INTRODUCTION
The Rarámuri people are one of the most conservative indigenous ethnic groups remaining in northwestern Mexico in Sierra Tarahumara, Chihuahua (Espinosa & Ake, 2013). These regions are of primary concern for biodiversity conservation, especially for the edible plants that Rarámuri consume (Arriaga et al., 2000). Hence, it is important to document the properties of foods that contribute to their diets in addition to other social, cultural, and economic values.
Pinole (kobisi in the Rarámuri language) is a fine flour made from toasted corn, and it constitutes approximately half of the diet of the Rarámuri (Bennet & Zingg, 2012). According to the data obtained in the research project about the food products of the Sierra Tarahumara in which some of the authors are involved, moisture of pinole ranged between 5.6 to 7.7%, protein between 7.8 to 8.2%, fat between 4.5 to 5.3%, and, per difference, carbohydrates accounted for 75.8 to 78%. The content of crude fiber was close to 2.5% and ashes ranged from 1.3 to 2%. Although the amount of protein is high, compared to other foods, it is important to mention that corn is characterized by a deficiency in lysine and tryptophan, and this remains in the traditional pinole where Lozano-Aguilar, Solórzano-Vega, Bernal-Lugo, Rebolledo-Robles & Jacinto-Hernández (2008), reported content of 2.2% of lysine and 0.3% of tryptophan which represents 39% and 30%, respectively, of the requirements established by the FAO. An important aspect to consider is that the traditional diet of the Rarámuri, in addition to pinole and other types of corn-based foods, includes beans, a legume rich in lysine (Guzmán-Maldonado, Acosta-Gallegos & Paredes-López, 2000) that complements the diet of the Rarámuri.
Pinole from Sierra Tarahumara is made from the endemic maize variety ‘Cristalino de Chihuahua’ (Burgess & Albino, 2010; Linares, Bye & Mera, 2016). The kernels are heated and popped to obtain popcorns which are then finely ground on a metate. This produces a hygroscopic powder that can be easily dispersed in water (Linares et al., 2016; Sánchez-Herrera, Martínez-Cano, Maldonado-Santoyo & Aparicio-Fernández, 2014).
Different corn varieties and several traditional methods are used to obtain pinole, which until now have been scarcely studied. Sánchez-Herrera et al. (2014) noted the pinole atole (porridge beverage) was higher in protein, fat, and phenolic compounds than common breakfast cereals, then pointed out the nutritional quality of the pinole. However, there is little information on the sensory characteristics and the volatile compounds of pinoles. Sensory characteristics are essential in food quality and also one of the keys for consumers to recognize the authenticity of traditional foods (Guerrero et al., 2009).
To evaluate the sensory characteristics, the use of rapid descriptive tests is increasing day by day, and their results correlate well with those of classical techniques (Aguiar, Melo & de Lacerda de Oliveira, 2019). One of these methods, modified Flash Profiling, was applied to study some traditional Mexican tortillas (Arnés, Severiano-Pérez & Astier, 2022). Furthermore, indirect techniques such as the olfactory profile (by electronic nose) or the volatile profile (by gas chromatography) are used to complement sensory information.
Electronic noses (e-noses) are quick and non-destructive tools made up of sensors that react to different volatile substances, which create an odour fingerprint, which is related to the sensory odour of food (Alasalvar, Pelvan, Bahar, Korel & Ölmez, 2012). Using different e-nose equipment, it has been possible to differentiate aromatic profiles in freshly squeezed strawberry juice during storage at 4 ± 1 °C by PEN3 (Airsense Analytics Gmbh, Schwerin, Germany) (Zhang, Pan & Tu, 2023); as well as different flavor profile of roasted beef meat using Alpha-MOS Fox Electronic Nose (E-nose Fox 4000, France) (Al-Dalali, Li & Xu, 2022). In another study, different volatile compounds were identified and characterized in five high-yielding soybean varieties using HERCALES Fast Gas Chromatography (GC) electronic nose (Ravi, Taheri, Khandekar & Millas, 2019). Gas chromatography (GC) allows identify and determine volatile organic compounds (VOCs) in foods. GC was used to evaluate the VOCs of different corn products like popcorn, corn starch and cereal foods (Pico, Tapia, Bernal & Gómez, 2018).
This work aimed to contribute to the knowledge of the sensory attributes of Tarahumara pinole and its volatile composition. Furthermore, the contributions of the raw material and its processing to the differentiation of Rarámuri pinole, an energetic ethnic food, from other similar products will be investigated.
