Introduction
The heart is the central organ of the circulatory system of vertebrates; it functions as a pump that aspirates and propels blood throughout the body. Of all the organs in the body, it is the first to acquire functionality during embryonic development, supplying nutrients and oxygen to the embryo, as well as eliminating waste substances1-3. In humans, the heart develops from the second to the eighth week of gestation; however, various physiological and structural modifications occur during and after birth that completes its formation3. Cardiogenesis is a complex process that involves the gradual integration of different cardiac cell populations, called heart fields4-6, and other non-cardiac cell populations, such as neural crest7 and proepicardial organ cells8,9.
The classical view of cardiac development was established mainly by descriptive studies of human embryos resulting from spontaneous abortions10-17. Although these studies provided valuable information, they did not reflect the true dynamism of the process of cardiogenesis as they were based on histological sections; moreover, the embryos likely presented errors associated with defects in cardiac development. These limitations were initially resolved using in vivo selective labeling experiments with plastic labels carried out in chick embryos18-28. This technique allowed longitudinal studies to be carried out since it is possible to temporally trace the structural changes of an embryonic region previously marked with a label. Furthermore, molecular labeling studies in mouse5,6,29 and chick4 embryos established the concept of the "heart field" as an embryonic region in which cells destined to become myocardium are located. With this new approach, in vivo, and in vitro selective labeling experiments in chick embryos30-32 have been developed, which collectively have succeeded in proposing new models of heart development.
It is important to emphasize that errors in the normal development of the heart cause congenital heart disease, a condition with a worldwide incidence of 8-10/1,000 live births, of which approximately 50% die during the first year if they are not clinically and surgically treated3,33. The frequency and lethality of congenital heart defects make it imperative to study normal cardiogenesis to improve diagnosis and treatment. In this review, we contrast some of the classical concepts of cardiac development with current anatomical descriptions and the general events of gene expression and their cellular repercussions. We hope this work will provide an overview for understanding the anatomical, cellular, and genetic processes of normal cardiogenesis.
Cardiogenic areas
The first signs of cardiogenesis appear at the blastula stage, when the embryonic disc consists of only two cell layers: the epiblast and the hypoblast (Fig. 1). Two compact groups of cells positioned in the epiblast on both sides of the primitive streak are known as pre-cardiogenic areas (Fig. 1A)34,35. The cells of the pre-cardiogenic areas remain undifferentiated through positive Wnt/β-catenin signaling36-38. Once gastrulation has begun, the cells of the cardiogenic precardiac areas migrate through the primitive streak until they are incorporated into the splanchnic mesoderm, where they form two cardiogenic areas located contralateral to the primitive streak at the level of Hensen's node (Fig. 1B)34,35,39. In the splanchnic mesoderm, cells from cardiogenic areas are already determined to differentiate into cardiac cells34 by molecular signals secreted by the underlying endoderm40, including bone morphogenetic protein (BMP), fibroblast growth factor (FGF), and Wnt signaling inhibitors, which together promote cardiac phenotype genes, such as NKX2-5, GATA4, and TBX5, and the chromatin remodeling protein SMARCD3 (BAF60c)36-38,41,42. Even ectopic activation of 3SMARCD3, GATA4, and TBX5 is sufficient to drive cardiomyogenesis in non-cardiogenic regions of the embryo43.
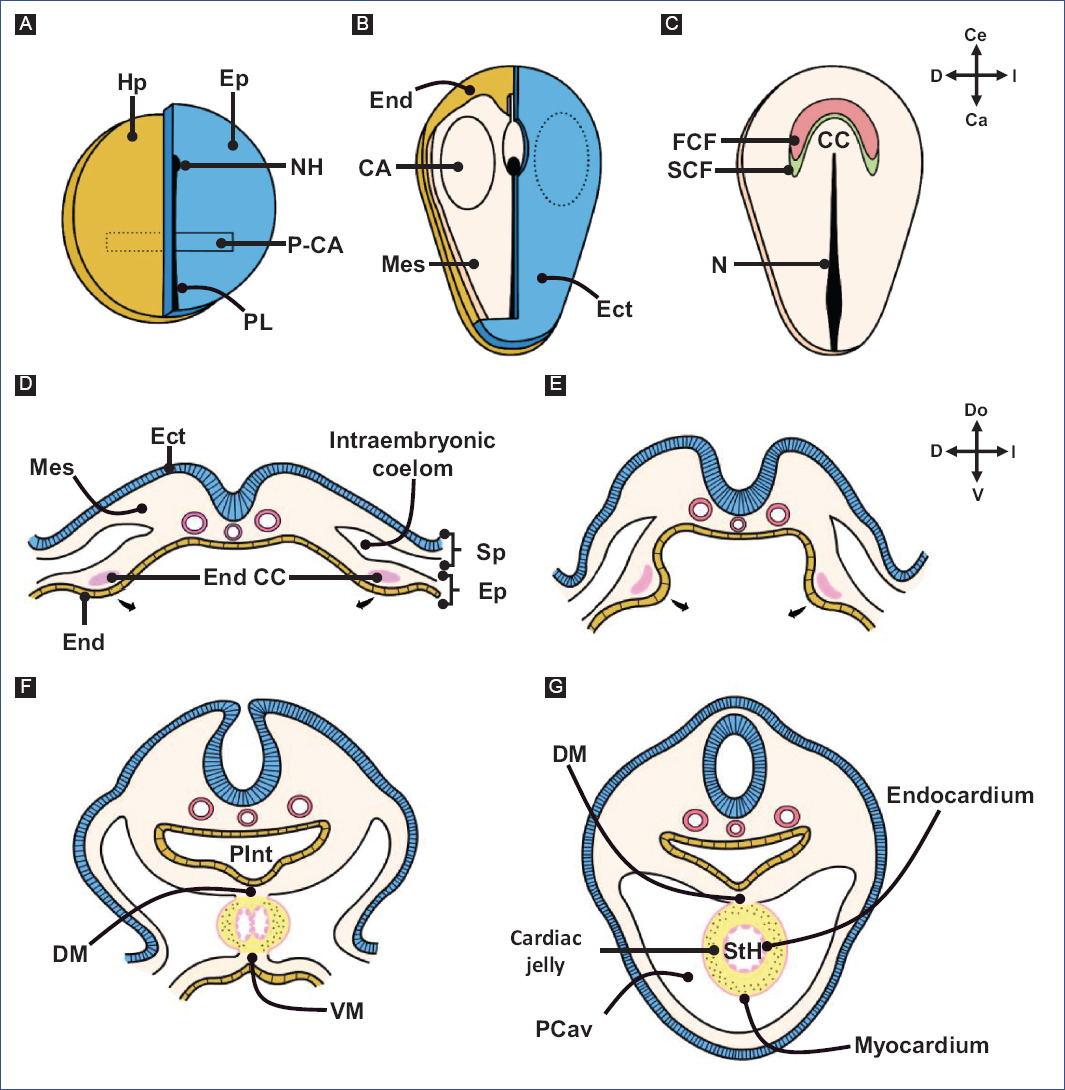
Figure 1 Early cardiogenesis. A: blastula. Two pre-cardiogenic areas are present in the epiblast. B: early gastrula. The pre-cardiogenic areas migrate through the primitive streak to incorporate into the splanchnic mesoderm, forming the cardiogenic areas. C: late gastrula. The cardiogenic areas migrate in an cephalomedial direction and fuse to form the cardiogenic crescent. D: the cardiogenic crescent is mobilized in the splanchnopleura, showing its ends as two endocardial tubes. E-G: the ends of the cardiogenic crescent move in a ventro-medial direction until they fuse and form a single myo-endocardial tube, called a straight-tube heart. Note that the dorsal wall of the heart is attached to the ventral wall of the primitive gut tube (A and B modified from García-Peláez82, D-G modified from Arteaga et al3.).CA: cardiogenic areas, DM: dorsal mesocardium, Ect: ectoderm, End CC: cardiogenic crescent endings, End: endoderm, Ep: epiblast, FHF: first heart field; Hp: hypoblast, Mes: mesoderm, N: notochord, NH: node of Hensen, P-CA: pre-cardiogenic areas, PCav: pericardial cavity, PGT: primitive gut tube; PS: primitive streak, SHF: second heart field, Sp: somatopleure, splanchnopleure, TH: straight-tube heart, VM: ventral mesocardium.
