Introduction
Starch is considered the main polysaccharide reserve in vegetables and constitutes the most abundant carbohydrate in human nutrition, because it plays an important role in supplying metabolic energy (Obadi et al., 2020). Due to its intestinal digestibility, starch is classified into three fractions: rapidly digestible (RDS), slowly digestible (SDS), and resistant starch (RS), which are digested in vitro within the first 20 min, and from 20 to 120 min, or not digested at all, respectively (Ratnaningsih et al., 2019). In the scientific literature, it has been reported that through a variety of physical treatments (heat-moisture treatment, autoclaving, and cooking/cooling cycles), chemical treatments (acid hydrolysis or lintnerization, phosphorylation, acetylation, oxidation, and carboxymethylation) and by enzymatic methods (using β-amylase, isoamylase, pullulanase enzymes), it is feasible to increase the starch RS content from different botanical sources (Chiu and Solarek, 2009). For its study, RS can be classified into five types: RS1 is the one that is physically inaccessible inside the plant tissue, trapped in a protein or cellulose matrix; RS2 is the native starch in the granules, resistant due to its semi-crystalline character; RS3 is considered as retrograde starch, and is produced by physical treatments like heat-moisture and temperature cooling cycles; RS4, is obtained by the chemical modification of native starch; and RS5 which is an amylose-lipid complex, assuming that the aliphatic part of the lipid is included within the amylose helix (Ma and Boye, 2018). The nutritional and physiological importance of RS relies on the fact that it is considered a functional ingredient. It can prevent cardiovascular diseases, colon cancer, type 1 and 2 diabetes, obesity, osteoporosis, hypertension, improve intestinal health, micronutrient absorption, lipid profile, satiety, and the development of probiotic microorganisms (Fuentes-Zaragoza et al., 2010; Ashwar et al., 2016c).
In addition, RS3 can be obtained by autoclaving-cooling cycles of native starch, a simple, safe, and economical physical method (Ratnaningsih et al., 2019). The RS4 resistance is achieved by starch chemical modifications such as lintnerization, esterification, etherification, and crosslinking (Ashwar et al., 2016a). Raungrusmee and Anal (2019) reported a 64% increase in rice starch RS content by autoclaving and lintnerization. Concerning this, in the scientific literature, there are reports on obtaining and characterizing RS from native starch of the main conventional sources of tubers and cereals, such as potato (Kwon et al., 2019), cassava (Abioye et al., 2017), rice (Raungrusmee and Anal, 2019), wheat (Punia et al., 2019) and corn (Li et al., 2020), being the latter the most widely cultivated cereal in the world and the main conventional source of starch (Bustillos-Rodríguez et al., 2019).
Within the feasible non-conventional sources, apple (Malus domestica B.) is one of the most consumed fruits worldwide; this fruit, in an immature physiological state and its waste (the part that is not industrialized) is rich in carbohydrates, mainly starch (Kringel et al., 2020). Although the native starch obtained from apples has been characterized (Stevenson et al., 2006; Tirado-Gallegos et al., 2016), to our knowledge there are no reports on obtaining RS from native apple starch. On the other hand, white malanga (Xanthosoma sagittifolium) and yellow malanga or taro (Colocasia esculenta), are perennial herbaceous plants widely cultivated in tropical regions, which form corms rich in starch. Although native and modified starches have been obtained from both Xanthosoma sagittifolium (Ojinnaka et al., 2009) and Colocasia esculenta (Deka and Sit, 2016), reports on obtaining RS from these species are insufficient. Simsek and El (2012) produced 35 % RS3 by autoclaving native starch from Colocasia esculenta. Espinosa-Solis et al. (2021) recently reported the feasibility of obtaining RS3 from malanga flour using the autoclave hydrothermal treatment and performing the RS characterization. Thus, this study aimed to assess the effect of the application of successive cycles of autoclaving/cooling and lintnerization (acid hydrolysis) on the formation of RS3 and RS4, respectively, from native corn, apple, and malanga starches and in evaluating its physicochemical properties and their in vitro digestibility. The information obtained could suggest the potential of these resistant starches in food applications, with possible benefits for human health.
Materials and methods
Materials
Commercial corn starch (MaizenaTM, Unilever, Mexico) was acquired at a local market (Cuauhtemoc City, Chihuahua, Mexico). Apples of the Golden Delicious Smoothee variety were used. These fruits were obtained 70 days after full flowering (DAFF) and provided by a commercial orchard in Cuauhtemoc City (Chihuahua State, Mexico). Flour from malanga corms (Xanthosoma sagittifolium) obtained from plants grown in the municipality of San Fernando (Chiapas State, Mexico) was used. All chemical reagents used were analytical grade and acquired from Sigma-Aldrich (Toluca, Mexico State).