Materials and Methods
Pinole samples
Pinole samples were obtained directly from Tarahumara families. Two batches of pinole (named in this study PA and PB) were collected in the Chihuahuan municipality of Bocoyna (Gonogochi), and a third one (PC) was obtained in the municipality of Urique. Batches of 2 kg of pinole were prepared following the traditional process. Grains can explode and form large, puffed flakes when heated with oak firewood in a special pot (olla esquiatera), open to the atmosphere (CONABIO, 2017; Linares et al., 2016). Then, popped corn is ground on a specific device called metate to get a powder formed by irregular particles. These expanded kernels make approximately up to 60% of the total metate-grounded mass, the rest being unpopped kernels. The elaborated pinole was conserved in carefully closed double plastic bags, under conditions similar to those the Rarámuri use to store pinole to be consumed for over a year.
Sensory analysis
A modified flash profile (MFP) methodology, as described by Väkeväinen et al., (2020) was used for sensory analysis of pinole. The MFP was carried out in the Sensory Evaluation Laboratory of the National Autonomous University of Mexico, which complies with the ISO 8589:2007 standard.
Eight panellists (half female and half male; 20-24 years old) from the Faculty of Chemistry of the National Autonomous University of Mexico participated in this experiment. The selection of panellists included screening and those with certain characteristics such as allergies, pregnancy, etc. were excluded. All panellists signed written consent and the project was approved by the Ethics and Scientific Responsibility Commission of the Faculty of Sciences of the UNAM. Folio PI_2021_11_18_Severiano.
Panellists were previously trained in the evaluation of products from Tarahumara milpas, including pinole-based foodstuffs like cookies, but not powdered pinole. Flash profiling allows working with trained judges to reach an agreement on how each attribute will be evaluated without generating a document with its definition and without using standards for them. For this reason, within the results of the MFP, it is important to include the consensus of the evaluation of the panellists (Väkeväinen et al., 2020).
Pinole samples, 12 g of powder, were weighed (Ohaus Series 700/800 balance, OHAUS, Mexico, D. F.) and placed in white plastic coded containers (50 mL). The samples were stored at room temperature (∼22 °C, ∼90 minutes) until the beginning of the sessions. Water was provided for mouth rinsing between samples. The order of the samples was random. Sensory evaluation sessions and questionnaires were designed using FIZZ software (Biosystems, version 2.51c, Acquisition and Judge modules, Courtenon, France).
In the first MFP session, the panellists individually generated the appearance, texture, odour, and taste attributes of pinole (the panellists did not use hedonic responses). Subsequently, they discussed and selected by consensus the attributes that best described the pinole. The panellists understood what would be evaluated on each attribute. In a second session, the panellists individually evaluated the samples in a monadic sequential way using a 9-point intensity scale, ranging from 1 (very low intensity) to 9 (very intense). All evaluations were carried out in duplicate.
Odour fingerprint
Each pinole sample (100 g) was ground in a grinder (Moulinex, Mod., Mexico, D. F.) and sieved on a 0.25 mm mesh sieve to obtain 44 g of sieved and homogenized sample. Odour fingerprint was measured directly on the powdered samples, following the procedure described by García-Lomillo, González-SanJosé, Del Pino-García, Ortega-Heras & Muñiz-Rodríguez (2016). Briefly, 1.5 grams of pinole were placed in 10 mL vials 125X10-CV P328 (Chromacol™, Thermo Scientific, Barcelona, Spain) and immediately sealed with a metallic cap with chlorobutyl/polytetrafluoroethylene seal (Chromacol™, Thermo Scientific). Samples were slightly heated under fixed conditions, after that a volume of the headspace was injected by an autosampler HS100 CTC-Combi-Pal (CTC Analytics AG, Zwingen, Switzerland) in a α-FOX 4000 electronic nose (AlfaMOS, Toulouse, France) equipped with 18 metal oxide sensors. Samples were analyzed five times. The sensor responses were collected for two minutes, and the maximum response intensity of each sensor was registered. The set of the average values of each sensor defined the odour fingerprint of each pinole.
Volatile compounds identification
Sifted pinole samples (1.5 g) were transferred to 10 mL headspace glass vials and sealed in the same way as mentioned in the odour fingerprint analysis. Five vials for each batch of pinole were prepared and measured. Headspace solid-phase dynamic extraction (HS-SPDE) was carried out based on the procedure reported by García-Lomillo et al. (2016), using an automated sample injector (CTC Analytics AG, Zwingen, Switzerland), in which the vials were incubated at 55 °C and volatile organic compounds were extracted from the powder headspace using a preconditioned SPDE-syringe. The VOCs accumulated inside the needle in a fiber coated with a non-polar stationary phase (90% polydimethylsiloxane (PDMS) and 10% activated carbon). Fifty syringe extraction cycles were made. Then, volatile compounds were thermally desorbed from the fiber in the injection port at 250 °C and into the gas chromatography-mass spectroscopy system (Agilent Technologies 6890N) using helium as carrier gas. The separation was conducted in a 007-WAX capillary column (Quadrex Corporation, New Haven, USA) (60 m length, 0.32 mm inside diameter, and 1 μm film thickness). Compounds were identified by comparison to the mass spectra of the Wiley 7th and NIST 98 libraries. The areas for each chromatographic peak were calculated, and mean values were reported along with their corresponding standard deviation.