Cardiogenic crescent
During late gastrulation, cells from the cardiogenic areas migrate in a cephalomedial direction and fuse to form the cardiogenic crescent,44 named for its lunar crescent-like appearance (Fig. 1C and 2A). Figure 2 depicts normal cardiac development based on observations in chick embryos. The cell population that makes up the cardiogenic crescent is recognized as the "first heart field" (FHF), which expresses genes characteristic of the cardiac phenotype and is the precursor of the left ventricle and part of the atria in the amniotic heart (reptiles, birds, and mammals)5,6. The rest of the heart in amniotes derives from another cell population located dorsal to the cardiogenic crescent, known as the "second heart field" (SHF)4-6,29,45. The cells of the SHF are undifferentiated and can be observed by molecular labeling of Isl1 or Tbx1, considered characteristic transcription factors of the SHF46. In fact, knockout mice for Isl1 develop hearts lacking the embryonic outflow tract (classically called conotruncus), right ventricle, and a large part of the atria47; also, mice with a deletion in Tbx1 present defects in the embryonic outflow tract48.
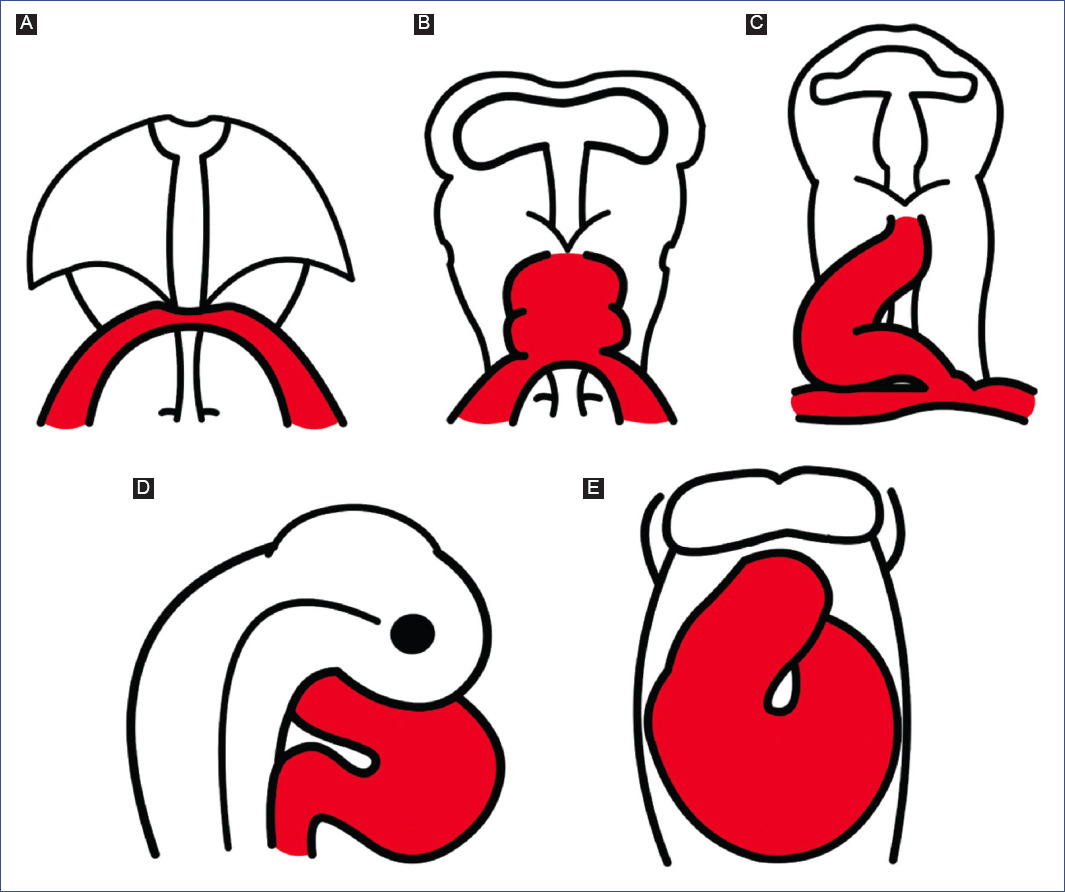
Figure 2 Schematic representation of normal cardiac development, based on observations of chick embryos. A: cardiogenic crescent. B: straight tube heart. C: C-loop. D: S-loop. E: advanced loop.