Isolation of native apple starch (NAS)
The native apple starch was obtained within 48 h after harvesting the fruit according to the methodology reported by Tirado-Gallegos et al. (2016). For this, apples were washed and cut into small pieces, then soaked in distilled water with 0.16 % potassium metabisulfite and ground in a blender (Osterizer, Blender Classic). The suspension was sieved through a 100 mesh sieve (ASTM) and allowed to stand under refrigeration (4 °C) for 12 h. Then, the supernatant was removed by decantation, and the settled starch layer was re-suspended in distilled water and centrifuged at 10,500 × g for 15 min. Next, the green surface of the obtained pellet was removed with the help of a spatula. The remaining pellet was homogenized with distilled water for 10 min and allowed to stand for 24 h under refrigeration. Then, the supernatant was decanted and washed again; this process was repeated at least three additional times until the supernatant was clear and free of fiber and pigment traces on the sedimented starch. The starch was collected and dried in an oven with hot air circulation at 40 °C for 24 h. Finally, it was ground and sieved in a 100 mesh (ASTM) with a pore opening of 0.149 mm.
Isolation of native malanga starch (NMS)
The starch isolation was carried out according to the methodology reported by Dai et al. (2015), with modifications. First, 100 g of malanga flour were suspended in 500 mL of distilled water and homogenized for 1 h under constant stirring at 300 rpm. The dispersion was sieved through organza cloth; the permeate was centrifuged (Allegra 64R) at 10,000 × g for 10 min. The supernatant was discarded, and the pellets re-suspended in 1,000 mL of distilled water and kept under stirring for 30 min; this dispersion was sieved through a 250 mesh sieve. The sieve was mixed with 500 mL NaOH 0.05 M and kept under stirring at 300 rpm for 60 min, then centrifuged (10 min at 10,500 × g). The supernatant was removed, and the brown layer (pigments, fibers, and proteins) on the pellet’s surface was scraped off with the help of a spatula. Subsequently, the starch was washed for 30 min with 0.1 M HCl for neutralization; the dispersion was centrifuged again at 10,500 × g for 10 min. The supernatant was discarded, and to remove traces of NaCl, the sedimented starch was washed with distilled water (30 min, 300 rpm) and centrifuged (10 min at 10,500 × g). The supernatant was decanted, and the starch dried in a forced air oven at 40 °C, ground in a mortar, and sieved through a 100 mesh (ASTM) to standardize the particle size.
Production of RS3 by autoclaving
The production of resistant starch type III (RS3) was done by physical modification of native starch by autoclaving, according to Berry (1986). First, 100 g of native starch were mixed with 350 mL of distilled water and stirred for 15 min (IKA, RW 20 digital). Next, the starch dispersion was heated in an autoclave (121 - 127 °C) for 1 h, allowed to cool, and stored at 4 °C for 24 h. This process was repeated three times. Subsequently, the starch was subjected to a freeze-drying process (LABCONCO) for 72 h and later ground in a mill (IKA M 20 S3). This procedure was applied for NAS to obtain lintnerized apple starch (LAS); also, for NMS to obtain lintnerized malanga starch (LMS) and to NCS to obtain lintnerized corn starch (LCS).
Production of RS4 by lintnerization
The production of resistant starch type IV (RS4) was achieved by chemical modification of native starches with acid hydrolysis, following the methodology of Rivas-González et al. (2008). First, 100 g of native starch were mixed with 200 mL of 1 M HCl and stirred for 6 h at 50 rpm and 35 °C. Then, 200 mL of 1 M NaOH (pH 6.0) were added. Next, the starch solids were centrifuged (Allegra 64R) 6 times using 2,000 mL of distilled water. Subsequently, they were dried in an oven at 37 °C for 48 h. This procedure was applied to NAS to obtain autoclaved apple starch (AAS); also, to NMS to obtain autoclaved malanga starch (AMS) and to NCS to obtain autoclaved corn starch (ACS).
Proximal análisis
The following analyses were performed on native and resistant starches (RS3 and RS4). The official AOAC (2002) methods of analysis described in the 17th Edition (2002), ether extract (954.18, subchapter 4.5.02.), moisture (method 934.01 subchapter 4.1.03), ash (method 942.05 subchapter 4.1.10), proteins (991.20 Subchapter. 33.2.11). Finally, the carbohydrate content was calculated by difference according to the equation 1:
Enzymatic determinations of total starch (TS), available starch (AS), resistant starch (RS), retrograde resistant starch (RRS)
Total starch (TS)
The TS content of each sample was quantified by means of a determination that estimates the total amount of starch available for enzymatic hydrolysis. This determination was according to Goñi et al. (1997). First, 50 mg of starch were dispersed in a 2 M KOH solution for 30 min, to gelatinize all the starch molecules. Subsequently, it was incubated at 60 °C for 45 min (pH 4.75) with an amyloglucosidase enzyme solution (Roché brand, no. 102 857, Roche Diagnostics, IN, USA); after this time, the released glucose was determined using the glucose oxidase/peroxidase assay (GOD/POD) (sera-PAK® Plus, Bayer de Mexico, SA de CV). TS content was calculated as glucose (mg) × 0.9. Potato starch was used as a reference.