Statistical Analysis
Multidimensional analysis of data was performed by XLSTAT statistical add-in for Microsoft Excel (version 2020.2.2, Addinsoft).
Statistical analysis of Flash Profile data was performed using the multivariate Generalized Procrustes Analysis (GPA) method to assess panel consensus and to visualize the relative sensory positioning of attributes and products (Väkeväinen et al., 2020).
To simultaneously analyse the results corresponding to chromatography, odour fingerprint and sensory analysis, Multiple Factor Analysis (MFA) was performed. Data from this latter analysis were logarithmically transformed before modelling.
Results
Sensory profile
The sensory profile of pinole was finally made up of seventeen attributes (Table I), including properties of appearance, odour, flavour, and texture. Significant differences between the sensory characteristics of the three pinoles studied were found.
Table I Sensory attributes generated by modified Flash Profile for pinole powders from Sierra Tarahumara.
| Attributes | |||
|---|---|---|---|
| Appearance | Odour | Texture | Flavour |
| Color (beige-coffee) Homogeneity Particle size Toasted Humidity Floury |
Corn Sweet Pinole Peanut Toasted |
Humidity Grainy |
Corn Sweet Pinole Toasted |
Results of Flash Profiling were analysed by GPA evaluating the multi-variability of the samples. A biplot graph (Figure 1) of the two principal components, which explain the total variability (59.4% PC1 and 40.6% PC2), summarizes the differences between the samples.
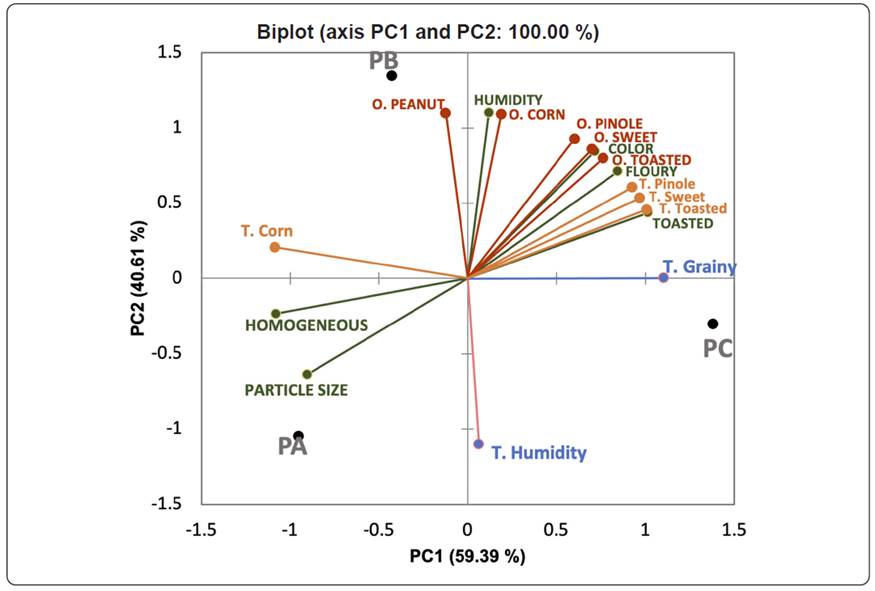
Figure 1 Sensory configuration obtained from the attributes data of three pinole powders (PA and PB from Gonogochi and PC from Urique). Appearance attributes are marked with blue line and capital letters, odour attributes (O) are marked with red line and capital letters, flavour (F) attributes are marked with orange line and texture (Tx) with green line.
In general, the three samples were clearly separated based on their sensory characteristics. Pinole C is richer in olfactory and gustatory notes, being correlated to the greatest number of sensory attributes. It is intensively different from pinoles A and B.
Odour fingerprint analysed by e-nose
Analysis by MOS electronic noses could be useful for studying similarities or differences between the “odour fingerprint” of products. Furthermore, if the sensor responses can be associated with volatile compounds and sensory parameters, they could provide approximate information on the odour profile.
The results of 11 of the 18 sensors that make up the device were used to obtain the odour fingerprint. Data from the other 7 sensors were not included in the analysis due to low responses recorded (response intensity < 0.05) (García-Lomillo et al., 2016). Statistically significant differences were found (p < 0.05) in only four sensors (T70/2, PA/2, T40/2, TA/2).
These data indicated that, although with some differences, the global volatile fraction of the three pinoles seemed to be very similar, probably because the three pinole samples were prepared from the same variety of corn.