STRAIGHT-TUBE HEART
Once gastrulation is completed, embryonic tubulation begins, a process by which the trilaminar embryo adopts a tubular and elongated morphology (Fig. 1D-G), manifesting the segmentation of the mesoderm into three layers (paraxial, intermediate, and lateral) and the development of the neural tube, the primitive gut tube and the walls of the body3. The lateral mesoderm is delaminated in two layers; one is associated with the ectoderm (somatopleure) and the other with the endoderm (splanchnopleure), causing the formation of the intraembryonic coelom (Fig. 1D)3. The cardiogenic crescent is located in the splanchnopleure11,39 and is organized to form two endocardial tubes (Fig. 1D-F), right and left26, which are the ends of the cardiogenic crescent. As a result of the embryonic tubulation process, the ends of the cardiogenic crescent move in a ventromedial direction until they fuse and form a single tube called a straight-tube heart (Fig. 1F-G and 2B)39.
The straight-tube heart is composed of a lumen delimited by a layer of endocardial cells and another layer of myocardial cells; in between these layers exists an extracellular matrix rich in mucopolysaccharides, glycoproteins, and collagen, called cardiac jelly (Fig. 1G)11,13. Several authors accept that cardiac jelly only has a purely septal function in the embryonic heart13,16,20, while others claim that it also has a provisional valvular activity49,50.
During this stage, the heart is incorporated within the cephalic portion of the intraembryonic coelom (primitive pericardial cavity) and is positioned ventrally to the primitive gut tube. In fact, the straight-tube heart initially has a canal shape because its dorsal wall corresponds to the ventral wall of the primitive gut tube; however, after the myocardium invades and closes the canal to form a tube, the heart remains attached to the primitive gut tube by a band of mesoderm, called the dorsal mesocardium (Fig. 1G)26,39,51,52. It is likely that this temporary junction, heart with primitive gut tube, allows the cardiac loop to twist to the right during embryonic flexion.
In the 1920s, the preformation model of cardiogenesis was proposed based on descriptive studies of human embryos. This model considered that all the components of the mature heart were already present in the straight-tube heart, which only grew during development (Fig. 3A). Decades later, through in vivo selective labeling studies in chick embryos20,25-27,53, it was described that the heart is formed by the gradual integration of several cardiac components (Fig. 3B-F). It was accepted that the straight-tube heart is composed of the trabeculated region of the right ventricle and the trabeculated region of the left ventricle, both regions flanked by interventricular grooves (Fig. 3B)26,27. However, Villavicencio et al.32 have recently proposed a segmental model of the heart (Fig. 4A-D) where they suggest that the straight-tube heart (Fig. 4A) is composed of the primordia of the interventricular septum, the left ventricle, and the atrioventricular canal (A-V canal). Figure 4 shows selective labeling experiments in chick embryos.
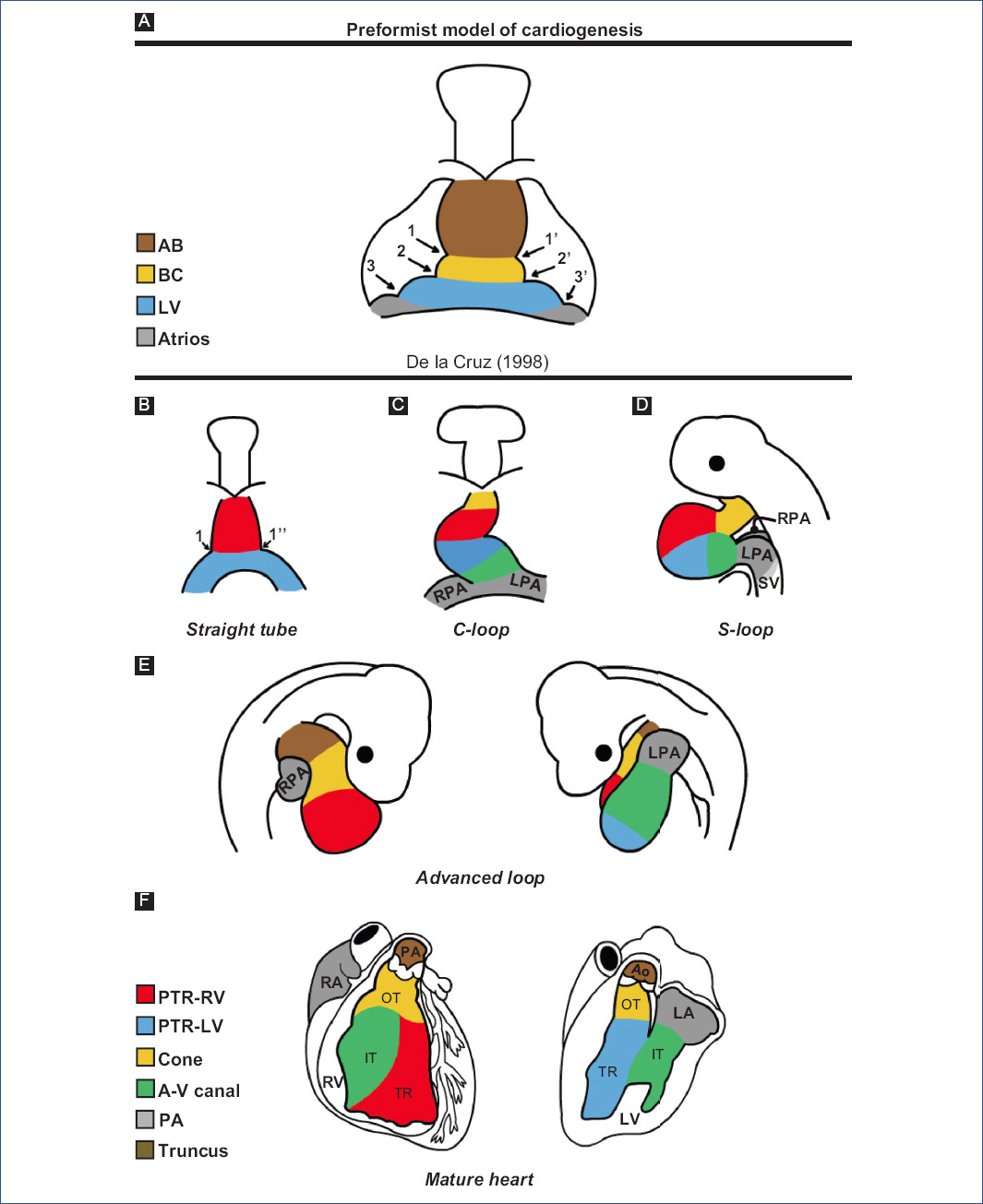
Figure 3 Theories of cardiac development A: according to descriptive embryology studies in humans and B-F: selective labelling studies in chick.1 and 1': right and left interbulbar sulci, 1 and 1'': right and left interventricular sulcus, 2 and 2': right and left bulboventricular sulcus, 3 and 3': right and left atrioventricular sulcus, A-V canal: atrioventricular canal, AB: aortic bulb, Ao: aorta, BC: bulbus cordis, IFT: inflow tract, LA: left atrium, LF: left ventricle, LPA: left primitive atrium, OFT: outflow tract, pA: primitive atria, PA: pulmonary artery, pTRRV: primordium of the trabeculated region of the LV, pTRRV: primordium of the trabeculated region of the RV, RA: right atrium, RPA: right primitive atrium, RV: right ventricle, SV: sinus venosus, TR: trabeculated region.(Figure A was modified from De la Cruz et al.26 and figures B-F from De la Cruz53.).