Available starch (AS)
This determination was carried out according to Holm et al. (1989). First, 20 mL of distilled water were mixed with a 500 mg sample, stirred for 10 min, and then 100 µL of Termamyl were added. Next, samples were placed in a boiling bath for 20 min and stirred every 5 min until they completed 20 min; then, samples were cooled to 30 - 40 °C and transferred to a 100 mL volumetric flask. Next, in a glass tube, 1 mL of sodium acetate buffer at pH 4.75, 25 µL of amyloglucosidase, and 500 µL of sample from the 100 mL volumetric flask were placed and incubated for 30 min at 60 °C, under constant stirring. Finally, the content of the tube was transferred to a 10 mL volumetric flask, and 50 µL of the sample was taken to determine the glucose released by enzymatic digestion, using the GOD/POD (glucose/oxidase peroxidase) method, reading the optical densities of the samples at 510 nm in a Spectronic Genesis 5 spectrophotometer (Spectronic Instrument, Inc Rocherster, NY, USA).
Resistant starch (RS)
The methodology described by Goñi et al. (1996) was used to determine the indigestible starch content. One hundred mg of starch were weighed in a centrifuge tube, and 10 mL of KCl-HCl pH 1.5 buffer were added, followed by 200 µL of pepsin solution (the pepsin solution prepared in a ratio of 25 mg of pepsin with 250 mL of KCl-HCl buffer per sample). The sample was mixed and left in a water bath at 40 °C for 60 min with constant stirring. Once the time had elapsed, the sample was left to stand at room temperature, and 9 mL of trismaleate buffer at pH 6.9 were added, followed by 40 mg of α-amylase (290 U/mg, from the Sigma-Aldrich brand, San Luis, Missouri, USA), mixed and incubated for 16 h in a water bath at 37 °C with constant agitation; subsequently, the sample was centrifuged (Allegra 64R) for 15 min at 3000 × g at 4 °C. The supernatant was discarded and washed twice with 10 mL of distilled water, centrifuged again, and the supernatant was discarded. Then, 3 mL of distilled water and 3 mL of 4 M KOH were added, and the mixture was left to stand for 30 min at room temperature with constant stirring, then adding 5.5 mL of 2 M HCl and 3 mL of 0.4 M Na acetate buffer (pH 4.75), and subsequently, 25 mL of amyloglucosidase were immediately added and left for 45 min in a water bath at 60 °C with constant stirring. The sample was centrifuged for 15 min at 3000 × g at 4 °C and the supernatant was collected and transferred to a 50 mL volumetric flask. The residue was washed twice with 10 mL of distilled water each time, and the supernatant was combined with that previously obtained. Subsequently, it was adjusted to 50 mL, and 50 mL of sample was taken to determine the glucose released by enzymatic digestion, using the GOD/POD method (glucose/oxidase peroxidase, SERA-PAK® Plus, Bayer de México, S.A. de CV), reading the optical densities of the samples at 510 nm in a Spectronic Genesis 5 spectrophotometer (Spectronic Instrument, Inc Rocherster, NY, USA).