Volatile compounds in pinole
A global of forty-nine VOCs could be identified, based on mass spectra data, in the three pinole samples (Table II). Twenty-two of the identified volatiles: acetic acid, 2-acetyl-pyridine, benzaldehyde, butyrolactone, 2,6-dimethyl-pyrazine, diethyl phthalate, dodecane, 2-ethyl-5-methyl-pyrazine, 2-ethyl-6-methyl-pyrazine, furfural, furfuryl alcohol, 5-methyl-furfural, 2-methyl-pyrazine, (E)-2-nonenal, nonanal, octanoic acid, 1-pentanol, 2-pentylfuran, propionic acid, 2,3,5-trimethyl-pyrazine, 4-vinyl-guaiacol, p-xylene had been previously detected in popcorn and corn derivates (Burdock, 2010; Buttery, Ling & Stern, 1997; Park & Maga, 2006; Pico et al., 2018). In general, the volatile profile of the pinole includes acids, alcohols, aliphatic aldehydes, alkanes, aromatic compounds, furans, lactones and nitrogen compounds.
Table II Peak area of volatile compounds identified by HS-SPDE/GC-MS in pinole samples (PA, PB and PC) from Sierra Tarahumara (mean+/- standard deviation, n=5).
| Code | Volatile Compound | Molecular ion | Pinole A | Pinole B | Pinole C | |||
|---|---|---|---|---|---|---|---|---|
| Alcohols | ||||||||
| VC1 | 1-pentanol | 31 | 5.5X105 | ±5.7X103b | 3.9X105 | ±3.1X103c | 1.4X106 | ±1.4X104a |
| Aliphatic aldehydes | ||||||||
| VC2 | nonanal | 41 | 2.9X106 | ±3.9X104 b | 1.0X106 | ±1.1X104c | 5.4X106 | ±7.9X104a |
| VC3 | (E)-non-2-enal | 43 | 2.4X104 | ±1.5X103c | 2.4X105 | ±4.4X103b | 5.7X105 | ±1.2X104c |
| Nitrogen compounds (pirazynes, secondary heterocyclic amine and pyridine) | ||||||||
| VC4 | 1-pyridin-2-ylethanone | 79 | 7.3X105 | ±2.0X104b | 6.9X105 | ±1.7X104b | 8.5X105 | ±3.5X104a |
| VC5 | (2E)-2-pyrrolidin-2-ylideneacetonitrile | 107 | 1.4X106 | ±7.6X103a | 2.4X105 | ±1.8X103c | 1.0X106 | ±6.6X103b |
| VC6 | 2,6-dimethylpyrazine | 108 | 2.1X106 | ±1.8X104b | n.d. | 3.7X106 | ±2.5X104a | |
| VC7 | 2-ethyl-6-methylpyrazine | 121 | 3.7X105 | ±5.9X103b | 1.5X105 | ±2.1X103c | 1.1X106 | ±1.9X104a |
| VC8 | 2-ethyl-5-methylpyrazine | 121 | 2.4X105 | ±1.0X103c | 1.4X107 | ±5.4X104a | 1.9X106 | ±6.1X103b |
| VC9 | 2-methylpyrazine | 94 | 3.2X106 | ±1.1X104b | 5.7X105 | ±3.3X103c | 8.9X106 | ±3.4X104a |
| VC10 | 2,3,5-trimethylpyrazine | 42 | 2.3X106 | ±1.9X104a | 3.1X105 | ±1.9X103c | 1.8X106 | ±8.0X103b |
| Furans and aromatic compounds | ||||||||
| VC11 | benzaldehyde | 106 | 1.1X106 | ±2.7X104b | 3.2X105 | ±6.5X103c | 3.1X106 | ±8.9X104a |
| VC12 | 1-methyl-2-propan-2-ylbenzene | 93 | 5.9X105 | ±3.1X103b | 3.3X106 | ±1.8X104a | 4.2X105 | ±2.9X103c |
| VC13 | 2,6-ditert-butyl-4-ethylphenol | 219 | 3.4X105 | ±1.1X103c | 3.8X106 | ±1.2X102a | 1.3X106 | ±1.3X104b |
| VC14 | 1-methoxy-4-prop-2-enylbenzene | 148 | 3.0X105 | ±1.4X103c | 1.2X107 | ±4.9X104a | 5.1X106 | ±2.6X104b |
| VC15 | furan-2-carbaldehyde | 97 | 1.2X107 | ±3.5X104a | 1.2X107 | ±3.2X104a | 9.9X106 | ±2.7X104b |
| VC16 | furan-2-ylmethanol | 81 | 1.9X106 | ±3.1X104b | 8.0X105 | ±2.0X104c | 2.4X106 | ±4.8X104a |
| VC17 | 4-ethenyl-2-methoxyphenol | 150 | 1.8X106 | ±4.1X104b | 1.7X105 | ±3.7X103c | 2.8X106 | ±9.8X104a |
| VC18 | 5-methylfuran-2-carbaldehyde | 111 | 9.1X105 | ±5.2X104b | 5.2X105 | ±2.2X104c | 1.0X106 | ±6.5X104a |
| Furans and aromatic compounds | ||||||||
| VC19 | 2-pentylfuran | 81 | 2.3X105 | ±4.7X103c | 4.1X105 | ±5.9X103b | 2.7X106 | ±1.7X104a |
