1. Introduction
A chromitite is a very peculiar case of magmatic rock where chromite is the predominant mineral (> 80 % vol). This type of rock is usually associated with mafic and ultramafic rocks, specifically peridotites and their serpentinized equivalents, with two main styles of ores distinguished by their geological setting: (1) stratiform or Bushveld-type chromitite associated with layered mafic intrusions (e.g., Mukherjee et al., 2017; Latypov et al., 2017; Mathez and Kinzler, 2017) and (2) “podiform” or ophiolitic chromitite hosted in fossil oceanic lithosphere (Leblanc and Nicolas, 1992; González-Jiménez et al., 2014; Arai and Miura, 2016). In the last few decades the interest for chromitite has greatly been increased because their intriguing genesis (e.g., Rollinson, 2016; O’Driscoll and Vantongeren, 2017) and economic interest as a unique source of the metal chromium and potential target for the recovery of the critical raw metal platinum-group elements (O’Driscoll and González-Jiménez, 2016; Mudd et al., 2018).
In Latin America, chromitites of the stratiform-type have been exclusively reported from large layered intrusions within Archean cratons and Neoproterozoic continental crust in Brazil (e.g., Ferreira-Filho et al., 2002). In contrast, chromitites of the ophiolitic-type are relatively frequent in many of the ophiolites widespread throughout the whole continent in North, Central and South America. The latters have been mainly found in suprasubduction-zone ophiolites (SSZ) corresponding to oceanic lithosphere that preserve an evolution or transition from back-arc to fore-arc environments or vice versa (i.e., MORB-island arc tholeiite-boninite sequence of igneous activity). Most of these chromitites were already exploited for chromium since middle 20th Century, although some ophiolites with strong potential for chromite deposits still remain underexplored. Examples of ophiolitic chromitites associated with SSZ ophiolites in North Latin America include those metamorphosed ones of Paleozoic age reported from Tehuitzingo and Loma Baya in Mexico (Proenza et al., 2004a; González-Jiménez et al., 2017a; Colás et al., 2017; Farré-de-Pablo et al., 2019), and the unaltered Triassic ophiolites from the Mexican Baja California (i.e., Puerto Nuevo; Vatin-Perignon et al., 2000; González-Jiménez et al., 2017b). In South Latin America, chromitite are known from the metamorphosed ophiolites of Neoproterozoic age of the Eastern Papean Ranges (Los Congos and Los Guanacos; Proenza et al., 2008; Colás et al., 2016), the Paleozoic ultramafic massifs of Tapo in Peru (Tassinari et al., 2011; Colás et al., 2017) and La Cabaña in Chile (Barra et al., 2014; González-Jiménez et al., 2016), and the Permian-Triassic Medellin Metaharzburgitic Unit in the Central Cordillera of Colombia (Proenza et al., 2004b; Correa-Martínez, 2007). A remarkable suite of relatively well-preserved chromite deposits cropping out in the (peri)-Caribbean region in North, Central and South America are associated ophiolites corresponding to remnants of the oceanic lithosphere of Late Jurassic-Early Cretaceous. These include the chromitite deposits of Baja Verapaz in Guatemala (Thayer, 1946), Siuna in Nicaragua (Flores et al., 2007; Baumgartner et al., 2004), Santa Elena in Costa Rica (Zaccarini et al., 2011), Northen Cuban ophiolite belt that includes a set of allochthonous massifs (Cajálbana, Habana-Matanzas, Villa Clara, Camagüey, Holguín, Mayarí-Baracoa) distributed along more than 1000 km in the mainland island of Cuba (Thayer, 1946; Proenza et al., 1999, 2018; Gervilla et al., 2005; González-Jiménez et al., 2011a), and Loma Peguera in the Dominican Republic (Proenza et al., 2007).
This paper provides the first scientific report of a chromitite body associated with the Cerro Colorado Ophiolite, in the Paraguaná Peninsula, in the northern part of Venezuela. Méndez (1960) investigated for the first time this chromitite body, reporting the exact location, morphology, size and field relations of this chromitite body; however he did not provide any geochemical data nor an interpretation on its petrogenesis. This paper presents and discusses whole-rock PGE data on this chromitite and novel micro-analytical data, obtained using electron-microprobe and in situ ablation technique, for a suite of key minerals and elements of the chromitite and host peridotites. These data are integrated with field information and compared with recently published experimental and empirical data on ophiolitic chromitites, in order to identify the nature of the parental melt of the chromitite and, indirectly, precisely constrain the tectonic setting of their emplacement in the geological framework of the (peri)-Caribbean region.
2. Geological setting and chromitite
2.1. Peri-Caribbean ophiolites in the northern coast of Venezuela
Jurassic-Cretaceous ophiolitic rocks crop out along three margins of the Caribbean plate (Figure 1). These have been interpreted as relicts of proto-Caribbean oceanic lithosphere formed after Pangea’s break-up or remnants of the oceanic lithosphere related to the origin and evolution of the Caribbean volcanic arc (135-70 Ma). In the northern coast of Venezuela, these “ophiolitic” sutures comprise, from east to the west, the ophiolites of the El Copey (Araya-Paria; Alvarado, 2010; Petrásh and Revanales, 2010), Paraguachí (Margarita Island; Rekowski and Rivas, 2006; Maresch et al., 2009), La Orchila (Cáceres, 2016), Carayaca (Cordillera de la Costa; Sisson et al., 1997; Urbani, 2018), Loma del Hierro (Caucagua-El Tinaco; Baquero et al., 2013; Urbani, 2018), Yaracuy (San Felipe; Bellizzia and Rodríguez, 1976), Siquisique (Rodríguez and Muñoz, 2009; Kerr et al., 2012) and Cerro Colorado (Paraguaná; Santamaría and Schubert, 1974; Mendi and Rodríguez, 2006; Baquero et al., 2013) (Figure 1).
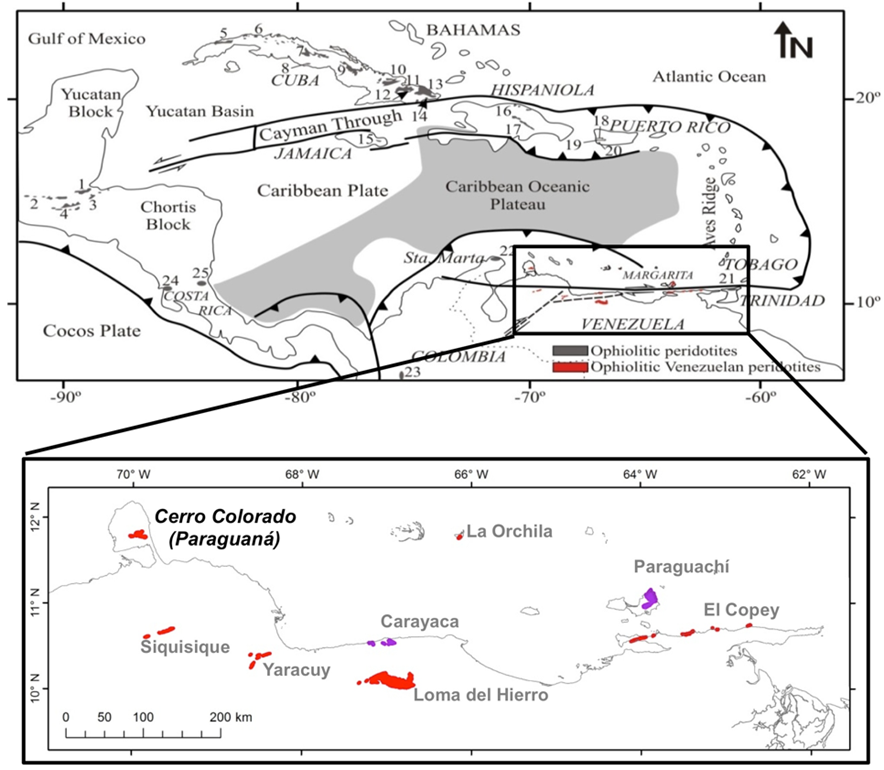
Figure 1 (a) Distribution of ophiolite-related ultramafic rocks around the margins of the Caribbean Plate (from Lewis et al., 2006): 1) Sierra de Santa Cruz, 2) Baja Verapaz, 3) Juan de Paz, 4) Grupo El Tambor, 5) Cajálbana, 6) Habana-Matanza, 7) Villa Clara, 8) Escambray, 9) Camagüey, 10) Holguín, 11) Mayarí-Cristal, 12) Alto de La Corea, 13) Moa-Baracoa, 14) Sierra del Convento, 15) Arntully, 16) North Coast Belt, 17) Loma Caribe, 18) Monte del Estado, 19) Río Guanajibo, 20) Bermeja, 21) San Souci, 22) Serpentinitas de Cabo de la Vela (Guajira), 23) Dunita de Medellín, 24) Santa Elena, 25) Río San Juan. Northern Venezuelan ophiolites are marked in red in (a) and zoomed in (b).
