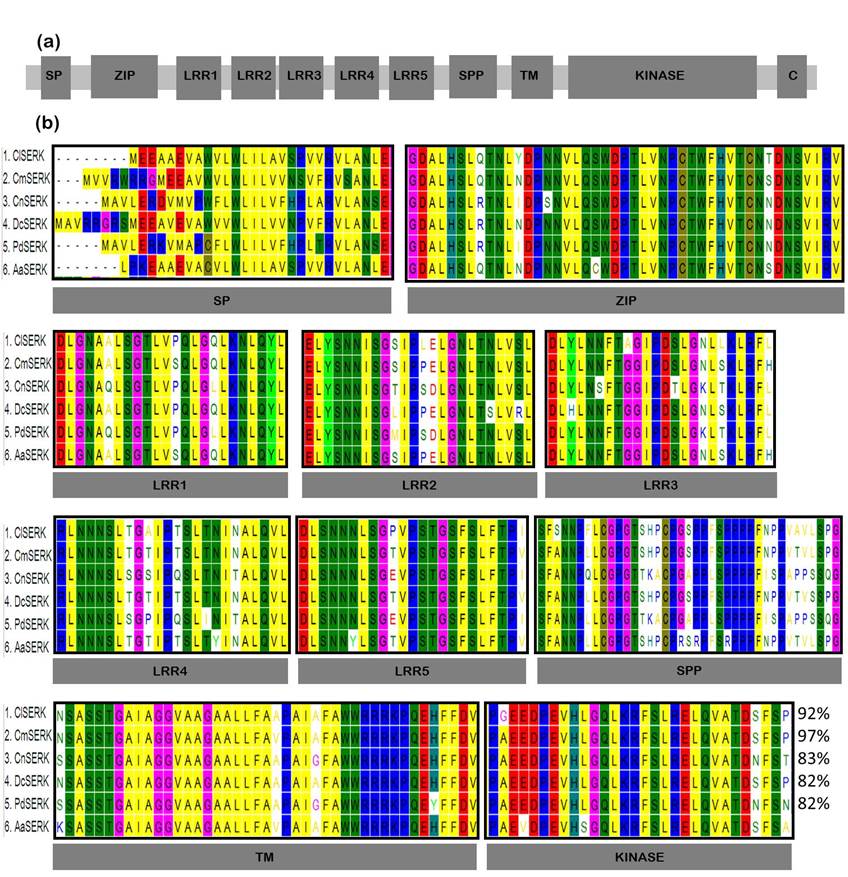
Figure 3 (a). Dibujo esquemático de los dominios típicos en un gen de la Embriogénesis Somática Receptor Similar a la Quinasa (SERK). Péptido señal SP, cremallera de leucina ZIP, repeticiones ricas en leucina LRR1 - LRR5, motivo SPP serina-prolina-prolina, motivo transmembrana TM, región C C-terminal. (b). Estructura de la SERK identificada de Agave angustifolia (AaSERK) y alineación de la secuencia de aminoácidos predicha que indica el grado de similitud (%) con algunos miembros de las proteínas quinasas de la familia SERK: (1. Cyrtochilum loxense SERK, ClSERK, 97%; 2. Cattleya maxima SERK, CmSERK, 89%; 3. Cocos nucifera SERK CnSERK, 82%; 4. Dendrobium catenatum SERK, DcSERK, 97%; 5. Phoenix dactylifera SERK, PdSERK, 82%; 6. AaSERK). Se destacan residuos de aminoácidos idénticos en todas las proteínas enumeradas.
Our phylogenetic analysis revealed a clear grouping of SERK proteins in accordance with two taxonomic categories. The first cluster included monocot SERK proteins (DcSERK, CmSERK, ClSERK, AaSERK, CnSERK and PdSERK). In contrast, the second cluster included dicot SERK proteins (AtSERK) (as shown in Figure 4). Additionally, we observed the highest similarity of AaSERK with the predicted DcSERK, CmSERK, and ClSERK.