| VC20 | 1,3-xylene | 91 | 9.2X105 | ±4.5X103c | 3.3X106 | ±1.6X104a | 1.5X106 | ±6.3X103b |
| VC21 | 1,2-xylene | 91 | 4.1X106 | ±3.7X103b | 1.9X106 | ±1.2X103c | 1.0X107 | ±7.3X103a |
| VC22 | 1,4-xylene | 91 | 3.1X106 | ±2.6X103c | 6.3X106 | ±3.8X103a | 5.6X106 | ±1.9X103b |
| VC23 | 1,2,3-trimethylbenzene | 121 | 1.0X107 | ±8.7X104a | 8.0X106 | ±6.6X103b | 2.3X106 | ±1.9X104c |
| Acids and lactones | ||||||||
| VC24 | acetic acid | 43 | 2.6X107 | ±5.5X104c | 5.3X107 | ±6.9X102a | 3.2X107 | ±9.9X104b |
| VC25 | benzoic acid | 105 | 1.3X106 | ±3.7X104b | 2.8X105 | ±3.3X103c | 2.5X106 | ±9.9X104a |
| VC26 | oxolan-2-one | 42 | 1.3X106 | ±7.4X103b | 2.3X106 | ±1.4X104a | 1.3X106 | ±6.6X103c |
| VC27 | 3H-2-benzofuran-1-one | 105 | 4.9X105 | ±2.5X103b | n.d. | 6.6X105 | ±9.9X104a | |
| VC28 | octanoic acid | 117 | 2.8X105 | ±1.4X103b | 2.7X105 | ±1.4X103b | 3.3X106 | ±2.8X104a |
| VC29 | propanoic acid | 28 | 8.4X105 | ±1.2X104a | 8.5X105 | ±1.4X104a | 8.3X105 | ±1.2X104a |
| Alkanes | ||||||||
| VC30 | 1-chlorohexadecane | 58 | 1.9X106 | ±8.9X103a | 2.5X105 | ±2.5X103c | 6.0X105 | ±5.4X103b |
| VC31 | decane | 43 | 1.0X107 | ±1.6X104a | 3.4X106 | ±8.4X103c | 7.6X106 | ±2.6X104b |
| VC32 | 3,3-dimethylhexane | 43 | 5.3X106 | ±9.5X103b | 2.0X106 | ±3.9X103c | 7.4X106 | ±2.9X103a |
| VC33 | 2,6-dimethyloctane | 57 | 7.7X105 | ±5.8X103b | 7.9X105 | ±3.6X103b | 8.9X106 | ±1.7X104a |
| VC34 | 3,9-dimethylundecane | 57 | 1.3X107 | ±1.6X103c | 1.8X107 | ±3.3X103b | 8.9X107 | ±6.3X103a |
| VC35 | dodecane | 57 | 1.0X106 | ±3.8X103c | 7.2X106 | ±1.8X104a | 2.8X106 | ±6.9X103b |
| VC36 | heptadecane | 57 | 2.1X107 | ±5.2X103c | 2.8X107 | ±7.5X103b | 1.7X108 | ±4.6X103a |
| VC37 | 1-iodo-2-methylundecane | 57 | 1.2X107 | ±1.1X104b | 1.4X107 | ±1.5X104a | 7.6X106 | ±3.6X103c |
| VC38 | 4-methyldodecane | 43 | 3.0X106 | ±1.5X104b | 2.3X106 | ±1.1X104c | 1.8X107 | ±1.2X104a |
| VC39 | 3-methylundecane | 57 | 3.9X106 | ±1.5X104a | 1.6X106 | ±5.9X103c | 2.9X106 | ±1.3X104b |
| VC40 | 2,6,10,14-tetramethyl-hexadecane | 57 | 5.1X106 | ±3.1X103c | 7.1X106 | ±3.8X103b | 1.0X107 | ±5.9X103a |
| VC41 | 2,2,8-trimethyldecane | 57 | 1.2X106 | ±8.9X103a | 2.4X106 | ±2.2X103b | 5.5X107 | ±3.5X103a |
| VC42 | 2,6,10-trimethyl-tetradecane | 57 | 4.6X106 | ±2.4X103a | 1.5X106 | ±1.3X103b | 5.6X105 | ±8.1X102c |
| Others | ||||||||
| VC43 | diethyl benzene-1,2-dicarboxylate | 177 | 6.5X105 | ±2.3X103b | 8.1X104 | ±6.3X102c | 6.8X105 | ±2.8X103a |
| VC44 | [(E)-2-methyltetradec-3-enyl] acetate | 43 | 5.3X105 | ±3.6X103c | 1.0X106 | ±8.9X103a | 8.9X105 | ±6.9X103b |
| VC45 | 2,2,4,4,6,6-hexamethyl-1,3,5,2,4,6-trioxatrisilinane | 207 | 3.4X106 | ±3.1X103a | 9.9X105 | ±8.3X102c | 2.7X106 | ±4.2X103b |
| VC46 | hexyl 2-methoxyacetate | 45 | 8.8X105 | ±1.8X104c | 3.3X106 | ±5.6X103b | 3.6X106 | ±6.4X104a |
| VC47 | methyl (Z)-N-hydroxy-benzenecarboximidate | 133 | 5.5X105 | ±1.7X103c | 1.4X107 | ±1.5X104a | 6.3X106 | ±1.8X104b |
| VC48 | 3,7,11,15-tetramethyl-hexadecan-1-ol | 57 | 5.9X105 | ±6.2X103b | 5.1X105 | ±4.8X103c | 8.3X106 | ±2.7X104a |
| VC49 | tridec-1-ene | 97 | 1.8X106 | ±3.6X103b | 4.6X106 | ±1.2X104a | 9.4X105 | ±1.7X103c |
a- c Different letters in the same row indicate significant differences by F test at p < 0.05.