The Cerro Colorado ophiolite, the subject of this paper, crops out in the southern part of the Paraguaná Peninsula corresponding to the most important topographic highs in the region (Figure 1, 2a and 2b). The ophiolite sequence consists, from bottom to the top (Figure 2c; Mistage, 1989; Mendi and Rodríguez, 2006), of strongly tectonized mantle clinopyroxene-bearing harzburgite containing sills and dykes of pyroxenites and gabbroic rocks (diabase, gneissic and pegmatitic gabbro, leucogabbro, olivine-bearing gabbro, norite and troctolite, anorthosite) as well as dunite lenses occasionally containing impregnation of clinopyroxenes and chromitite ore. This section of upper mantle rocks is tectonically overlain by a sequence of layered gabbros and extrusive basaltic and hypabyssal gabbroic rocks.
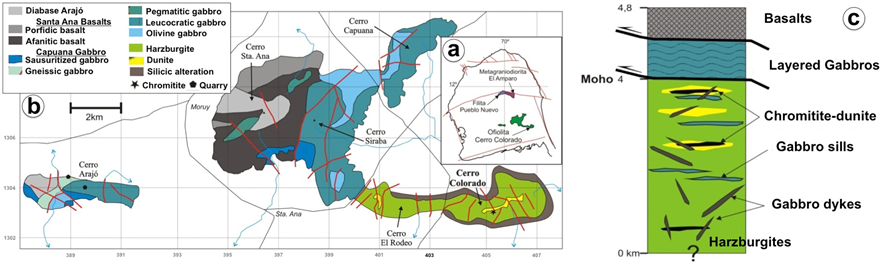
Figure 2 (a) Geological map of the studied area in the northern part of the Pagaguaná Peninsula along the north coast of Venezuela showing the location of the chromite body studied herein (b) and the pseudostratigraphy of the Cerro Colorado ophiolite (c). The map and the stratigraphic column are adapted from Mendi and Rodríguez (2006).
2.2. The chromitite body of cerro colorado ophiolite
The chromitite body investigated in this work is located at the Cerro Colorado, west to the Santa Ana Village, in the southern part of the Paraguaná Penisula, Falcon state in north Venezuela (Figure 2a to 2c). The chromitite is a podiform-like body of 4 x 12 m that extends over about 45 m long trending N50°E and dipping 15° to the northwest concordantly with the host dunite-harzburgite and a sill of gabbro (Figure 3a). It is strongly fractured and locally intruded by peridotite dykes. The body chiefly consists of massive chromitite, although semimassive, disseminated and nodular-textured ores are also found in the external parts of the orebody (Figure 3b to 3f). The contact between massive chromitite and dunite is usually sharp (Figure 3b) but in some zones of the body there is gradation from massive chromitite towards semimassive, disseminated and nodular-textured ore (Figure 3d and 3e). In the northern part of the chromitite body, there is a satellite vein made up of disseminated and nodular chromitite (up to 3 m long) emerging irregularly from the main body and penetrating into the host dunite.

Figure 3 Photographs showing morphologies and textures of the Cerro Colorado chromitite body. (a) Chromitite pod within dunite enclosed in harzburgite. (b-c) Massive chromitite showing sharp contact with enclosing dunite. (d-e) Semi-massive chromitite hosted in dunite. (f-g) nodular chromitite hosted in dunite.
3. Petrography
3.1. Chromitite
Massive chromitite (> 80% vol. chromite) forms the main part of the ore body and consists of aggregates of strongly fractured anhedral chromite (1-5 mm in size) grains. In some of the samples, the interstitial silicate matrix (representing < 20 vol.%) consists of olivine partially transformed to mesh lizardite cross-cut by late chrysotile veins (Figure 4a). Semimassive samples (70-80 vol. % chromite) consist of anhedral crystals of chromite of up to 4 mm across embedded in a serpentine matrix, whereas nodular chromitites (~50 vol. % chromite) are made up of aggregates of anhedral-subhedral, frequently ovoid-like, chromite crystals with grain sizes ranging between 2 and 6 mm (Figure 4b). In the nodular textures, the chromitite nodules are embedded in a serpentinite matrix and exhibit a well-developed crack-seal filled by chrysotile and opal (Figure 4c). Noteworthy, chromite forming the different chromite textures is unaltered.
3.2. Dunite and harzburgite
The dunite envelope around the studied chromitite reaches up to 1.5 m thick and chiefly consists of olivine (up to 0.5 cm) partly transformed to lizardite (Figure 5a to 5d), although some domains with disseminated chromite may contains impregnations (10-15 % modal) of variably bastitized anhedral clinopyroxene (Figure 5e to 5f). Regardless of its pyroxene content dunite contain subhedral grains of accessory chromite (up to 3% modal) and it is crosscut by a well-developed network of late magnesite, chrysotile and opal veins.
Harzburgite displays porphyroclastic texture; it is clinopyroxene-poor and contains ubiquitous crystals (1-2 mm) of subhedral chromite (< 2% modal). Olivine is heavily serpentinized (70%) to a lizardite-magnetite assemblage displaying mesh textures, whereas orthopyroxene and clinopyroxene are pseudomorphed by batiste. The harzburgite also contains accessory crystals of chromite with subhedral and anhedral (i.e., vermicular) morphology, although in lower amounts (< 2% modal) than dunite (Figure 5e to 5f).Similarly to chromite forming the chromitite body, accessory chromite in dunite and harzburgite is unaltered despite of the intense cracking that affected them.
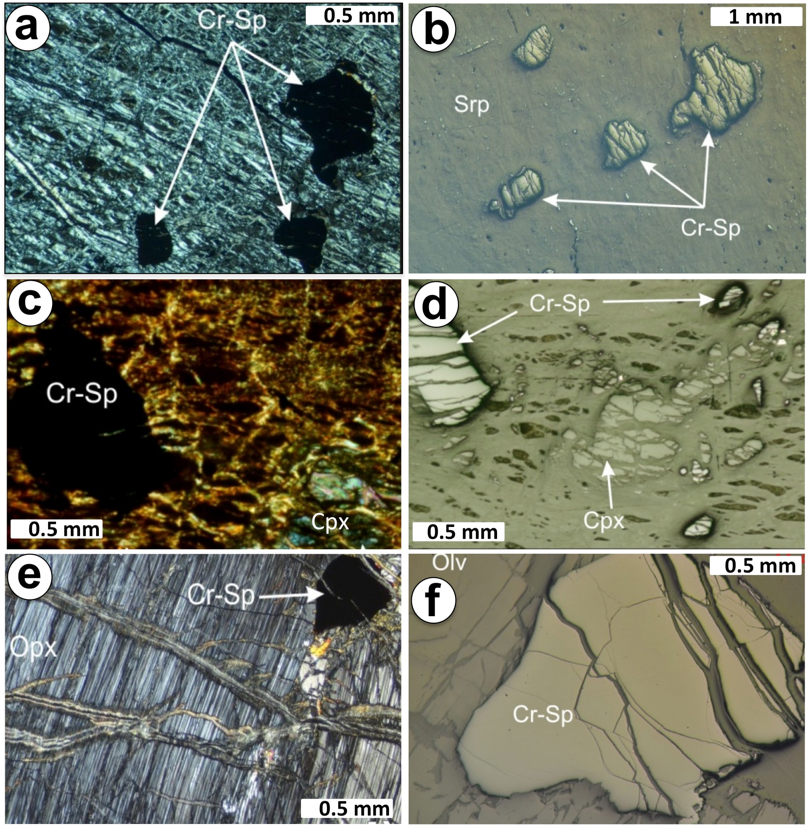
Figure 5 Photomicrographs (transmitted right side and reflected light left side) of chromie in dunite and harzburgite enclosing the Cerro Colorado chromitite. (a-b) irregular chromite in dunite hosting the high-Al chromitite of Cerro Colorado. (c-d) anhedral chromite in clinopyroxene-bearing dunite. (e-f) subhedral chromite in harzburgite. Keys: Cr-sp: chromian spinel; Srp: serpentine.