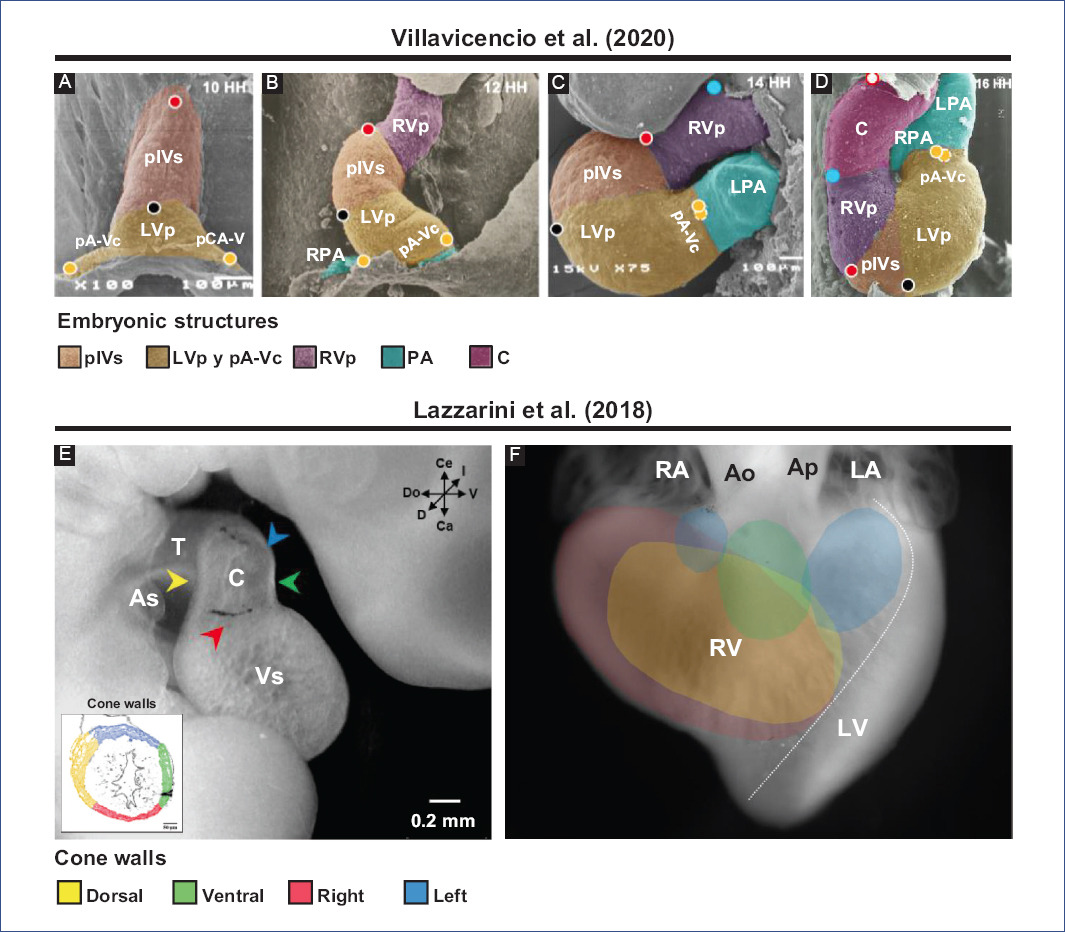
Figure 4 Selective labeling experiments in chick embryos. Segmental model of the heart development proposed by Villavicencio et al.32. A: straight tube heart. B: C-loop. C: S-loop. D: U-loop (advanced loop). The circles represent the selective markings performed, in A (red, black and yellow) and in C (blue), while in B-D the tracing of these markings is shown. Destination of myocardial cone walls by Lazzarini et al.31. E: location in space of the different cone walls: dorsal (yellow), ventral (green), left (blue), and right (red). F: map of the destination of all conal walls in the right ventricle, ventral view of the heart.Ao: aorta, As: atrial segment, C: conus, LA: left atrium, LPA: left primitive atrium, LV: left ventricle, LVp: LV primordium, pA-Vc: primordium of the atrioventricular canal, pA: primitive atria, PA: pulmonary artery, pIVs: primordium of the interventricular septum, RA: right atrium, RPA: right primitive atrium, RV: right ventricle, RVp: RV primordium, T: truncus, Vs: ventricular segment.Figures A-D were modified from Villavicencio et al.32 and E-F from Lazzarini et al.31. Since these are different experiments, the interpretations are not the same, so different terminologies are used.
Molecular expression studies have described that the straight-tube heart contains the primordia of the left ventricle and part of the atrial segment5,6. Concomitantly, SHF cells in the pharyngeal mesoderm are in a state of continuous proliferation and delayed differentiation due to positive FGF signaling, which stimulates proliferation by inhibiting the pro-differentiation signal of BMP47,54.
It is currently accepted that the straight-tube heart undergoes a series of morphological changes, mainly the addition of new segments from its venous and arterial ends, and consequently undergoes a process of torsion that establishes the definitive spatial position of the cardiac cavities. Cardiac torsion is divided into three stages: C-loop (Fig. 2C), S-loop (Fig. 2D), and advanced loop (Fig. 2E). The letters correspond to the similarity of the pathway followed by blood flow within the heart.