Volatile compounds in bold had previously been detected in popcorn and corn derivatives.
Abbreviation: n.d., not detected.
Comparative analysis of descriptive sensory and instrumental odour data
An MFA was carried out to explore correlations between sensory (odour and flavour attributes) and instrumental measures (VOCs and e-nose sensors). The obtained results are summarised by a graphical report (Figure 2) in which the total variability was explained by the two main axes of the MFA (52.41% and 47.59%, respectively).
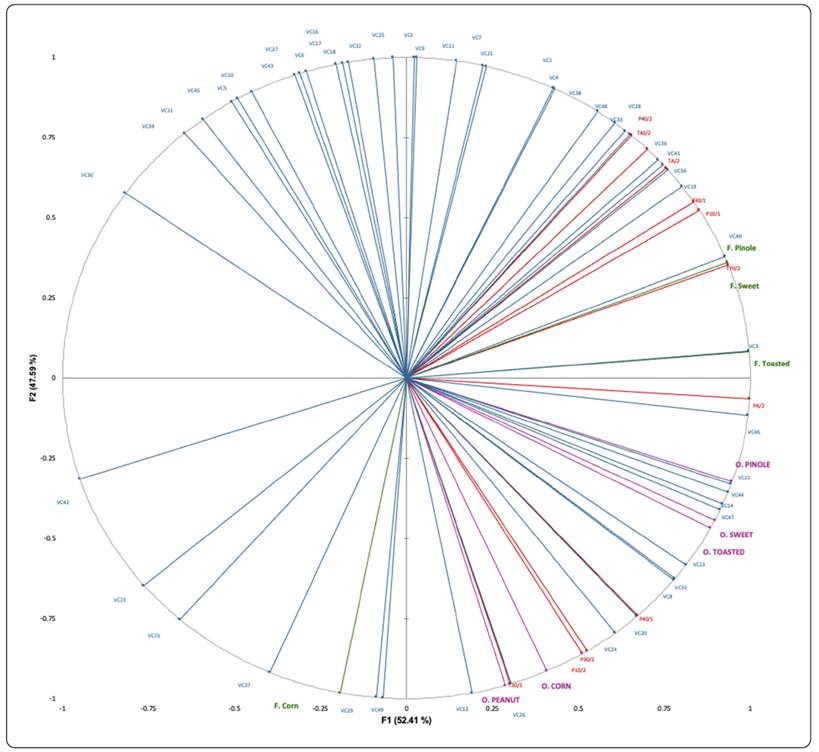
Figure 2 Mapping from Multiple Factor Analysis of the instrumental (electronic nose sensors: EN and volatile compounds: VC) and sensory (odour and flavour) data in pinole powder. EN are market with red line and capital letters, VC are marked with blue line and capital letters. Odour attributes (O) are marked with purple line and capital letters, Flavour (F) attributes are marked with green color. The volatile compound codes correspond to those shown in Table II.
The results obtained by correlating all the variables under study (MFA), corroborated the results of the sensory and instrumental evaluation in their discrimination capacity of the three samples, finding that the PC pinole is positively correlated to both factors, while the PB is positively correlated to factor 1 and negatively to factor 2. On the other hand, pinole PA was negatively correlated to both factors.
Discussion
Sensory profile
The results of the sensory profile of pinole (Figure 1) show that the three studied pinoles, PA, PB, and PC, were well separated. PA was mainly characterized by the appearance parameters showing high homogeneity and particle size. Lozano-Aguilar et al (2008) indicated that the particle size was an important attribute in the acceptance of regular consumers of pinole, reporting a particle size of 1 mm as the most appropriate. The three pinoles presented low granulometry, mainly PA and PB, in which 50% of the sample presented a particle size of less than 0.5 mm when sieving, while in pinole C only 40% of the sample passed through the 0.5 mm mesh. Although pinole C was perceived as grainy, this is not a problem from the point of view of consumer acceptance since conventional consumers of pinole recognize this feature.