4. Analytical procedures
4.1. Electron-probe microanalysis
The quantitative analyses of chromite and silicates were obtained by wavelength-dispersive spectrometry (WDS) analysis using a JEOL JXA-8230 at the Centres Científics i Tecnològics of the University of Barcelona (CCiTUB, Barcelona, Spain), operated at 20 kV acceleration voltage, 15 nA beam current and with a beam diameter of 1 μm. Calibration standards were Cr2O3 (Cr), corundum (Al), rutile (Ti), periclase (Mg), hematite (Fe), rhodonite (Mn), NiO (Ni), and metallic V. The PAP correction procedure was used for obtaining concentrations (Pouchou and Pichoir, 1985).
4.2. In situ laser ablation ICPMS
Minor and trace elements in chromite were obtained using Photon Machines Analyte Excite 193 nm laser system connected to an Agilent 8800 QQQ ICP-MS in the Instituto Andaluz de Ciencias de la Tierra, Granada, Spain, following the method described in Pagé and Barnes (2009). The chromite analyses were focused on the masses 45Sc, 47Ti, 51V, 55Mn, 59Co, 60Ni, 66Zn and 71Ga, and were conducted using a ~65 µm beam diameter, 10 Hz frequency, and fluence of 10 mJ/cm2, during 90 s analysis (30 s for the He gas blank and 60 s on the chromite).
The data obtained during ablation runs were processed using the IoliteTM V2.5 program (Paton et al., 2011), and aluminum values obtained by electron microprobe were used as the internal standard for chromite. The instrument was calibrated against the NIST 610 silicate glass (National Institute Standards and Technology; Jochum et al., 2011) using Al previously analyzed with EMPA. The basaltic glass BCR-2g (Norman et al., 1996; Gao et al., 2002) and the in-house secondary standard chromite G15-28 (Mercedita, Cuba; Colás et al., 2014) were analyzed as unknowns during each analytical run to check the accuracy and precision of the chromite analyses.
4.3. Whole-rock analysis of platinum-group elements
Whole-rock chromitite samples from the studied chromitite body were analyzed for platinum-group elements by at Genalysis Ltd (Perth, Western Australia) using nickel sulfide fire assay collection with ICP-MS (detection limits, 1 ppb for Rh and 2 ppb for Os, Ir, Ru, Pt, and Pd), following the method described by Chan and Finch (2001).
5. Chemistry of chromian spinel and silicates
About 300 single-spot electron-microprobe analyses were carried out on chromite and silicates from polished thin sections from the studied chromitite body as well as from the enclosing dunite and host harzburgite. Additionally, 45 in-situ analyses using LA-ICMP were performed on chromite from chromitite and host peridotites. Representative analysis of chromite and silicates are given in Tables 1 to 3.
Table 1 Ranges of major elements in chromite from the Cerro Colorado chromitite body and enclosing peridotites. Values of major elements are shown in wt.% as obtained from electron-microprobe.
| Massive n = 75 | Semimassive n = 30 | Disseminated n = 15 | Nodular n = 15 | Dunite n = 30 | Harzburgite n = 15 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Max | Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | Min | |
| SiO2 | 0.06 | 0 | 0.06 | 0 | 0.23 | 0 | 0.04 | 0 | 0.63 | 0 | 0.04 | 0 |
| Al2O3 | 25.37 | 21.01 | 25.54 | 24.49 | 31.2 | 29.59 | 31.16 | 29.61 | 27.55 | 19.25 | 25.41 | 21.84 |
| Cr2O3 | 46.15 | 41.63 | 43.18 | 41.19 | 37.32 | 36.27 | 40.12 | 36.41 | 41.86 | 34.6 | 44.34 | 40.24 |
| FeO | 13.43 | 12.05 | 12.6 | 11.81 | 11.44 | 10.66 | 15.44 | 11.52 | 20.82 | 15.2 | 16.03 | 14.25 |
| Fe2O3 | 4.27 | 2.9 | 4.47 | 3.26 | 3.84 | 3.05 | 3.87 | 0 | 10.97 | 6.07 | 4.85 | 4.04 |
| MgO | 15.34 | 14.14 | 15.56 | 14.82 | 16.82 | 15.96 | 16.19 | 12.84 | 13.64 | 8.83 | 13.97 | 12.38 |
| TiO2 | 0.35 | 0.1 | 0.37 | 0.18 | 0.19 | 0.1 | 0.18 | 0.1 | 0.31 | 0.19 | 0.1 | 0 |
| Cr# | 0.6 | 0.52 | 0.54 | 0.52 | 0.46 | 0.44 | 0.48 | 0.44 | 0.59 | 0.46 | 0.58 | 0.52 |
| Mg# | 0.69 | 0.65 | 0.7 | 0.68 | 0.74 | 0.72 | 0.71 | 0.6 | 0.62 | 0.43 | 0.63 | 0.58 |
Table 2 Ranges of minor and trace elements in chromite from the Cerro Colorado chromitite body and enclosing peridotites. Values are shown in ppm as obtained from LA-ICMPS.
| Massive n = 11 | Semimassive n = 12 | Dunite n = 11 | Harzburgite n = 7 | |||||
|---|---|---|---|---|---|---|---|---|
| Max | Min | Max | Min | Max | Min | Max | Min | |
| 45Sc | 3.3 | 2.9 | 3.78 | 2.73 | 6.09 | 4.16 | 2.61 | 1.61 |
| 47Ti | 1406 | 1040 | 1848 | 1631 | 1392 | 1233 | 413 | 254.4 |
| 51V | 1096 | 1017 | 1106 | 1022 | 1404 | 1260 | 1405 | 1247 |
| 55Mn | 1162 | 1096 | 1148 | 966 | 1824 | 1556 | 1603 | 1271 |
| 59Co | 217.3 | 205.2 | 212.9 | 184.6 | 365 | 315.3 | 488 | 306.1 |
| 60Ni | 1178 | 1008 | 1138 | 1042 | 1726 | 1369 | 1068 | 925 |
| 64Zn | 434 | 378 | 422 | 377 | 1229 | 1006 | 1707 | 1073 |
| 69Ga | 44 | 41.3 | 46.7 | 42.4 | 41.84 | 35.8 | 34.2 | 31.99 |
Table 3 Ranges of major and minor elements in silicates from the Cerro Colorado chromitite and enclosing dunite and harzburgite. Values are shown in wt.% as obtained from electron-microprobe.
| Olivine | Clinopyroxene | Orthopyroxene | ||||||
|---|---|---|---|---|---|---|---|---|
| Chromitite n = 85 | Cpx-bearing dunite n = 15 | Harzburgite n = 4 | Harzburgite n = 11 | |||||
| Min | Max | Min | Max | Min | Max | Min | Max | |
| SiO2 | 39.95 | 41.27 | 50.69 | 52.45 | 51.65 | 52.4 | 54.44 | 55.51 |
| TiO2 | 0 | 0.06 | 0.16 | 0.24 | 0 | 0.09 | 0 | 0.02 |
| Al2O3 | 0 | 0.39 | 2.73 | 4.24 | 2.82 | 3.14 | 2.14 | 2.56 |
| V2O3 | 0 | 0.05 | 0 | 0.07 | 0.01 | 0.07 | 0 | 0.04 |
| Cr2O3 | 0 | 0.07 | 0.79 | 1.21 | 1.32 | 1.53 | 0.67 | 0.96 |
| Fe2O3t | 5.9 | 7.2 | 2.15 | 2.55 | 2.41 | 2.5 | 5.62 | 6.09 |
| MgO | 51.23 | 53.1 | 15.58 | 16.7 | 16.48 | 16.81 | 32.2 | 34.33 |
| CaO | 0.02 | 0.07 | 24.06 | 24.67 | 23 | 23.26 | 0.8 | 3.68 |
| MnO | 0.07 | 0.14 | 0.01 | 0.08 | 0.08 | 0.11 | 0.14 | 0.17 |
| NiO | 0.39 | 0.52 | 0.02 | 0.09 | 0.05 | 0.09 | 0.08 | 0.12 |
| Na2O | 0 | 0.04 | 0.35 | 0.54 | 0.59 | 0.68 | 0.02 | 0.15 |
| K2O | 0 | 0.01 | 0 | 0.01 | 0 | 0.01 | 0 | 0.01 |
| Mg# | 0.93 | 0.94 | 0.93 | 0.92 | 0.92 | 0.92 | 0.91 | 0.91 |
5.1. Major and trace elements of chromite in chromitite, dunite and harzburgite
The chemistry of chromite in the Cerro Colorado chromitite body shows a relatively narrow compositional field (Figure 6a to 6d). The Cr# [(Cr/Cr+Al) atomic ratio] varies from 0.44 to 0.60 (corresponding to 36.3-45.2 Cr2O3 and 21-31.2 Al2O3) and Mg# [(Mg/Mg+Fe2+) atomic ratio)] from 0.60 to 0.74 (Figure 6a and 6c). There is a trend of increasing of Cr# from the outer part to the core of the body consisting of disseminated (0.44-0.46) and nodular textures (0.44-0.48) to semimassive (0.52-0.54) and massive textures (0.52-0.6) (Figure 6c). The accessory chromite of dunite enclosing this high-Al chromitite has even lower Cr# (0.46-0.59) than chromite of the chromitite and harzburgite (0.52-0.58) (Figure 7). The TiO2 contents analyzed with EMPA in chromite of the chromitite are relatively higher (up to 0.37 wt.%) than in dunite (0.19-0.31 wt.%) and harzburgite (<0.10 wt.%).