C-SHAPED LOOP HEART
The straight-tube heart increases in size due to the differential aggregation of cells from the SHF29,45 from its venous and arterial poles26,27. Different studies have shown that Isl147 and Tbx155,56 play a fundamental role in the admixture by promoting cell migration from the SHF to the developing heart. Cell differentiation toward the myocardium is mediated by positive BMP signaling, which by inhibiting FGF signaling and silencing Isl1 and Tbx1, promotes the expression of cardiac phenotype genes, such as NKX2-5 and GATA447,54. The increase in size causes the middle portion of the heart to begin to twist to the right, thus obtaining the characteristic shape of the letter "C" (Fig. 2C)51,53. The recruitment of cell populations that are added to the poles of the heart at this stage results in the emergence of new anatomical components. Initially, it was proposed that three new structures were incorporated into the heart: the A-V canal25-27, the primitive atria at the caudal end18,27,39 and the cone at the cephalic end (Fig. 3C)20,26,27. In contrast, it is currently proposed that during the C-loop stage, the conus, recognized as the right ventricular primordium including its outflow tract (Fig. 4E and F)31, is recruited cephalad and the primitive atria are recruited caudally (Fig. 4B)32.
S-SHAPED LOOP HEART
The continuous differential growth of the heart and the separation of the dorsal mesocardium from the cardiac midline cause the torsion of the heart to become increasingly accentuated until it acquires the shape of the letter "S" (Fig. 2D)51,53. De la Cruz et al.25-27 point out that the atria acquire a dorsal position due to their upward displacement (Fig. 3D). Similarly, Villavicencio et al.32 mention that during this stage, the primordia of the ventricles and the interventricular septum begin to descend, causing the primitive atria to ascend (Fig. 4C). Conversely, the results of Lazzarini et al.31 suggest that the incorporation of the myocardial conus into the ventricular segment is responsible for the ascent of the atria in the dorsal cephalic direction.
ADVANCED LOOP HEART
During the advanced loop stage (Fig. 2E), the atria and ventricles acquire their definitive position and spatial relationship51,53. De la Cruz et al.28 describe that the conus finishes incorporating into the heart, which contrasts with the recent findings of Lazzarini et al.31, who describe how the myocardium of the conus transforms into a large part of the myocardium of the right ventricle. Moreover, the distal segment of the conus begins to appear, being recognized as a truncal segment that joins the heart with the aortic sac (Fig. 3E and 4D)20. This segment, like the conus, originates from the incorporation of cells from the SHF29,45. In most of the literature, the conus and the truncus are considered to be the same structure, called the conotruncus or embryonic outflow tract. However, despite having anatomical homology, both structures undergo different developmental processes, for example, apoptosis31.
During the advanced loop heart stage, the proepicardial organ appears, which is recognized as a bulge of mesothelial cells positioned on the surface of the venous sinus8. The cells of the proepicardial organ invade the myocardium so that they cover it and subsequently transform into the epicardium8,9,57. In addition, some of these cells contribute to the formation of coronary arteries and veins58.
CARDIAC SEPTATION
Cardiac septation is a process by which the heart with a single blood flow is physically divided into four chambers, thus creating a dual pathway flow: systemic circulation and pulmonary circulation, characteristic of birds and mammals. Thus, three septums are recognized in the heart: the interatrial septum (IAS), the interventricular septum (IVS), and the atrioventricular septum (AVS). In addition, aorticopulmonary septation and conus remodeling are important events for cardiac septation and the establishment of definitive cardiac circulation.
According to De la Cruz et al.23,28, the first indication of the cardiac septum is the primitive cardiac septum (pCS), which they describe as being shaped like an eye mask (Fig. 5A). This septum is constituted by the septum primum (SP), the ventro-superior (VSc) and dorso-inferior (DIc) cushions of the A-V canal and the primordium of the muscular IVS (pmIVS)23,28. However, the pCS is likely an erroneously described structure since it does not fulfill a proper septal function due to the temporary valvular function of the cardiac jelly and its derivatives: the cushions of the A-V canal and the conal and truncal crests49,50. Thus, we propose that all the structures that constitute the pCS have an indispensable role in cardiac septation but do not form a single septum.
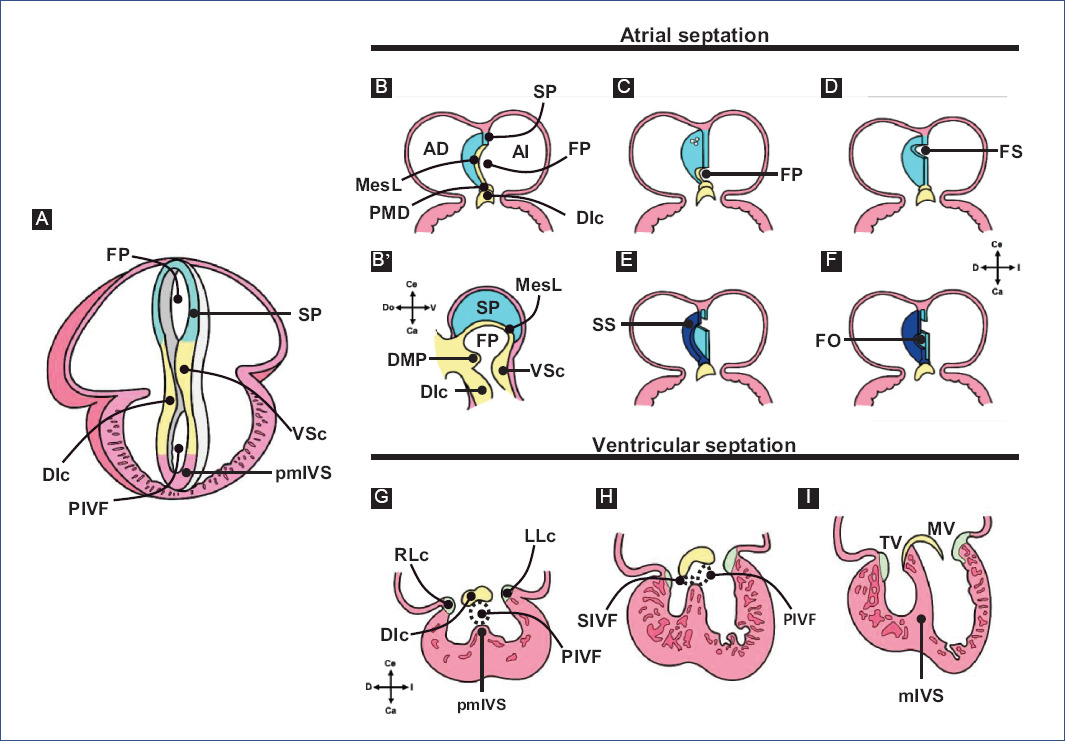
Figure 5 Cardiac septation. A: primitive cardiac septum proposed by De la Cruz et al. B-F: atrial septation. G-I: ventricular septation.DIc: dorso-inferior cushion, DMP: dorsal mesenchymal protrusion, FO: fossa ovalis, FP: foramen primum, LLc: left lateral cushion, MesC: mesenchymal cap, mIVS: muscular interventricular septum, MV: mitral valve, PIVF: primary interventricular foramen, pmIVS: primordium of the muscular interventricular septum, RLc: right lateral cushion, SIVF: secondary interventricular foramen, SP, septum primum, SS: septum secundum, TV: tricuspid valve, VSc: ventro-superior cushion.Figure A was modified from De la Cruz et al.28 and B-I from Arteaga et al.3.