PB was characterized by peanut odour and corn flavour and was negatively correlated with the humidity texture attribute, ranging the values for the three samples between 1-2, with PB being the one with the lowest humidity. PC was mainly characterized by a toasted and floury appearance. The texture was considered very grainy. On the other hand, the flavour was defined by the pinole, toasted and sweet parameters, and by the low intensity of corn flavour.
Lozano-Aguilar et al. (2008) working with pinoles made with a combination of different cereals, pointed out that the participants demanded the characteristic flavour of corn. In this sense, the sensory results seem to affirm that, despite having differences, the three pinoles studied in this work can meet the consumer expectations because they showed the attributes of corn (taste and odour), although in different intensities.
Sweet corn has sweet odour and taste notes derived from its chemical composition, with up to 40% sugars such as fructose, glucose, and sucrose (Žilić et al., 2020). In ‘Dulcillo de Noreste’ corn, a variety used to make popcorn and pinole, polysaccharides represent 71.5% of the grain, presenting a high content of free sugars and a peculiar flavour (Lozano-Aguilar et al., 2008). In this sense, the more intense sweet taste of PC could be due to climatic conditions. PC comes from Urique, a warmer area than Gonogochi where PA and PB come from, hence the corn can ripen better and achieve a greater accumulation of sugar, increasing the intensity of the sweetness observed in that sample. The sweet note is important in pinole, as indicated by the fact that, in other regions of Mexico, sugar is added to pinoles when eaten as a treat (Litaye, 2016). So, PC may better satisfy the demands of consumers than PB and PA, regarding sweet taste.
On the other hand, the toasted attribute comes from the production process in which oak firewood is used to toast the corn and pop it into popcorn. A common compound of wood smoke is acetic acid (Toledo, 2008), which would agree with the high area of this compound found in the chromatographic analysis, as will be described later.
The evaluated pinoles do not present a high dryness as is perceived in other pinoles made in Mexico, which when introduced into the mouth absorb all the saliva (Sánchez-Herrera et al., 2014), making it difficult to consume. This may be because they are made with popcorn and not roasted corn. According to Sweley, Rose & Jackson (2013), in popped corn, there is evidence of incomplete starch gelatinization because a large part of the starch granules remains intact. Based on that knowledge, popped grains of Cristalino maize could be less prone to retrogradation because a portion of their endosperm does not gelatinize nor change its structure which could explain the absence of a dry texture in the samples. It also explains the tendency of the pinole from Sierra Tarahumara to rehydrate easily. The exposed results for the sensory profile of pinole are novel. Some previous studies on pinole used affective sensory methods, such as the level of liking and the preference test (Lozano-Aguilar et al., 2008). However, to our knowledge, this is the first descriptive analysis of pinole performed by a trained panel.
Volatile compounds in pinole
The pinole produced by the Rarámuri families is substantially different from others produced in various regions of the country since native maize from this region is used (i.e. the Cristalino maize race from Chihuahua). Due to the hardness of the pericarp and the crystallinity of the endosperm, this maize is capable of popping like popcorn when toasted in an open pot to the atmosphere heated using firewood. These popped grains constitute more than 60% of the total mass that is subjected to fine grinding and is as such the final product. Taking this into account and knowing that the traditional Tarahumara pinole has been described as having the odour and flavour of popcorn (Linares et al., 2016), this discussion will focus on comparison to other VOCs found in popcorn, as there is no chemical information of ethnic food.
Twenty-two of the identified volatiles, in bold in Table II, had previously been detected in popcorn and corn derivatives (Burdock, 2010; Pico et al., 2018). These compounds constitute 48%, 53%, and 51% of the total peak area for PA, PB, and PC, respectively. The highest yielded peak areas were mainly from furan compounds, pyrazines, and organic acids, which accounted for 27 - 36% of the relative areas. The remaining 27 identified compounds are reported for the first time in corn-derived products. Most of them are alkanes.
It is widely known that alkylated pyrazines can be produced when amino ketones condense after the first stages of Maillard reactions (García-Lomillo & González-SanJosé, 2018). Furan compounds are other types of products from these browning reactions that can be readily generated in high-carbohydrate foods. Both classes of compounds could exist in pinole, given the fact that corn endosperm contains not only starch granules but also a protein matrix as well as several reducing sugars (Serna-Saldívar & Perez Carrillo, 2019).
Moreover, the presence of signals from acetic acid (Table II), which ranges between 6.2 to 22% of the total peak area for each sample, can be an indicator of oak wood pyrolysis, being retained in the smoke-exposed pinole. Additionally, lipid degradation in flours/starches can produce volatiles with odour contributions, such as nonanal and (E)-2-nonenal.