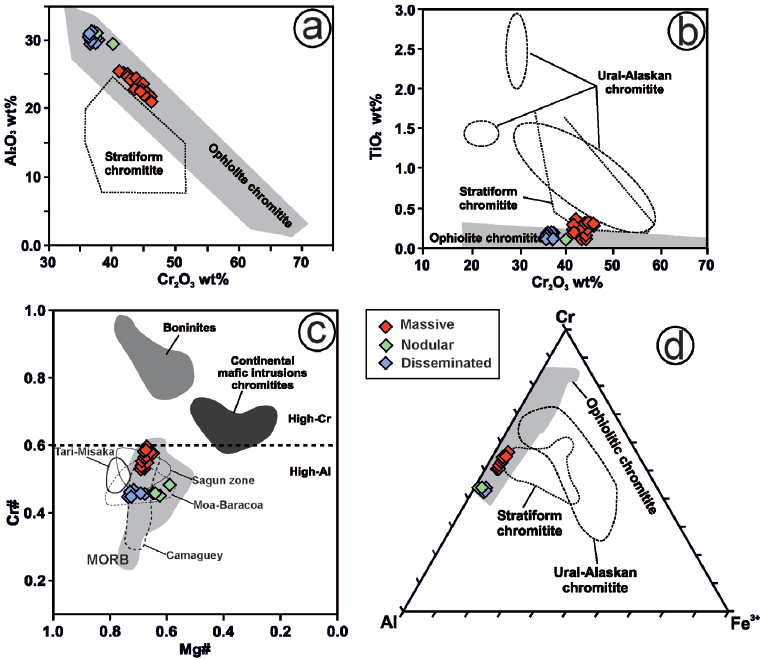
Figure 6 Chemistry of primary chromites from the Cerro Colorado chromitite as compared to chromite from various tectonic settings in terms of (a) Al2O3 vs. Cr2O3, (b) TiO2 vs Cr2O3, (c) Cr# [Cr/(Cr+Al) atomic ratio] vs Mg# [Mg/(Mg+Fe) atomic ratio] and (d) Al-Cr-Fe3+ compositions. Data sources for chromian spinel of different tectonic settings are Bonavia et al. (1993), Kamenetsky et al. (2001) and Proenza et al. (2007). Legend is inset in the figure (note that semi-massive samples shown in Table 1 include also the nodular chromitite plotted in the figure).
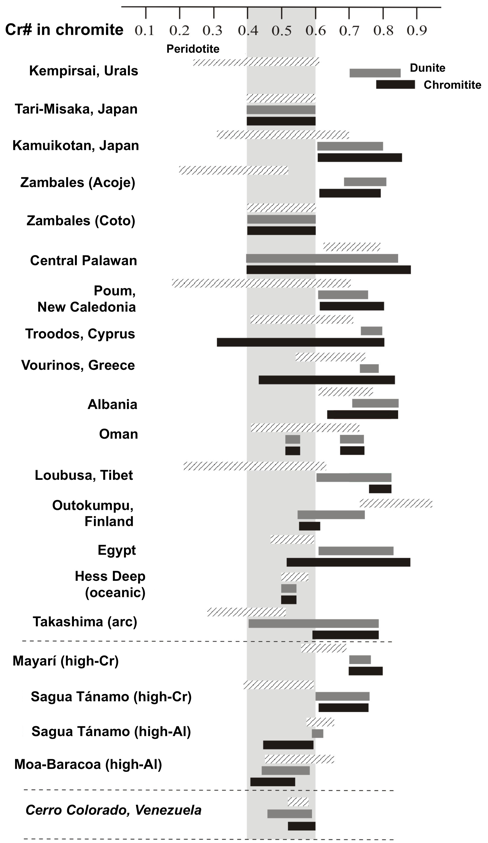
Figure 7 Comparison of Cr# in chromite from chromitites and enclosing host rocks of Cerro Colorado, and in podiform high-Cr and high-Al chromitites of some ophiolitic complexes. Data sources for high-Cr chromitites: CED, Egypt (Ahmed et al., 2001); Kempirsai, Kazakhastan (Melcher et al., 1994, 1997, 1999); S. Kamuikotan, Japan (Arai, 1997); Acoje, Philippines (Rammlmair et al., 1987); Troodos, Cyprus (Augé and Johan, 1988; McElduff and Stumpfl, 1991); Vourinos, Greece, (Economou, 1983; Economou et al., 1986; Economou-Eliopoulos, 1996); N. Oman (Ahmed and Arai, 2002; Rollinson, 2005); Albania (Kocks et al., 2007); Poum, New Caledonia (Leblanc, 1995); Luobusa, China (Zhou and Bai, 1992; Zhou and Robinson, 1994; Peng et al., 1995; Zhou et al., 1996); Mayarí and Sagua de Tánamo in Cuba (Proenza et al., 1999; Gervilla et al., 2005; González-Jiménez et al., 2011a). Data sources for high-Al chromitites: CED, Egypt, Outokumpu, Finland, and Tari-Misaka, Japan (Arai, 1997 in Ahmed et al., 2001); Sartohay, China (Zhou and Bai, 1992; Zhou and Robinson, 1994; Peng et al., 1995; Zhou et al., 1996); Moa-Baracoa, Cuba (Proenza et al., 1999; Gervilla et al., 2005).
In-situ laser ablation ICP MS analysis was used to determine concentrations of a suite of minor and trace elements (Ti, Ga, Ni, Zn, Co, V and Sc) of chromite from massive chromitites, dunite and harzburgite. To facilitate a comparison of all elements, the data were normalized to the composition of chromite from a mid-ocean ridge basalt (MORB) (Figure 8a to 8b), and plotted in order of compatibility in chromite (Pagé and Barnes, 2009; González-Jiménez et al., 2017b). Chromite forming massive chromitite has levels of Ti (1040-1848 ppm), Ga (41-47 ppm), Zn (377-434 ppm), Co (185-217 ppm) and Mn (966-1162 ppm) similar to chromite from MORB, but slightly higher V (1017-1106 ppm) and lower Sc (< 4 ppm) and Ni (1008-1178 ppm) (Figure 8a; Table 2). Accessory chromite in the dunite also has Ti (1233-1392 ppm) and Ni (1369-1726 ppm) similar to MORB but higher V (1260-1404 ppm), Zn (1006-1229 ppm), Co (315-365 ppm) and Mn (1556-1824 ppm) and slightly lower Sc (4-6 ppm), Ga (36-42 ppm) and Ni (1369-1726 ppm) (Figure 8b; Table 2. Chromite in harzburgite has even higher content of V (1247-1405 ppm), Zn (1073-1707 ppm), Co (306-488 ppm) and Mn (1271-1603 ppm) but much lower of Sc (2-3 ppm), Ga (32-34 ppm), Ti (254-413 ppm) and Ni (925-1068 ppm) than chromite in chromitite, dunite and MORB reference (Figure 8b; Table 2).