Retrograde resistant starch (RRS)
The methodology described by Saura-Calixto et al. (1993) was used to determine the content of retrograde resistant starch in samples. First, 100 mg of starch were placed in a centrifuge tube, adding 10 mL of 0.08 M phosphate buffer at pH 6.0 and 10 mL of thermostable α-amylase (290 U/mg, Sigma-Aldrich brand, San Luis, Missouri, USA), and incubated in a boiling water bath for 30 min with constant stirring. Subsequently, they were allowed to cool to room temperature, then 2 mL of 0.0275 N NaOH and 100 μL of protease (170 U/mg, Sigma-Aldrich brand, San Luis, Missouri, USA) were added and incubated at 60 °C for 30 min with constant stirring, and allowed to cool to room temperature. Two mL of 0.325 N HCl and 60 μL of amyloglucosidase (15 U/mL, Roche, IN, USA) were added and incubated at 60 °C for 30 min. The sample was centrifuged for 15 min at 3000 × g at 4 °C, and the supernatant discarded. The residue was washed with 10 mL of distilled water, 10 mL of 96 % ethyl alcohol, and 10 mL of acetone, then centrifuged, and the supernatant discarded. Three mL of distilled water were added to the residue and dispersed. Subsequently, 3 mL of 4 M KOH were added and kept under stirring for 30 min at room temperature (25 ± 3 °C). Then, 5.5 mL of 2 N HCl, 3 mL of acetate buffer at pH 4.78 and 60 μL of amyloglucosidase (15 U/mL, Roche brand, IN, USA) were added and incubated at 60 °C for 30 min with constant agitation. Then, it was centrifuged at 3000 × g for 15 min at 4 °C, the supernatant saved, and the residue re-suspended in 10 mL of distilled water; the centrifugation was repeated 2 more times. Three mL of distilled water and 3 mL of 4 M KOH were added to the residue, and was kept under constant stirring for 30 min at room temperature. Then, 5.5 of 2N HCl, 3 mL of acetate buffer at pH 4.78 and 60 mL of amyloglucosidase (15 U/mL, Roche, IN, USA) were added, followed by an incubation at 60 °C for 30 min under constant stirring. Subsequently, it was centrifuged at 3000 × g for 15 min at 4 °C, the supernatant saved and re-suspended in 10 mL of distilled water. Centrifugation was repeated, and the supernatants were collected and calibrated at 100 mL. Fifty µL were used to determine the glucose content by the GOD/PAD method (glucose/oxidase peroxidase, SERA-PAK® Plus, Bayer de México, S.A. de C.V.), reading the optical densities of the samples at 510 nm in a Spectronic Genesis 5 spectrophotometer (Spectronic Instrument, Inc Rocherster, NY, USA).
Color evaluation
It was carried out using a Minolta colorimeter (Model CR-300, Osaka, Japan), and the color variables were reported according to the CIELAB system (L, a*, b*) in addition to the chroma variables (color saturation) and ºhue (hue) according to the methodology reported by Aboubakar et al. (2008). The instrument was calibrated using a white reference tile. Measurements were made in a glass cell containing the powdered starch on the light source (this cell was placed on a white base), and values recorded in quintuplicate for each starch.
Determination of apparent amylose content
For this determination, the starches were defatted in a Soxhlet with an 85 % methanol solution for 24 h. Subsequently, they were washed with ethanol and recovered by filtration. Iodine affinities of defatted starches were measured using an automatic potentiometer (702 SM Tirino, Metrohm, Herisau, Switzerland). Apparent amylose content was calculated by dividing the iodine affinity of defatted starches by 20 %.
Determination of amylose molecular weight
The methodology reported by Torruco-Uco et al. (2016) was used to determine the molecular weight of amylose. Pullulan standards of various molar masses (1.6 × 106, 3.8 × 105, 1.8 × 105, 1.0 × 105, 4.8 × 104 and 1.2 × 104 g/mol) were used to obtain a linear calibration. Pullulans were dissolved in HPLC-grade water at 25 ºC, kept in the dark (overnight), filtered using a 0.2 μm nylon syringe filter, and injected into the HPSEC-RI system. The amylose solubilization procedure was performed with 50 s of microwave heating. The supernatant solution was filtered through a 5 µm nylon syringe filter. The solution was injected (50 μL) into the HPLC AT 1100 (Agilent Technology, Deutchland GmbH Waldbronn, Germany) with gel permeation chromatography-size exclusion chromatography (HPLC-SEC) PL aquagel-OH mixed, 8 μm column (7.5 mm ID × 300 mm; Agilent Technologies Deutchland GmbH Waldbronn, Germany).
The column and detector were kept at 30 °C. HPLC-grade water was used as diluent, carefully degassed, and filtered through Durapore GV membranes (0.2 μm) before use. A flow rate of 1.0 mL/min was used. Data analysis was performed using Agilent’s GPC software (Agilent Technologies Deutchland GmbH Waldbronn, Germany). After filtration, the carbohydrate concentration of the supernatant solution was measured by the sulfuric acid-phenol colorimetric method. The procedure was performed at least five times for each sample.
In vitro digestibility
The in vitro digestibility was determined by the methodology reported by Zamudio-Flores et al. (2015) with modifications, which in turn is based on the methods reported by Englyst et al. (1992) and Regand et al. (2011). The enzyme solution for digestion was prepared as follows: 0.9 g of porcine pancreatin (EC 232.468.9, from pig pancreas, activity 8 × USP/g, Sigma-Aldrich, St. Louis, MO) in 4 mL of distilled water and centrifuged at 1,500 g for 10 min.