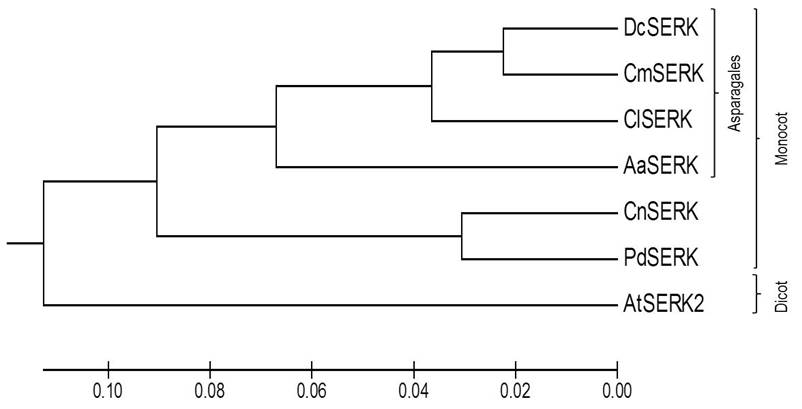
Figure 4 Phylogenetic tree of AaSERK with other reported SERK sequences depicting the interrelationship of different SERK: Dendrobium catenatum SERK (DcSERK), Cattleya maxima SERK (CmSERK), Cyrtochilum loxense SERK (ClSERK), Agave angustifolia (AaSERK), Cocos nucifera SERK (CnSERK), Phoenix dactylifera SERK (PdSERK) and Arabidopsis thaliana SERK (AtSERK).
Discussion
Somatic embryogenesis serves as the cornerstone of cellular totipotency in higher plants. In the controlled environment of in vitro conditions, a single or a few somatic cells within an explant must possess the competency to receive a developmental signal, whether endogenous or exogenous, that triggers the transformation toward the embryogenic pathway (Mikuła et al., 2022; Von Arnold et al., 2002). In the case of the Agave genus, somatic embryogenesis protocols have been established for various species, including A. angustifolia (Reyes-Díaz et al., 2017). Likewise, epigenetic mechanisms, such as DNA methylation and histone modifications in the micropropagation of A. angustifolia (De-la-Peña et al., 2012; Duarte-Aké et al., 2016) has been studied, but the absence of reports on the molecular mechanisms such those regulate the somatic embryogenesis process is still unclear.
Understanding the regulation of somatic embryogenesis during tissue and organ culture could significantly enhance the development of more efficient regeneration and transformation protocols. Schmidt et al. (1997) reported a correlation between the expression of the SERK gene and the potential of cells to exhibit embryogenic characteristics, especially in the presence of 2,4-D, a known promoter of totipotency. The genetic expression, such as key transcription factor, is crucial for triggering and regulating the processes involved in embryo formation from somatic cells (Kikuchi et al., 2006). However, not all the samples express those genes at sufficient levels to initiate and complete the process of somatic embryogenesis (Méndez-Hernández et al., 2019; Schwessinger & Rathjen, 2015), as it was observed in our assay in embryogenic and none embryogenic calli, respectively. From a genetic variability perspective, differences in the expression of these specific genes may be due to the presence of allelic variants, epigenetic modifications, or differences in transcriptional regulation (Pikaard & Mittelsten Scheid, 2014; Sivanesan et al., 2022). Some cells may have the necessary genetic combination to activate these genes and trigger somatic embryo formation, while other cells may lack this combination or be subject to transcriptional repression (Fambrini et al., 2022). Additionally, the physiological mechanisms (endogenous production of acid abscisic) that regulate the cellular ability to form somatic embryos are closely related to the genetic activity (Kikuchi et al., 2006). From this point, cells must be in a receptive physiological state, characterized by a high rate of cell division and plasticity, to respond to the activation of SERK genes related to somatic embryogenesis (Meira et al., 2024). Also, external factors such as nutrient availability, the presence of auxins in the culture medium, and environmental signals can also modulate the expression of these genes and thus influence in the cellular ability to initiate the process of somatic embryo formation (Long et al., 2022).