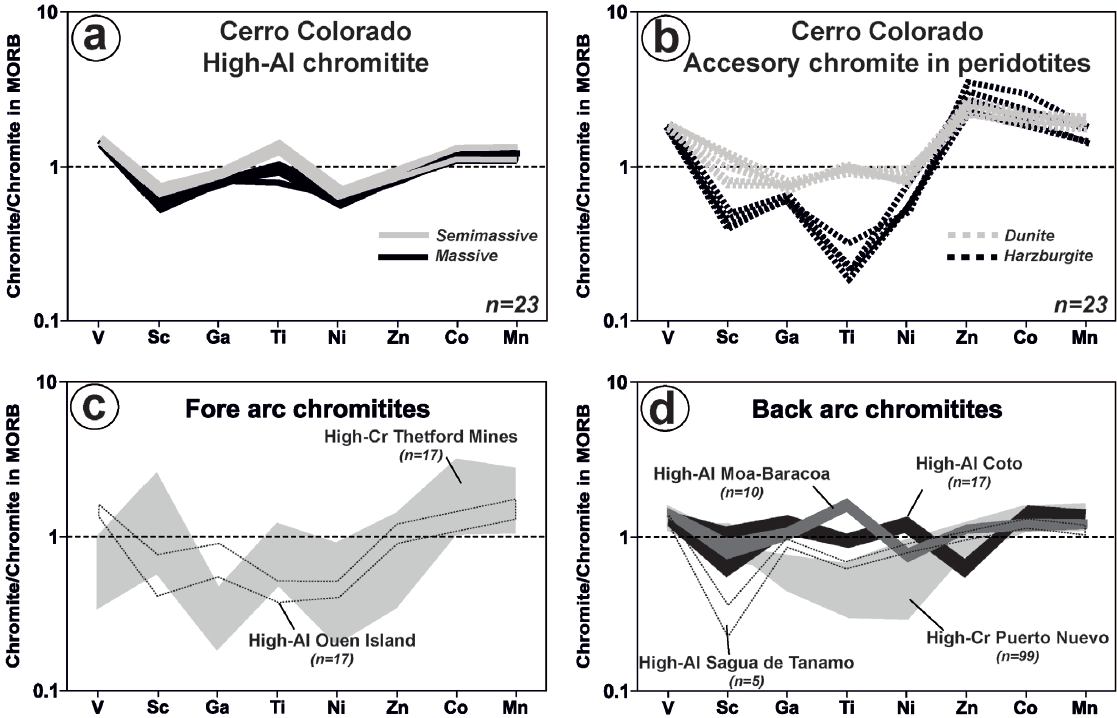
Figure 8 Spider diagrams showing the composition of minor and trace elements of chromite of massive and semimassive chromitite of the Cerro Colorado chromitite body and enclosing dunite and harzburgite (a-b) and comparison with other representative low-pressure chromitites from fore-arc and back-arc regions of supra-subdution zone ophiolites (c-d). Data sources for chromitites from the forearc mantle are Thetford Mines (Pagé and Barnes, 2009), Ouen Island in New Caledonia (González-Jiménez et al., 2011b) whereas those from back-arc mantle are from the eastern Cuban mining districts of Sagua de Tánamo (González-Jiménez et al., 2015) and Moa-Baracoa (Colás et al., 2014), the Mexican ophiolite of Puerto Nuevo (González-Jiménez et al., 2017b) and Coto in Philippines (Yao, 1999).
5.2. Silicates in chromitite, dunite and harzburgite
Unaltered olivine useable for electron-microprobe analyses has only been preserved in the matrix of massive chromitite. These olivines are forsterite (Mg# = 0.93), containing NiO (0.43 wt.%) and MnO (0.11 wt.%) (Table 3).
Clinopyroxene in dunite has relatively high Mg# (0.92-0.93) which roughly correlates inversely with Cr2O3 (0.79-1.21 wt.%) and Al2O3 (2.73-4.24 wt%); the TiO2 content reaches 0.24 wt.% and Na2O is lower than 0.54 wt.% (Figure 9a to 9c; Table 3). Clinopyroxene in harzburgite exhibits similar high Mg# (0.92), although with higher Cr2O3 (1.32-1.53 wt.%) and overall lower Al2O3 (2.82-3.14 wt.%) and TiO2 (<0.09 wt.%) (Figure 9a to 9c; Table 3).
Orthopyroxene in harzburgite enclosing the pair chromitite-dunite has slightly lower Mg# (0.91), Cr2O3 (0.66-0.96 wt.%), Al2O3 (2.14-2.56 wt.%) and TiO2 (<0.02 wt%) and Al2O3 than clinopyroxene (Figure 9e to 9f; Table 3).
6. Bulk-rock geochemistry of platinum group elements
The total PGE abundances in the studied chromitite body range between 60 and 109 ppb (average: 93 ppb), having the nodular chromitites samples lower values (60 ppb) than massive ones (96-109 ppb) (Figure 10; Table 4). The gold contents vary between 2 and 13 ppb. Overall, the analyzed samples have almost identical total contents of IPGE (Os+Ir+Ru=38-56 ppb; average: 39 ppb) and PPGE (Pt+Pd+Rh=65-16 ppb; average: 35). This distribution of the PGEs produces relatively flat PGE-chondrite normalized patterns, although nodular chromitites exhibit remarkable negative anomalies in Os, Pt and Pd (Figure 10).
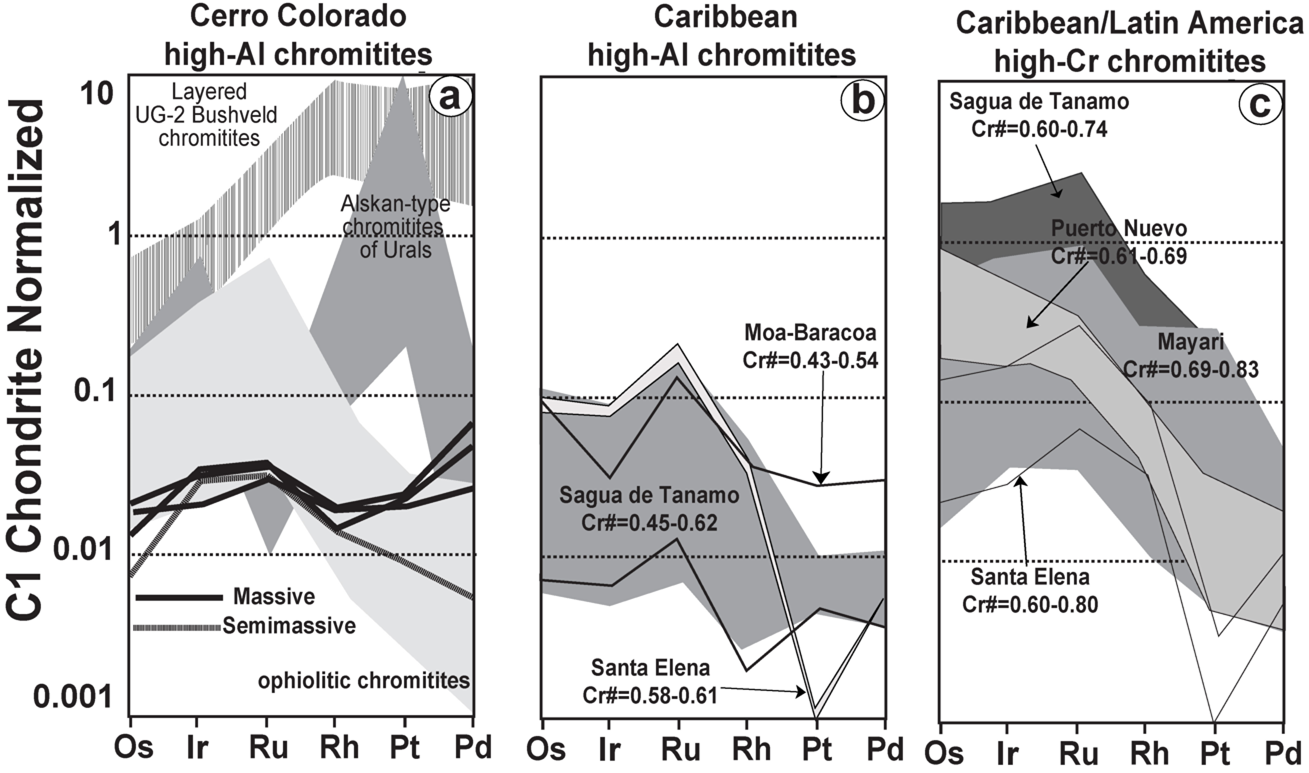
Figure 10 C1-chondrite (Naldrett and Duke, 1980) normalized patterns of the Cerro Colorado chromitite and comparison with chromitites from different crustal settings and hosted in the mantle section of ophiolites. (a) chromitites in Ural-Alaskan-type complexes (Garuti et al., 2005) of the Urals and the Bushveld (UG2) Layered Complex (Naldrett et al., 2012) and ophiolites (Proenza et al., 2007). (b) Caribbean high-Al chromitites (Sagua Tánamo and Moa-Baracoa in Cuba; Gervilla et al., 2005; González-Jiménez et al., 2011a), Santa Elena in Costa Rica (Zaccarini et al., 2011). (c) Caribbean and other Latin American high-Cr chromitites including Sagua Tánamo and Moa-Baracoa in Cuba (Gervilla et al., 2005; González-Jiménez et al., 2011a), Santa Elena in Costa Rica (Zaccarini et al., 2011) and Puerto Nuevo in Mexico (González-Jiménez et al., 2017b).