Figure 5 A shows the primitive cardiac septum proposed by De la Cruz et al.28; figures 5B-F the atrial septum, and 5G-I the ventricular septum.
EPITHELIAL-MESENCHYMAL TRANSITION
The proliferation, delamination, and invasion of endocardial cells into the cardiac jelly of the A-V canal cushions and conotruncal crests are considered fundamental events for septation and are also part of a process known as epithelial-mesenchymal transition (EMT)49,59. In the embryonic heart, the surrounding myocardium stimulates endocardial EMT when it secretes adherens that induce the loss of cell adhesion molecules, such as E-cadherin, causing the cells to delaminate and acquire an invasive mesenchymal phenotype characterized by the expression of N-cadherin, vimentin, and fibronectin60-62. The transforming growth factor beta (TGF-β) signaling pathway is considered the most important in EMT; it has even been shown that in cell cultures, TGF-β treatment is sufficient to induce EMT in epithelial cells63. However, numerous studies propose that this process is regulated by a complex network consisting of TGF-β/Smad, BMP, Wnt/β-catenin, Notch, and Smad-independent TGF-β signaling pathways, which together induce the expression of transcription factors, such as Snail, Slug and Twist, and promote or inhibit EMT64-68.
ATRIAL SEPTATION
Atrial septation is a cardiac event that, despite its complexity, has been almost completely described since its pioneering studies10,69-71. However, one of the drawbacks to understanding this phenomenon is the developmental variations presented in the animal models, which result from evolutionary modifications72,73. For this reason, we will focus only on IAS formation in birds and placental mammals (Fig. 5B-F), as these are the animal models most commonly used for research.
Atrial septation begins with the appearance of the SP, a muscular structure in the dorsal cephalic wall of the common atrium (Fig. 5B)69,73,74. The SP has a crescent shape, with one end directed toward the VSc and the other toward the DIc. In addition, the SP is covered at its leading edge by a mesenchymal cap originating from the dorsal mesenchymal protrusion (DMP)73-75, a mesenchymal bulge expressing Isl1 derived from the SHF (Fig. 5B and B')76. The orifice bounded by the SP's mesenchymal cap and the A-V canal's two cushions is known as the foramen primum (FP)23,28,73. Some authors include the DMP as part of the perimeter of this foramen73,74,77,78 and even mention that these structures form the atrioventricular mesenchymal complex (A-VMC)74. The growth of the SP and the subsequent fusion of the A-VMC components results in the FP closure (Fig. 5C and D)23,69,70,74,78,79.
According to Anselmi and De la Cruz80, experiments by De la Cruz et al.22,23 demonstrated that only the DIc of the A-V canal cushions participates in the closure of the FP. Before the closure of the FP, several perforations appear in the cephalic region of the SP (Fig. 5C), which allow unidirectional blood flow between the atria69,73. In the chicken, the interatrial septation process remains in this state until the time of eclosion, which is when these perforations are eventually closed by the growth of the myocardial and endothelial tissues at their margins69,72,81. In contrast, in placental mammals, the perforations of the SP coalesce to originate the foramen secundum (FS) (Fig. 5D)10,73. Subsequently, another muscular structure, the septum secundum (SS), appears to the right of the SP (Fig. 5E) due to the folding of the right atrial roof and myocardial differentiation of the mesenchyme that closed the FP10,75,77,79. The ends of the SS grow until they meet and fuse, resulting in the formation of an orifice just below the FS, known as the fossa ovalis (FO) (Fig. 5F)3,71. The location of the FS and FO determines that the SP functions as a valve, which maintains right-to-left blood flow3,73. Finally, during birth, physiological closure of the interatrial communication occurs due to increased pressure in the left atrium, which ends up compressing both septa3,73. In humans, anatomical closure of the interatrial communication occurs in the first 6 months after birth3.
VENTRICULAR AND ATRIOVENTRICULAR SEPTATION
The IVS is an anatomically and embryologically complex structure because it originates from different embryonic structures in a "mosaic" fashion. In general, the IVS comprises a muscular portion, the muscular interventricular septum (mIVS), and a fibrous portion, known as the membranous septum (MS), with the mIVS being the most prominent component of the IVS1. The muscular and fibrous nature of the IVS is responsible for the lack of consensus on its embryonic origin; however, it has been reported that the pmIVS, the DIc and VSc cushions of the A-V canal, and the left conal crest (LCc) participate in its formation12,14,15,21-23,28,77.
Regarding the formation of the MS, it is considered that the cushions of the A-V canal and the conal crests participate21,22. Therefore, the fusion of the main cushions of the A-V canal (Fig. 6) is the most relevant event in the formation of the MS15,17. Initially, due to the appearance of cardiac jelly and its subsequent transition to mesenchyme, four endocardial cushions are formed in the A-V canal: one dorso-inferior, oneventro-superior, and two lateral3,82. Afterward, the DIc fuses with the VSc, which occurs in a cephalocaudal direction, leaving no demarcation line to distinguish one from the other (Fig. 6A-C)3,24,82. The fusion of the cushions divides the A-V canal into the right and left atrioventricular orifices, (A-VV) will form, tricuspid valve (VT) and mitral valve (MV), respectively (Fig. 6D and E)3,82. In addition, the DIc participates in the formation of the septal leaflet of the TV and the septal portion of the anterior leaflet of the MV, whereas the VSc contributes to the origin of the free portion of the latter leaflet23. For their part, the lateral cushions contribute to the development of the valvular rings and the lateral leaflets of the A-VV3,82. The A-VV does not coincide in the horizontal plane within the fibrous skeleton, as the septal leaflet of the TV inserts closer to the cardiac apex than the anterior leaflet of the MV1,3,82,83. This misalignment of A-VV insertion is attributed to the development of lateral protrusions in the DIc and VSc cushions, termed the right and left tubercles, during the fusion process. Specifically, the DIc is curved at its caudal edge, so the right tubercle is also positioned closer to the cardiac apex than the left tubercle (Fig. 5H)3,82. It is relevant to mention that this difference in the level of the A-VV leaflets delimits the AVS region, which separates the right atrium from the left ventricle1,3,82,83. Despite being recognized as independent septa, the MS and AVS share the same embryonic origin, which is reflected in the lack of an anatomical boundary beyond the gap between the atrioventricular leaflets.