The supernatant (5.4 mL) was mixed with 0.8 mL of diluted amyloglucosidase [0.64 mL of amyloglucosidase (EC 3.2.1.3, 3300 U/mL, Megazyme) diluted to 0.8 mL of distilled water], and 0.2 mL of distilled water was added. This enzyme solution was prepared for each enzyme digestion. One hundred mg of native starches, RS3, RS4, and control (white bread made with wheat flour) were weighed in 50 mL tubes in triplicate, and 10 glass beads (diameter = 5 mm) were added to each tube. Two mL HCl (0.05 M) and 10 mg pepsin were added to the tubes and incubated (37 °C, 30 min) in a shaking bath. Subsequently, 4 mL of sodium acetate buffer solution (0.5 M, pH = 5.2) were added to each tube; freshly prepared enzyme solution (1 mL) was added at 1 min intervals. The mixtures were incubated at 37 °C in a shaking water bath. Aliquots (100 μL) were taken at intervals of 0, 10, 20, 30, 60, 90, 120 and 180 min and mixed with 50 % ethanol (1 mL). These solutions were centrifuged at 800 × g (10 min), and the hydrolyzed glucose content of the supernatant was determined using the glucose oxidase/peroxidase assay (Megazyme). The equation 2 determined the percentage of total starch hydrolysis:
Statistical analysis
The experiments were carried out using a completely randomized design. A one-way analysis of variance (ANOVA, p ≤ 0.05) was applied using the statistical program Sigma-Stat, version 2.03 (Fox et al., 1995). The size of the analyzed samples was a minimum of three (n > 3), and when significant differences were found, the Tukey test was applied to compare the means (Walpole et al., 1999).
Results and discussion
Proximal chemical analysis
The proximal chemical analysis is shown in Table 1. The NMS and NAS, as well as their respective lintnerized starches (LMS and LAS), presented the highest moisture contents with values that ranged from ≈ 13 % (NAS) up to ≈ 16 % (NMS) although no significant differences were observed between them (p > 0.05). On the other hand, every starch subjected to the physical processing of autoclaving presented the lowest moisture percentages, in the following descending order: AAS > ACS > AMS; however, no significant differences (p > 0.05) were observed among them. In this regard, lower moisture values have been reported in native corn starches (6.5 - 8.5 %) (Bustillos-Rodríguez et al., 2018), native oat starch (10.03 - 10.63 %) (Shah et al., 2016) and native (4.89 %) and lintnerized (7.99 %) plantain starch (Aparicio-Saguilán et al., 2005). Recently, it was reported that the moisture content in powders is an essential factor in flow and other mechanical properties and is highly dependent on the method used for its determination, the degree or level of drying, and the humidity in the surrounding atmosphere (Juarez-Enriquez et al., 2019).
Table 1 Proximal chemical analysis of native, autoclaved, and lintnerized starches from corn, apple, and malanga&.
Tabla 1: Análisis químico proximal de almidones nativos, autoclaveados y lintnerizados de maíz, manzana y malanga&.
| Sample | Analysis | ||||||
| Moisture (%) | Protein (%) | Lipid (%) | Ash (%) | CHO1 (%) | Amylose (%) | MW (kDa)2 | |
| NCS | 11.12 ± 0.62bc | 0.35 ± 0.02b | 0.16 ± 0.03abcd | 0.09 ± 0.02bcd | 88.29 ± 0.11bc | 28.62 ± 0.11b | 108.28 ± 0.92a |
| ACS | 8.73 ± 0.46cd | 0.30 ± 0.01bc | 0.11 ± 0.07abcd | 0.07 ± 0.01cde | 90.77 ± 0.09ab | 23.65 ± 0.10f | 86.39 ± 0.08g |
| LCS | 11.45 ± 0.90bc | 0.20 ± 0.01de | 0.12 ± 0.01abcd | 0.03 ± 0.01e | 88.20 ± 0.19bcd | 23.55 ± 0.14f | 100.72 ± 0.12b |
| NMS | 16.46 ± 0.22a | 0.42 ± 0.02a | 0.27 ± 0.03a | 0.14 ± 0.01ab | 82.71 ± 0.05e | 29.84 ± 0.10a | 95.53 ± 0.14d |
| AMS | 6.05 ± 0.72d | 0.33 ± 0.01b | 0.23 ± 0.03ab | 0.15 ± 0.01a | 93.24 ± 0.14a | 24.82 ± 0.07d | 80.44 ± 0.15h |
| LMS | 14.02 ± 1.02ab | 0.29 ± 0.02bc | 0.10 ± 0.00bcd | 0.07 ± 0.00cde | 85.52 ± 0.19cde | 24.18 ± 0.06e | 91.64 ± 0.09f |
| NAS | 13.21 ± 0.63ab | 0.26 ± 0.01cd | 0.17 ± 0.03abc | 0.11 ± 0.01abc | 86.26 ± 0.12cd | 27.37 ± 0.08c | 98.23 ± 0.38c |
| AAS | 9.24 ± 0.53cd | 0.19 ± 0.01e | 0.00 ± 0.00d | 0.06 ± 0.00cde | 90.51 ± 0.10ab | 22.88 ± 0.03g | 81.58 ± 0.09h |
| LAS | 14.77 ± 0.54a | 0.18 ± 0.02e | 0.07 ± 0.03cd | 0.05 ± 0.00de | 84.94 ± 0.10de | 22.69 ± 0.06g | 93.59 ± 0.13e |
&Mean of at least three repetitions ± standard error. Values with the same letter within columns are not significantly different according to Tukey’s test (p > 0.05). 1Total carbohydrates (CHO) were obtained by difference (100 % - % rest of the other components). 2MW = Molecular weight.