Table 4 Concentration (ppb) of platinum-group elements in the Cerro Colorado chromitite.
| Sample | Type | Os | Ir | Ru | Rh | Pt | Pd | Total | IPGE | PPGE | Pd / Ir |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PP3 | Nodular | 10 | 12 | 22 | 3 | 10 | 3 | 60 | 44 | 16 | 0.3 |
| PP5C | Massive | 4 | 17 | 23 | 4 | 24 | 37 | 109 | 44 | 65 | 2.2 |
| PP5D1 | Massive | 11 | 18 | 26 | 4 | 22 | 15 | 96 | 55 | 41 | 0.8 |
| PP10B | Massive | 7 | 19 | 26 | 3 | 23 | 27 | 105 | 52 | 53 | 1.4 |
7. Discussion
7.1. Parental melts of the chromitite
Chromite of the Cerro Colorado chromitite displays relatively high Al2O3 (21-31.2 wt.%) and low Fe2O3 (<4.5 wt.%) and TiO2 (< 0.37 wt.%) contents (Figura 6a, 6b and 6d; Table 1). This composition is typical of the chromite forming the high-Al chromitites that are usually found hosted in the mantle section of ophiolite complexes (Figure 6a to 6d), overlapping - at least in terms of major elements- that of chromian spinel from MORB sources (Figure 11a to 11d). Nevertheless, the slight differences in the chemistry of chromite forming massive, disseminated and nodular chromitite (Figure 6a and 6d) may reflect significant element exchange (mainly Fe2+ and Mg) between chromite and host peridotites during subsolidus re-equilibrium upon cooling of the chromitite body (Bussolesi et al., 2019 and references therein). Therefore, only composition of massive samples (i.e., nearly monomineralic chromite) should be used to estimate the nature of the parental melts.
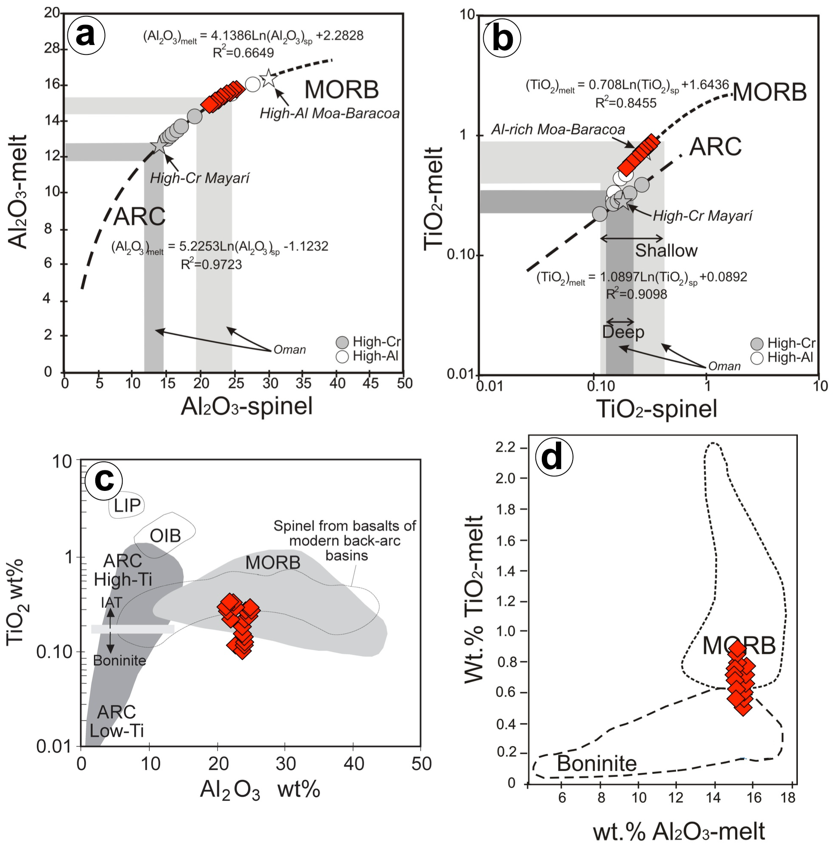
Figure 11 Al2O3 (a) and TiO2 (b) contents of the melt in equilibrium with the Cerro Colorado high-Al chromite. The regression lines are from Zaccarini et al. (2011) using data on chromite-melt inclusions in MORB and arc lavas reported by Kamenetsky et al. (2001) and Rollinson (2008). The range of chromite and the calculated melt compositions from the shallow and deep Wadi Rajmi chromitites in Oman (Rollinson, 2008), and for high-Al and high-Cr chromitites from Mayarí-Baracoa ophiolitic belt in eastern Cuba (González-Jiménez et al., 2011a) are shown for comparison. Only data of massive chromitite samples were used for computation. (c) Chemistry of chromite of massive chromitites forming the Cerro Colorado chromitite plot in terms of TiO2 versus Al2O3. Data sources for chromian spinel of different tectonic settings were obtained after Kamenetsky et al. (2001) and from the compilation of plots shown in Proenza et al. (2007). (d) TiO2 and Al2O3 (wt. %) content of the melt calculated to be in equilibrium with chromite from the Cerro Colorado chromitite compared to the fields for chromites from boninites and MORB sources (after Pagé and Barnes, 2009).
Several experimental (Maurel and Maurel, 1982; Wasylenki et al., 2003) and empirical works (e.g., Kamenetsky et al., 2001) have shown that Al2O3, TiO2 contents and FeO/MgO in chromites is a direct function of the contents of Al2O3, TiO2, FeO and MgO in the melt from which chromite had crystallized. A comparison of the geochemistry of chromite forming chromitites hosted in mantle rocks and associated extruded lavas has validated the feasibility of this approach showing that there is a link between the composition of the melt and chemistry of chromite (e.g., Rollinson, 2008; Pagé and Barnes, 2009; Farahat et al., 2011). Thus, the Al2O3 content in the melt can be estimated from the Al2O3 content from chromite using the equation proposed by Maurel and Maurel (1982):
or alternatively/complementary by using the empirical power-law expression proposed by Rollinson (2008) for MORB melts, which was partially derived from Kamenetsky et al. (2001):
The application of these two formulations to massive chromitites forming chromitites in different geotectonic settings (e.g., continental and oceanic lithosphere) have shown that the Al2O3 contents of the melt in equilibrium with chromite estimated by both methods correspond closely (e.g., Uysal et al., 2009; González-Jiménez et al., 2011a; Mukherjee et al., 2010; González-Jiménez et al., 2017b). Additionally, the TiO2 content in the melt from which the high-Al chromitite of Cerro Colorado crystallized can be also extracted using the TiO2 content measured in the high-Al chromite by applying the empirical equation obtained by Kamenetsky et al. (2001) for chromite of MORB sources:
while the FeO/MgO ratio of the melt can also be computed using the formula proposed by Maurel and Maurel (1982):
with FeO and MgO in wt.%, Al#=Al/(Cr+Al+Fe3+) and Fe3+#=Fe3+/(Cr+Al+Fe3+).