The studied pinoles scarcely differed from each other in the volatile compounds detected, varying only in the absence of 2,6-dimethyl-pyrazine and 1(3H)-iso-benzo-furanone in PB (Table II).
The commented results provide novel information on the chemical composition of a poorly studied ethnic food, the pinole from Sierra Tarahumara.
Comparative analysis of descriptive sensory and instrumental odour data
A minority number of VOC were well correlated with some odour or flavour parameters, so as with some specific sensors of the e-nose (Figure 2). That is the case of o-cymene, 2,6-ditertbutyl-4-ethylphenol, butyrolactone, m-xylene, dodecane, 2-ethyl-5-methylpyrazine, estragole, acetic acid, E-2-methyl-3-tetradecen-1-ol- acetate, hexyl 2-methoxyacetate, oxime methoxy phenyl, and p-xylene which were well correlated with T30/1, P10/2, P30/1, P40/1, and PA/2 sensor responses; and with peanut, corn, toasted, sweet and pinole odours. Some of these findings are in line with those of García-Lomillo et al. (2016), where T30/1, P30/1 and P40/1 were associated with high responses prompted by alkylated pyrazines. Furthermore, butyrolactone has been reported to have a sweet odour, while 2-ethyl-5-methyl-pyrazine has a roasted nutty aroma (Burdock, 2010).
Compounds nonanal, (E)-2-nonenal, 2-pentylfuran, 5-methylfurfural, octanoic acid, 1-pentanol, 2-acetylpyridine, 2-ethyl-6-methylpyrazine, 2-methylpyrazine, o-xylene, 2,6-dimethyloctane, 3,9-dimethylundecane, heptadecane, 4-methyldodecane, phytane, 2,2,8-trimethyldecane, 3,7,11,15-tetramethylhexadecan-1-ol and benzaldehyde correlated with e-nose sensors T70/2, P10/1, T40/1, TA/2, T40/2, and P40/2 and with toasted, pinole and sweet flavour attributes. Previous research has found pyrazines have a characteristic toasty note (García-Lomillo & González-San José, 2018), then they could also contribute to popcorn odour. VOC 5-methyl-furfural can have a sweet, caramel-like odour and has been reported in many foods, while 2-ethyl-6-methylpyrazine has a roasted and baked odour. Even more, benzaldehyde has a characteristic almond odour and 1-pentanol is lightly sweet when smelled (Burdock, 2010).
The volatiles identified as furfural, 1,2,3-trimethylbenzene, propanoic acid, 1-iodo-2-methylundecane, 2,6,10-trimethyl-tetradecane, and 1-tridecene got a positive correlation to the attribute corn flavour. These compounds can be indicative of the raw material.
Regarding the compounds 2-(cyanomethylene)-pyrrolidine, 2,6-dimethylpyrazine, 2,3,5-trimethylpyrazine, furfuryl alcohol, 2-methoxy-4-vinylphenol, 5-methyl-2-furfural, benzoic acid, 1(3H)-isobenzofuranone, 1-chlorohexadecane, decane, 3,3-dimethylhexane, 3-methylundecane, and diethyl phthalate, they show a partial association with the corn flavour attribute.
It should be noted, however, the fact that multiple odour compounds together can generate a different smell than the isolated components. The perception of a volatile may change based on its concentration, and even more, concentrations of volatiles can be below their detection or recognition thresholds. Hence, further study of this type of product is needed. We suggest improving the volatile profile through experiments that give actual sensory odour data for individual chemicals in pinole (such as gas chromatography-olfactometry), which are necessary to confirm associations between pinole odorants and their perception.
Conclusions
This work constitutes the first characterization of the pinole from the Sierra Tarahumara, with respect to its sensory profile and composition of volatile compounds.
The three pinoles have different sensory profiles. Even the two pinoles from the same geographic area differed in some odour and texture properties. The pinole C, which is from other origin had the greatest differences in odour, flavour and texture. However, the e-nose fingerprints of the pinoles were quite similar, presenting differences only in the response of four sensors.
The volatile compound composition of the three pinoles was similar, although the concentrations of the compounds differed in each one of them. Twenty-two volatile compounds were previously identified in popcorn. Around 50% of the relative area corresponded to products of the Maillard reaction, as well as compounds derived from Strecker degradation and lipid oxidation. According to the identified volatiles, the aromatic and taste properties that characterize the Tarahumara pinole are closely linked to the roasting and popping processes that take place during its elaboration.
Multivariate analysis permitted the visualizations of some correlations between the sensory and instrumental descriptors. The odours peanut, corn, toasted, sweet and pinole attributes were the main attributes apparently correlated to some organic volatile compounds found in the pinole. Although correlations between sensory properties and instrumental analysis, the latter does not allow the differentiation of the three pinoles as sensory analysis does.











 nueva página del texto (beta)
nueva página del texto (beta)