The components (mainly lipids and proteins) can influence the functionality of the starch granule; protein is associated with grain hardness; while lipids can significantly reduce the swelling power of starch (Bustillos-Rodríguez et al., 2018). The native and modified starches of corn, malanga, and apple presented proteins, lipids, and ashes values lower than 1 %, with which these polluting components of the starch would be reduced, indicating that these starches were extracted with high purity (Lawal, 2004). Various investigations have reported similar results in native starches from corn, potato, cassava (Waterschoot et al., 2015), malanga (Deka and Sit, 2016), and rice (Ashwar et al., 2016c; Van Hung et al., 2016).
Table 1 also shows that the lintnerization process decreased proteins, lipids, and ashes content in LMS, with significant differences (p < 0.05) with respect to its native counterpart; however, in LCS and LAS, this reduction was only observed in proteins and ashes (being statistically significant, p < 0.05). This decrement is attributed to the acid used in the lintnerization process, which solubilized an important portion of these components (Aparicio-Sanguilán et al., 2005). After autoclaving modification of native starches, the protein, lipid, and ash content decreased because the thermal treatment caused proteins denaturation and lipids saponification (Aparicio-Sanguilán et al., 2005). In the AMS, the reduction in protein content was significant (p < 0.05) compared to NMS. In addition, the AAS protein and lipid content decreased significantly (p < 0.05) compared to their native counterpart. At the same time, in the NCS, NMS, and NAS samples, no significant differences were found (p > 0.05) in ash content in relation to ACS, AMS, and AAS, respectively, after undergoing the autoclaving process; this was possibly due to the temperature used in the autoclaving process not being high enough to destroy the minerals present in the starch (Aparicio-Saguilán et al., 2005). These results are consistent with those reported by Aparicio-Saguilán et al. (2005) in autoclaved plantain starches and by Deka and Sit (2016), who reported a similar behavior in starches modified by heat-moisture treatment from malanga.
In relation to the carbohydrate content, no clear or defined trend was observed. In this sense, the autoclaved samples, in general, presented the highest values, while the samples lintnerization did not cause significant changes (p > 0.05) with respect to their native counterparts. This could be due to the fact that the values of these components were obtained by difference, which is related to the value of the other components (moisture, proteins, lipids, and ashes). This has been previously reported by Vargas et al. (2016) in acetylated potato starches. Regarding the similarities between the conventional and unconventional sources in this determination, NMS and NAS did not present significant differences (p > 0.05) in lipids and ashes content compared to NCS.
Apparent amylose content
The amylose contents for the NCS, NMS, and NAS samples were ≈ 29, 30, and 27 %, respectively (Table 1). Some studies have reported lower amylose content in corn (22.5 %) (Jane et al., 1999) and malanga starch (22.7 %) (Lawal, 2004), while a value within the interval of 26 - 29.1 % for apple starch was reported by Stevenson et al. (2006). The differences found may be due to the variety, harvesting time, and place of cultivation (Simsek and El, 2012). On the other hand, in the modified starches (autoclaved and lintnerized), the amylose content decreased significantly (p < 0.05) due to the selective elimination of amorphous zones during those modifications. The reduction in amylose content in the autoclaved starches is consistent with that reported in the literature for malanga starch modified by a convection process with a hot air oven, where that decrement was attributed to the union of the amylose chains already present with each other or to the amylopectin molecule (Deka and Sit, 2016). However, Babu et al. (2014) reported the opposite of this study in autoclaved banana starch where the amylose content increased after autoclaving, which they attributed to a possible partial debranching of amylopectin by the drastic heating and pressure during autoclaving.