The estimated melt compositions in equilibrium with the Cerro Colorado high-Al massive chromitites of the main body have 14.88-15.7 wt.% Al2O3 and highly variable TiO2 (0.5-0.9 wt.%; average 0.72 wt%) and FeO/MgO ratios (0.95-1.13) (Figure 11a to 11d; Table 5). These results suggest that the Cerro Colorado high-Al chromitite body crystallized from melts with composition broadly similar to MORB in terms of Al2O3 (15-16 wt.%). However, the obtained TiO2 and FeO/MgO are overall lower than in MORB melts, suggesting an affinity closer to back-arc basin basalts (e.g., Dick and Bullen, 1984; Willson, 1989; Mudholkar and Paropkari, 1999). High-Al cromitites that have crystallized from melts with identical Al2O3 contents to those that formed the chromitite body studied here are known from other Mesozoic ophiolites of the Great Antilles Arc (Moa-Baracoa and Sagua de Tánamo mining districts in eastern Cuba; Proenza et al., 1999; González-Jiménez et al., 2011a as well as in Muğla and Elekdağ in Turkey (Uysal et al., 2009; Dönmez et al., 2014) and Oman (Rollinson, 2008) (Table 5). Similarly to observations in this study, all these high-Al chromitite were found located in the shallowest portion of the upper mantle section of the oceanic lithosphere, within the mantle-crust transition zone (i.e., Moho Transition Zone or MTZ) of the ophiolite. All these chromitites were interpreted to have precipitated from N-MORB or BABB melts in the shallow upper mantle, within the MTZ of oceanic lithosphere originated or modified in a SSZ back-arc setting.
Table 5 Calculation of Al2O3 and TiO2 contents and FeO/MgO ratios of the melts in equilibrium with chromite from the Cerro Colorado chromitite and other high-Al chromitites from Mesozoic ophiolites. The values for boninites and MORB are also presented for comparison. Ti values in the melt have been computed using the values obtained from the electron-microprobe analysis.
| Al2O3 liquid (wt%) | TiO2 liquid (wt%) | FeO/MgO liquid (wt%) | |
|---|---|---|---|
| Cero Colorado | 14.8-15.7 | 0.5-0.9 | 0.9-1.3 |
| Sagua de Tánamo (a) | 15-16 | 0.3-0.5 | 0.9-1.1 |
| Moa-Baracoa (a) | 16.4 | 0.9 | 0.9 |
| Elekdağ(b) | 14.7-17.3 | 0.5-1 | 0.3.08 |
| Muğla (c) | 15.2-15.9 | 0.7-1.1 | |
| Oman (d) | 14.5-15.4 | 0.4-0.9 | |
| Boninitite (e) | 10.6-14.4 | 0.1-0.5 | 0.7-1.4 |
| MORB (f) | 15-16 | 1.2-1.6 |
*a)Proenza et al. (1999) and González-Jiménez et al. (2011a, 2011b), (b)Dönmaz et al. (2014), (c)Uysal et al. (2009), (d)Rollinson (2008), (e)Hicky and Frey (1982), and (f)Wilson (1989).
7.2. Trace element fingerprints in chromitite for the tectonic setting of formation
Valuable information on the geochemical signature of the parental magmas and the tectonic setting of their genesis can be also obtained by studying the distribution of a suite of minor and trace elements in chromite (including V, Sc, Ga, Ti, Ni, Zn, Co and Mn) and platinum-group elements (Os, Ir, Ru, Rh, Pt and Pd) in the chromitite. A comparison of the MORB-normalized patterns of the chromitites analyzed in this study with other chromite from well constrained tectonic settings (Figure 8) reveals that they are clearly distinct to those displayed by igneous chromite forming the high-Cr chromitites found in the back-arc (e.g., Sagua de Tánamo in Cuba) or fore-arc (e.g., Thetford Mines in Canada) (Figure 8c) mantle sections from some ophiolites, whose chromite spidergrams have more affinity with those chromite from fore-arc boninites (Figure 8c). Rather the chromitites studied here are akin to those hosted in the back-arc mantle of the Cuban and Philippine ophiolites (Figure 8d). It is worth to note that Cerro Colorado chromitite exhibit MORB-normalized patterns strongly similar to that reported for chromitites of the Mercedita chromite deposit in the Mayarí-Baracoa ophiolitic belt in Cuba. The Mercedita chromitite is also hosted in a mantle with frequent gabbro sills and dikes, which has been interpreted as the petrological Moho Transition Zone of an oceanic lithosphere originated in the rear of the Cretaceous Great Antilles island arc (Proenza et al., 1999; Gervilla et al., 2005; Pujol-Solà et al., 2018).
Empirical and experimental estimations (e.g., Bockrath et al., 2004; Prichard et al., 2008; Fonseca et al., 2012, 2017; Luguet and Reisberg, 2016) have shown that appreciable concentration of the PGE > 100 ppb can only occur in mantle melts that have been from relatively depleted mantle source after relatively high rates of partial melting, at about 20%. The fact that in the Cerro Colorado chromitite the total PGE content is overall less than ~100 ppb (< 109 ppb; Table 4) suggest that partial melting degrees were not far beyond 20%. These rates of partial melting are typically produced in fast-spreading centers in mid-ocean ridges or relatively mature back-arc basins originated in the rear of intra-oceanic island arcs.
In the latter settings, melting of fertile or moderately depleted peridotite takes place in relatively anhydrous conditions, which are not enough to remove PGE-bearing minerals such as sulfides and alloys and release PGE into the produced silicate melt (e.g., Prichard et al., 2008; Luguet and Reisberg, 2016). These types of melts are usually S-saturated and therefore partition all the PGE almost equally, therefore chromitites crystallized from them are characterized by PGE-normalized patterns with no significant fractionation between IPGE (Os, Ir, Ru) and PPGE (Pt, Pd, Rh) such as observed in the Cerro Colorado chromitite.
These clearly contrast with the typical negative-slope (i.e., enrichment in Os-Ir-Ru over Pt-Pd-Rh) of the PGE-patterns that usually display mantle chromitite originated in the fore-arc or back-arc mantle in supra-subduction zones (e.g., González-Jiménez et al., 2014), whose parental melts are usually extracted from relatively depleted or moderately depleted sub-arc peridotites that have experienced higher rates of hydrous partial melting aided by the infiltration of slab-derived fluids.
On the other hand, some of the chromitite samples analyzed from the studied chromitite body display relative enrichment in Pd and Au (Figure 10; Table 4). This is a relatively unusual characteristic of mantle-hosted chromitite, which is usually attributed to post-magmatic remobilization of these metals. Secondary enrichment in Pd and gold is a distinctive feature of chromitites altered by hydrothermal fluids related with serpentinization and/or metamorphism (Thalhammer et al., 1990; Tarkian et al., 1991; Yang and Seccombe, 1993; Graham et al., 1996; Malitch et al., 2001; Proenza et al., 2008; González-Jiménez et al., 2016). Of particular interest is the silica-carbonate (litsvenitization) alteration that has precipitated magnesite and opal in the secondary crack-seal of the Cerro Colorado chromitite (Martín-Belliza, 1960; Franco and Torrealba, 1987; Mistage et al., 1989). Several studies have shown that this style of post-magmatic alteration is a very effective in the remobilization and concentration of gold (of up to 1000 times the original value) in ultramafic rocks and associated chromitites (Buisson and Leblanc, 1986; Escayola et al., 2009; Buckman and Ashley, 2010; Azer, 2013).
Summarizing, the Cerro Colorado chromitite has geochemical fingerprints for chromite and bulk-rock PGE that are compatible with those chromitites hosted in the oceanic mantle (back-arc mantle) section of SSZ ophiolites. This is consistent with the fact that the chromitites associated with gabbro sills with no evidence of HP/UHP metamorphism, similarly the ordinary low-pressure high-Al chromitites reported from the Moa-Baracoa ophiolitic massif in eastern Cuba (e.g., Pujol-Solà et al., 2018).