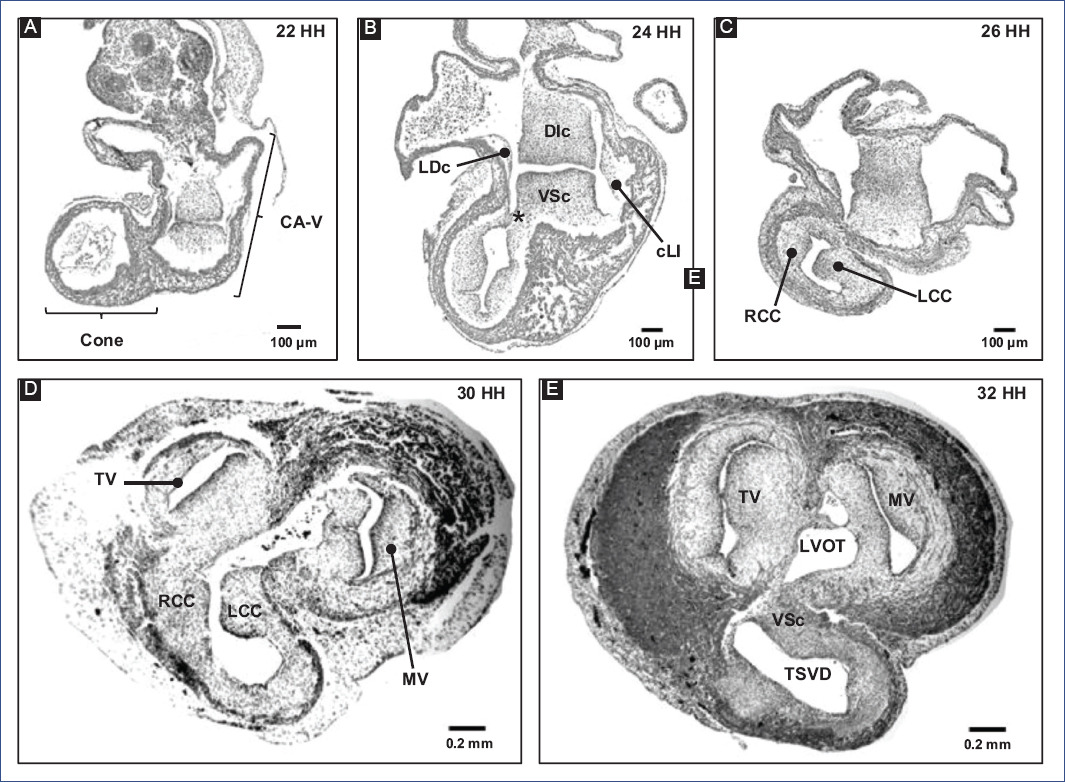
Figure 6 A-E: remodeling of the conus and A-V canal in chick embryo.DIc: dorso-inferior cushion, LCc: left conal crest, LVOFT: left ventricular outflow tract, MV: mitral valve, RCc: right conal crest, RVOFT: right ventricular outflow tract, SVr: supraventricular ridge, TV: tricuspid valve, VSc: ventro-superior cushion (modified from Lazzarini et al.31).
Descriptive cardiac embryology work concluded that the AVS is formed by the contribution of the DIc and VSc cushions of the A-V canal15,17. In contrast, an in vivo labeling experiment of the A-V canal cushions described that the DIc forms the entire AVS and the adjacent portions of the IAS and IVS22,23. Despite both perspectives, Webb et al.84 concluded that simple fusion of the A-V canal cushions is not the only event involved in atrioventricular septation. This process requires remodeling different cardiac structures, including the development of the venous sinus, the formation of the atrial and ventricular septa, the expansion of the right atrioventricular junction, and the junction of the LCc with the VSc of the A-V canal84.
It is commonly described that the first sign of mIVS is a myocardial ridge located between the trabecular pouches of the ventricles; in fact, the formation of these trabecular pouches (a process called diverticulization) is responsible for its appearance3,82. Classical studies of human embryos12,14,15 attributed the emergence of the mIVS to the coalescence of the trabecular pouches. Likewise, the authors suggested that this septum grows passively toward the ventricular cavity as the trabecular pouches develop. However, this coalescence origin was not fully supported by the in vivo selective labeling results in chick embryos19,21,22,28. De la Cruz et al.28,85 described that pmIVS appears in the straight-tube heart, precisely at the midline of fusion of the cardiac primordia and at the level of the interventricular grooves (Figure 3B). They also observed that the first morphological manifestation of this septum appears in the apical region of the interventricular groove and that its growth occurs in a caudal-cephalic direction due to the continuous incorporation of cells from the ventricular free walls and cell multiplication. Despite this, Villavicencio et al.32 recently proposed that the IVS primordium occupies the entire cephalic segment of the straight-tube heart. In contrast, the results of Contreras-Ramos et al.30 showed neither the coalescence of the trabecular pouches suggested by classical studies12,14,15 nor the continuous incorporation of cells from the ventricular free walls proposed by De la Cruz et al.28,85. Conversely, these authors proposed that the formation is due to the association of the trabeculae to the pmIVS suggesting that the septum grows in a cephalic-caudal direction, not caudal-cephalic as described by De la Cruz et al.28,85.
Regardless of its development, anatomically, it is recognized that the mIVS has two ends: a dorsal one that continues with the DIc and a ventral one that joins with the VSc and LCc3,28,82. Thus, the mIVS and these mesenchymal structures delimit the perimeter of the primary interventricular foramen (PIVF) (Fig. 5G)3,28,82.
Subsequently, remodeling and fusion of the A-V canal cushions determine the inclination of the PIVF and the formation of the secondary interventricular foramen (SIVF) (Fig. 5H)3,61. The SIVF will eventually close by fusion of the DIc with the dorsal end of the mIVS, while the PIVF will form the left ventricular outflow tract (Fig. 5I)3,82. In addition to the above, Lazzarini et al.31 suggest that the supraventricular crest fulfills a septal function at the level of the ventricular outflow tracts (Fig. 6E), thus discarding the idea that the supraventricular crest separates the inflow outflow tracts of the right ventricle, as described by De la Cruz et al.20.