The lintnerization treatment decreased the amylose content by approximately 5 % (due to partial hydrolysis) in the three analyzed starches. The results of this study are consistent with those reported by Lawal (2004) in lintnerized malanga starches and by Wang and Wang (2001) in lintnerized potato, corn, and rice starches, who reported a decrease in amylose content during the acid treatment, relative to native starch. The H+ ions acted mainly on the amorphous regions within the starch granule, and both amylose and amylopectin were hydrolyzed in the acid presence (Wang and Wang, 2001).
No significant differences were observed between the amylose content of the autoclaved and lintnerized corn and apple starches (p > 0.05), contrary to what was observed in the modified malanga starches where the lowest amylose content was presented in the lintnerization treatment. In general, we can say that unconventional-source starches differ from those obtained from conventional sources (corn) in terms of amylose content, both in their native and modified forms. It is important to mention that malanga starches (native, autoclaved, and lintnerized) presented higher percentages of amylose compared to the type of modification. Finally, amylose content has been shown to have a significant correlation with higher resistant starch content or lower digestibility (Li et al., 2019).
Determination of molecular weight
Starch is a polymer consisting of a mixture of macromolecules of different chain lengths with a very similar molecular structure, so it is difficult to precisely define their molecular weight (MW) (Cai et al., 2019). The native starches MW was higher than that of the modified samples (Table 1), which indicated that the macromolecules (amylose and amylopectin) were fragmented or hydrolyzed due to degradation by the applied treatments. The autoclaved starches from the three botanical sources showed a significant decrease (p < 0.05) in MW, with respect to their native counterparts, and this is because the autoclaved starches present a lower content of amylose compared to the native ones (Zeng et al., 2015). This result is consistent with what was reported in autoclaved starch from lotus seeds and in starch subjected to ultrasonic-autoclaved treatment, where a decrease in MW was observed compared to its native starch and high-amylose corn starch (Zeng et al., 2015). The starches modified by acid hydrolysis (lintnerization) showed a significant MW decrease (p < 0.05) compared to the native ones; this is attributed to the fact that the amylose chains are more susceptible to acid hydrolysis instead of the amylopectin chains (Shi et al., 2016).
In the initial attack that occurs in this modification, most of the amylose chains could have been hydrolyzed into shorter chains, while the amylopectin chains were less susceptible to acid attack (Xia et al., 2017). The findings in this study are similar to those reported in hydrolyzed cassava (tapioca) starch at different reaction times, and those in rice starches modified by reactive extrusion through esterification with octenyl-succinylation and acetylation, and in hydrolyzed corn starches, where they presented a decrease in MW, after the chemical modifications (Cai et al., 2019; Xia et al., 2017; Ozturk et al., 2011). In general, we observed that the MW between the various unconventional botanical sources (as well as their respective modifications), presented significant differences (p ≤ 0.05) compared to the conventional source (corn), being malanga starches (native, autoclaved, and lintnerized) those that presented lower MW.
Color evaluation
Table 2 shows the color attributes of native and modified (resistant) corn, apple, and malanga starches. Color is a quality indicator criteria in the industrial and commercial use of starches, where white starches indicate a lower protein and pigment contents and, therefore, higher purity (Jan et al., 2017). Therefore, it should not present any coloration for a greater acceptance of the starch (Radley, 1976). Most of the starches showed lightness values (L*) higher than 90, so it can be considered that they presented high whiteness if it is estimated that a value of L* = 100, it is white (Tirado-Gallegos et al., 2016); however, AAS and AMS presented lower L* (90.47 and 85.55, respectively) values. This result agrees with what was described by Deka and Sit (2016), where their physically modified starch presented lower luminosity values (L*) according to its native counterpart. In starches subjected to the lintnerization treatment (LCS, LAS, and LMS), the whiteness or luminosity (L*) was similar to the native starches (NCS, NAS, and NMS) due to the use of NaOH to neutralize the lintnerized samples, which had a whitening effect (Ashwar et al., 2017). Regarding the coordinates a* (red/green) and b* (yellow/blue) values, AMS exhibited the highest ones (1.72 and 4.53, respectively), and AAS at coordinate b* (5.21). These results are similar to the values reported by Deka and Sit (2016) and Aboubakar et al. (2008) in malanga starches. The color change observed in the autoclaved samples, could be attributed to the Maillard reaction between the starch reducing sugars and the protein amino groups during hydrothermal processing (Shah et al., 2016; Gani et al., 2016). In relation to the hue angle variable (°hue); this has a value of 0° for red, 90° for yellow, 180° for green, and 270° for blue (Whale and Singh, 2007). The LAS presented the highest value in this variable (°hue ≈ 281), conferring blue tones, followed by LCS, ACS, and NCS, showing yellow tones; while the lowest values were observed in LMS (41.80), NMS (61.46) and AMS (68.36) starches, presenting red tones. These shades in the starch may be due to the presence of pigments in each botanical source used (Tirado-Gallegos et al., 2016).