7.3. Formation of the Cerro Colorado chromitite body
As noted above, in the high-Al chromitite of Cerro Colorado the relations between Al2O3 and TiO2 shows that the composition of chromite in chromitite overlaps the fields of MORB and spinel from modern back-arc basin basalts (Figure 11c). Similarly, the compositions of accessory chromite found in the host dunite envelope and harzburgite also span over the overlapping field of MORB and spinel from back-arc basin basalts (Figure 8b to 8d). This suggests a back-arc affinity, consistent with the composition of the melt estimated in equilibrium with chromite of the chromitite, distribution of trace element in chromite and bulk-rock PGE. Moreover, in plots Cr# vs. Mg# the chemistry of clinopyroxene and orthopyroxene found in the country peridotite overlap the fields of abyssal (MORB) and back-arc (Figure 11c), whereas coexisting gabbro and basalts have also back-arc affinity (Santamaría and Schubert, 1974, Baquero et al., 2013). Interestingly, chromite from chromitite and dunite exhibit almost identical Cr contents, which are broadly similar and higher respectively than chromite from host harzburgite (Figure 7). This observation indicates that chromitite and dunite very likely crystallized in equilibrium from a common parental melt that was slightly different from those extracted during the formation of the harzburgite. Indeed the formation of the chromitite-dunite pair involved the reaction of mantle harzburgite with a melt slightly richer in Al and Ti (BABB) than the one involved in the formation of accessory chromite (MORB affinity) in harzburgite. In the chromitites, the higher Cr# and TiO2 in massive samples compared with nodular and disseminated samples may reflect the fact that migrating melts with BABB affinity have had their composition by the reaction with the country harzburgite. Thus, during melt-rock processes under increasing melt/rock ratio the primitive BABB-like melt became progressively enriched in silica content and Cr#, thus explaining the observed chemical trend in chromite from country harzburgite to the chromitite-dunite pair (Figures 7 and 8; Zhou et al., 1994; Morishita et al., 2011; González-Jiménez et al., 2011a). These chemical trends in the major elements were also accompanied by increasing of Sc, Ga and Ni but decreasing Zn, Co Mn from country harzburgite to the chromitite-dunite pair (Figure 8a and 8b). It has been shown by empirical (Pagé and Barnes, 2009, Dare et al., 2009) and experimental studies (Mallmann, et al., 2009) that Sc and Ga contents correlate positively with f O2in chromian spinel. This leads us to suggest that the selective enrichment of incompatible elements shown by chromian spinel of the Cerro Colorado chromitites was associated with a progressively increasing of f O2 in the parental melt(s). This situation is typical in the back-arc mantle wedge of suprasubduction zones, where relatively more oxidized melts can form compared with typical MORB (Parkinson and Arculus, 1999) In this scenario, the crystallization of the chromitite would account by hybridization as a result of mixing/mingling of different batches of BABB melt within the dunite conduit continuously replenished by hotter primitive melts. The disseminated and nodular chromitite would record different steps of the progression of the primitive melt infiltration that have promoted melt-rock reaction, dunite formation and melt mixing/mingling within the dunite channel (González-Jiménez et al., 2014).
7.4. Geodynamic and metallogenic implications
The structure of the present margin of the northern part of South America in Venezuela is the result of accretion of several convergent-basin complexes between the Jurassic-Cretaceous. The geology and tectonic evolution of the Caribbean plate indicate that during Lower Cretaceous multiple spreading centers were developed in the realm (hanging wall) of the intra-oceanic Great Antilles Arc (Pindell et al., 2012). Remnants of these back-arc basins (LREE-depleted MORB) are now preserved in ophiolites exposed in the continental crust of Venezuela, Costa Rica and Guatemala, and in the mainland of the islands of Cuba and La Hispaniola (Giunta et al., 2002). Many of these ophiolites contain chromitites with compositions varying between high-Cr and high-Al, which have been related with the existence of melts that originated in different magmatic sources of the ophiolite environment (i.e., MOR, SSZ and arc regions) at different times during the formation and/or evolution of this oceanic lithosphere (e.g., Proenza et al., 1999; Lewis et al., 2006; Gervilla et al., 2005; González-Jiménez et al., 2011a; Marchesi et al., 2011). For example, the formation of the high-Al chromitites of the Moa-Baracoa ophiolite in eastern Cuba has been associated with tholeiitic magmas (BABB) originated in a relatively evolved back-arc basin (Proenza et al., 1999; Gervilla et al., 2005). The formation and evolution of this ophiolite is dated between 90-125 Ma (Iturralde-Vinent et al., 2006; Rojas-Agramonte et al., 2016) and igneous zircons recovered from these high-Al chromitite yield ages of 99-118 Ma that overlap, within uncertainty, the U-Pb age of zircons from a Fe-Ti-rich gabbro intruding the mantle peridotite section at the Moa-Baracoa ophiolitic massif (124 ± 1Ma; Rojas-Agramonte et al., 2016). Interestingly, K-Ar ages obtained for basalts and gabbros of the oceanic crust of Cerro Colorado ophiolite overlap that of the Moa-Barcoa ophiolite (118-129 Ma; Santamaría and Schubert, 1974) while U-Pb dating of zircons from these gabbros yield 121.7 ± 2 Ma (Baquero et al., 2013). Assuming that at Cerro Colorado the chromitites formed co-genetically with the crustal gabbros, the scenario above suggest that the high-Al chromitites of Cerro Colorado may have formed synchronically and in an analogous tectonic setting than high-Al chromitites from eastern Cuban ophiolites (Figure 12).
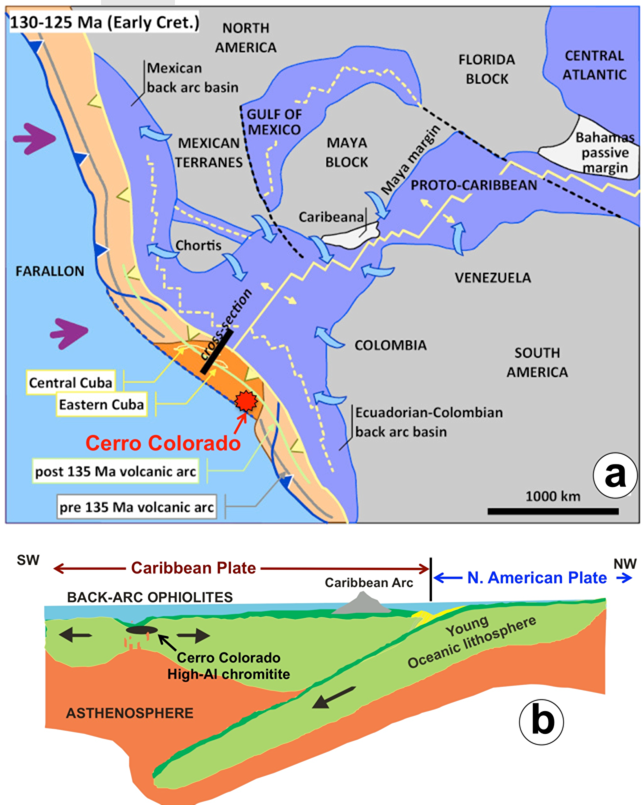
Figure 12 (a) Paleogeography and tectonic reconstruction of the Caribbean region (modified from Proenza et al., 2018 and references therein) showing the Lower Cretaceous onset of subduction of the Proto-Caribbean lithosphere with reference to the paleogeographical location of the Cerro Colorado and equivalent Cuban ophiolites. (b) Cross-section drawing showing the tectonic setting of formation of the Cerro Colorado high-Al chromitite in the rear of the Caribbean arc (i.e., Greater Antilles arc) during Lower Cretaceous.
On the other hand, the podiform-like chromitite body in the Cerro Colorado ophiolite with Cr2O3= 35-45 wt.%, low Fe contents (Cr/Fe= 2.6), Al2O3 >20 wt.% and Cr2O3 + Al2O3> 60 wt%, although MgO content is relatively high (average 15 wt.%). A combination of results obtained from field observations and study of drill-boreholes allow an estimation of 4000 tons of chromite ore. These data indicate that the Cerro Colorado ophiolite holds the promise of a source of refractory chromite in Venezuela. Such possibility needs to be further addressed in order to better define the possible existence of other chromitite bodies in the region.
8. Conclusions
The Cerro Colorado chromitite is a podiform-like body of ophiolitic affinity, which is classified compositionally as the high-Al type (Cr#< 0.6). Major, minor and trace element fingerprints in chromite and bulk-rock PGE contents indicate that this chromitite very likely formed from a melt of tholeiitic affinity within a back-arc setting. It is suggested that the back-arc mantle experienced partial melting degrees below 20%, which were not high enough to extract significant amounts of Cr and noble metals.
Previous age constraints of the Cerro Colorado ophiolite together with the geochemical fingerprints elucidated here for the chromitite allow to link the formation of the Cerro Colorado chromitite with the evolution of the Great Antilles Arc during Lower Cretaceous. The formation of the Cerro Colorado chromitite can be geologically linked with melts that infiltrated the mantle of a relatively mature back-arc basin originated at the rear of this intra-oceanic island arc. The Cerro Colorado high-Al chromitites, and therefore their associated ophiolitic mantle and crustal rocks, may indeed be similar to those reported in Cuba. This is a feature that should be further assessed and accounted for future palinspastic restorations for the region.
The scientific characterization of the Cerro Colorado chromitite is also valuable from a metallogenetic point of view, revealing the existence of a source of chromite of refractory grade in Venezuela. This discovery holds the promise for new discoveries of chromium resources in the region.











 nueva página del texto (beta)
nueva página del texto (beta)