AORTOPULMONARY SEPTATION
Aorticopulmonary septation is a process that has been controversial for different reasons: the variety of techniques used, spatiotemporal misinterpretation of embryonic events, morphological differences between biological models, and lack of consensus on the terms used86. In addition, for years, a disagreement prevailed regarding the number of truncal crest. Some authors have described two crests in the chick20,87,88, while others suggest the existence of three crests89-92, and it has even been reported that two of the three truncal crests are fused in their cephalic position to form a common crest90,91. In contrast, two truncal crests have been described in mammals13,17,93, although it has even been mentioned that both crests should be considered conotruncal because they continue longitudinally to the conus16. Both biological models mention the existence of intercalary crests, which, together with the truncal crests, participate in the formation of the leaflets of the arterial valves13,92. In addition, there are different proposals regarding the pattern of fusion of the truncal and intercalary crests, as well as the participation of the aorticopulmonary septum (APS) and conal crests. It is currently accepted that aorticopulmonary septation involves the participation of both the truncal crests and the APS, the latter being a contribution of non-cardiac cells.
Kirby et al.7 discovered that APS formation requires a cell population that originates from the neural crest between the otic placode and the third somite through ablation experiments. The neural crest cells delaminate from the neural tube and migrate to the third, fourth, and sixth pharyngeal arches (Fig. 7A)91,94, where they support the endothelium of the aortic arch arteries95. A neural crest cell subpopulation in the pharyngeal arches continues to migrate through the aortic sac until settling in the truncus, where they invade the vascular border of the truncal crests and give rise to APS (Fig. 7)78,96. The truncal crests and APS initially function as a septum, dividing the aortic and pulmonary components; however, they will eventually lose this function and separate the truncus into the trunk of the great arteries (Fig. 7B)92.
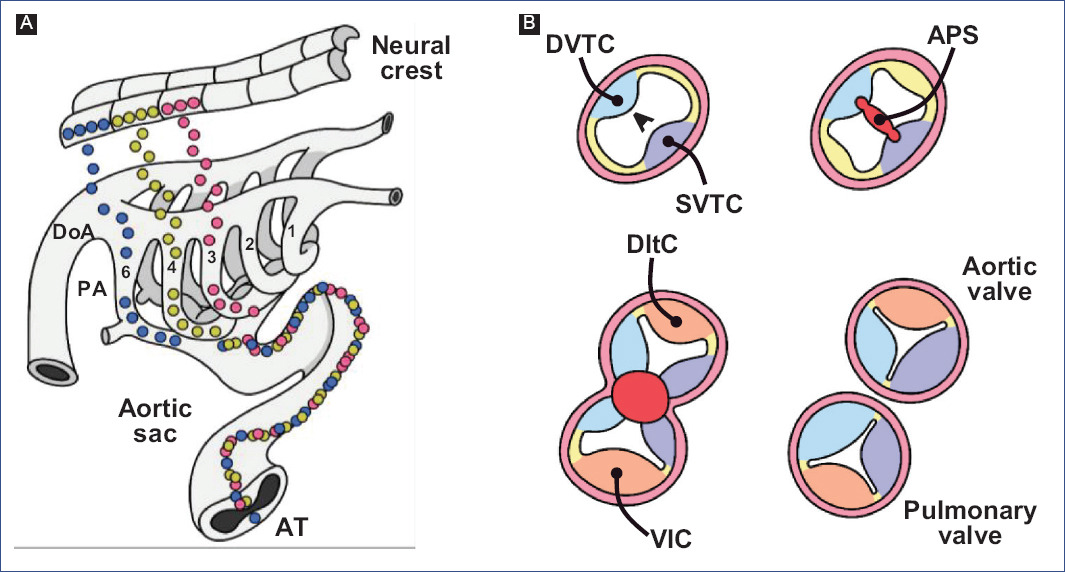
Figure 7 Aortic-pulmonary septation. A: migration of neural crest cells into the truncus (modified from Kirby and Waldo96). B: formation of the trunk of the great arteries, at the level of the arterial valves.APS: aortic-pulmonary septum, T: Truncus, DInc: dorsal intercalated crest, DoA: dorsal aorta, DDoTc: dextro-dorsal truncus crest, PA: pharyngeal arches, SVTc: sinistro-ventral truncus crest, VInc: ventral intercalated crest.
Neural crest cells express specific genes, including FoxD3, Snai1, and AP-297-100, induced by several signaling pathways such as BMP, FGF, Notch, and Wnt101,102. It is considered a pre-EMT stage, where the Slug promoter is activated, a SOX9-dependent event103. During delamination and migration, the action of the extracellular secreted signaling molecule WNT1 is considered essential104,105. Wnt1 is expressed in early migrating cells, but expression declines rapidly as they reach their final destinations106.
CONUS REMODELING
Over the years, the understanding of embryonic conus development has been misinterpreted by classical studies in human embryos and selective labeling experiments in chick embryos. It is widely accepted that the fusion of the right and left conal crests gives rise to two independent conduits, anterior and posterior; thus, the "anterior conus" would become the right ventricular outflow tract (RVOFT) and the "posterior cone" the left ventricular outflow tract (LVOFT)13,16,20,107. It has also been claimed that the conus is shortened longitudinally11,16,17,20,108, and it has even been proposed that it disappears completely108-110. Similarly, it has been suggested that the conotruncus's shortening and rotation result from cardiomyocyte apoptosis111,112. However, Lazzarini et al.31 describe that the spatiotemporal apoptotic pattern affects mostly the truncus, even suggesting that the myocardium of the conus of tubular structure loses continuity in its dorsal-left wall by an independent process of apoptosis and is transformed into a lamellar structure that corresponds to a large part of the anterior free wall of the right ventricle (Fig. 4E and F). Internally the cone crests fuse in their dorsal portions and participate in the formation of the RVOFT31 (Fig. 6), an event that contrasts with classical selective labeling experiments20. It is currently suggested that the ventricular outflow tracts have distinct embryonic origins, the RVOFT originating in the conus and the LVOFT in the cushions of the A-V canal31, descriptions that are consistent with the concepts of the FHF and SHF4-6,29.
Final considerations
Cardiac development is a highly complex process that depends on the active and orderly contribution of different cardiac and non-cardiac cell populations. This complexity makes cardiogenesis sensitive to developmental defects, which tend to give rise to various congenital heart diseases. For this reason, it is essential to elucidate the events involved in cardiogenesis to improve the diagnosis and treatment of these conditions. In this review, we discussed the contrast between information from classic studies and recent findings that propose new models of heart development. Interestingly, research is now being conducted that studies cardiogenic events, which have been assumed to be understood since the last century.











 nueva página del texto (beta)
nueva página del texto (beta)