SERK proteins play a pivotal role in regulating various facets of plant growth and development, encompassing the differentiation of somatic embryos. While the SERK gene family has been extensively scrutinized in well-established model plants like arabidopsis, rice, and maize, it remains comparatively less explored in non-model plant species (Baudino et al., 2001; Hecht et al., 2001; Singla et al., 2009). In the present study, a Somatic Embryogenesis Receptor-Like Kinase gene from Agave angustifolia (AaSERK) present only in embryogenic callus was isolated, characterized, and reported for the first time, similar expression was reported in carrot (Guzzo et al., 1994; Schmidt et al., 1997) and arabidopsis (Salaj et al., 2008). This pattern of SERK gene expression in cultured cells indicates its involvement in a signaling pathway responsible for orchestrating developmental modifications in response to specific culture conditions. These alterations include the activation of cell division and the reprogramming of cellular properties (Hu et al., 2005). It is believed that the level of expression of the SERK gene plays a fundamental role in determining cell fate, which can influence processes such as organogenesis or in this case somatic embryogenesis (Singla et al., 2009), thus underlining its importance in cell differentiation (Santos et al., 2009), as well as it is regarded as an indicator of cells with the ability to generate somatic embryos (Porras-Murillo et al., 2018).
This AaSERK gene encodes a Leucine-Repeat Receptor protein kinase and exhibits similarities to other SERK (Baudino et al., 2001; Cueva et al., 2012), suggesting that it functions as a Leucine-Rich Repeat Receptor-Like Kinase (LRR-RLK). In the model for RLK function, plant RLKs typically exist as monomers until the binding of an extracellular signal molecule induces receptor dimerization (Becraft, 2002). This brings the intracellular kinase domains of individual monomers into proximity, allowing for transphosphorylation, activation of kinase domains, and the regulation of cellular responses (Becraft, 1998; Maulidiya et al., 2020; L. Zhao et al., 2020).
LRR-RLKs are essential in regulating various physiological processes, including embryo pattern formation, microsporogenesis, vascular tissue differentiation, maintenance of meristematic cells, organ morphology, inflorescence structure, brassinosteroid (BR) signaling, and cellular death control (Cueva et al., 2012; De Oliveira Santos et al., 2005; Hecht et al., 2001; Koehler et al., 2020; Santos et al., 2009).
Structural and sequence analyses (as shown in Figure 3b) reveal that AaSERK encodes a typical SERK protein with conserved roles in plant development, displaying a high degree of sequence and protein domain homology with other SERK proteins. The characteristic distribution of SERK protein domains, including a signal peptide; a ZIP domain known to play a role in protein oligomerization (Shah et al., 2001); five LRR units that are exposed at the cell membrane, and these are presumed to be responsible for interactions with other signaling molecules, such as brassinosteroid receptors, as well as for the perception of external signals (auto- and transphosphorylation events between respective intracellular kinase domains), and signal transduction during somatic embryogenesis induction (Kobe & Deisenhofer, 1994; Schwessinger & Rathjen, 2015); a Pro-rich domain containing the SPP motif, a transmembrane domain, and a Serine - Threonine protein kinase, is also evident in AaSERK.
Phylogenetic analysis (as seen in Figure 4) indicates that AaSERK shares a close relationship with SERK sequences from other plant species, particularly with monocot SERKs belonging to the order Asparagales, such as DcSERK, CmSERK, and ClSERK. This could suggest a common evolutionary origin of SERK among these plants.
Conclusions
It can be inferred that AaSERK plays a crucial role in mediating somatic embryogenesis, where cells with embryogenic competence respond to biochemical signals triggered by the presence of auxins in a culture medium and transform into somatic embryos. The expression of SERK genes has been shown to be associated with various stages of embryogenesis in other plant species, further supporting this hypothesis.











 nueva página del texto (beta)
nueva página del texto (beta)