Table 2 Color variables of native, autoclaved, and lintnerized corn, from apple, and malanga starches&.
Tabla 2: Variables de color de los almidones nativos, autoclaveados y lintnerizados de maíz, manzana y malanga&.
| Samples | Color variables | ||||
| L* | a* | b* | Croma | °hue | |
| NCS | 99.56 ± 0.01a | - 1.39 ± 0.01g | 2.81 ± 0.00c | 3.13 ± 0.01c | 116.32 ± 0.18c |
| ACS | 97.52 ± 0.00b | - 0.81 ± 0.00e | 2.06 ± 0.02d | 2.21 ± 0.01d | 111.40 ± 0.27cd |
| LCS | 99.20 ± 0.01a | - 1.01 ± 0.01f | - 0.49 ± 0.01g | 1.32 ± 0.01e | 139.44 ± 0.63b |
| NMS | 96.00 ± 0.33c | 0.63 ± 0.01b | 1.18 ± 0.07e | 1.33 ± 0.07e | 61.46 ± 1.42f |
| AMS | 85.55 ± 0.13f | 1.72 ± 0.03a | 4.53 ± 0.32b | 4.66 ± 0.13b | 68.36 ± 0.21f |
| LMS | 97.43 ± 0.09b | 0.39 ± 0.07c | 0.36 ± 0.07f | 0.53 ± 0.10f | 41.80 ± 3.74g |
| NAS | 96.42 ± 0.03c | - 0.81 ± 0.02e | 2.37 ± 0.03cd | 2.51 ± 0.03d | 108.82 ± 0.61d |
| AAS | 90.47 ± 0.09e | 0.71 ± 0.00b | 5.21 ± 0.06a | 5.26 ± 0.06a | 82.3 ± 0.11e |
| LAS | 94.43 ± 0.09d | 0.09 ± 0.02d | 0.49 ± 0.04g | 0.49 ± 0.04f | 280.72 ± 2.39a |
&Mean of at least five repetitions ± standard error. Values with the same letter within columns are not significantly different according to Tukey’s test (p > 0.05).
In the chroma variable (color saturation or intensity), no trend was observed between native and modified starches. AAS presented the highest chroma value (5.26), which was statistically significant compared to the rest of the analyzed starches, followed by AMS (4.66). In native starches, the chroma value was determined in a range of 1.33 - 3.13; these values are lower than those reported by García-Tejeda et al. (2011) in native banana starch (10.95); however, the chroma values evaluated in our study are higher than those obtained by Tirado-Gallegos et al. (2016) in apple starch (ranges 0.20 - 1.70). The autoclaved starches presented higher values (2.21 - 5.26) than the lintnerized ones (0.49 - 1.32). Garcia-Tejeda et al. (2011) reported a chroma value of 5.75 for oxidized plantain starch, which is higher than those found in this study for starches chemically modified by lintnerization. Some researchers have reported that chroma values close to zero are related to the whiteness of the starch (García-Tejeda et al., 2011; Tirado-Gallegos et al., 2016). This result implies that the lintnerized starches of the present study are of greater whiteness than the autoclaved starches, being favorable for their industrial application since this is representative of quality and purity.
In general, the native and lintnerized malanga and apple starches showed differences in the color variables L*, a*, chroma, and °hue with the native starch and the LCS, respectively, except for the b* coordinate; where a similar value was observed in NAS to that obtained in NCS, and LAS was similar to LCS. Regarding the autoclaved malanga and apple starches, these presented differences in L*, a*, b*, chroma, and °hue with respect to ACS.
Enzymatic determinations of total starch (TS), available starch (AS), resistant starch (RS) and retrograde resistant starch (RRS)
Table 3 shows the contents (determined as a percentage from enzymatic evaluations) of total starch (TS), available starch (AS), resistant starch (RS), and retrograde resistant starch (RRS) quantified in the native and modified starch samples (lintnerized and autoclaved). The TS is considered a qualitative determination of starch purity since it represents an important criterion in its quality, while AS represents the starch susceptible to enzymatic attack (Tirado-Gallegos et al., 2016). We observed that the lintnerization and autoclaving treatments decreased the TS and AS contents in all the samples analyzed, which was more significant in the corn starch sample (≈ 5 %). On the contrary, RS and RRS contents presented an inverse behavior to the quantified values of TS and AS since RS and RRS increased in all the samples when they were subjected to the lintnerization and autoclaving treatments. Interestingly, we observed that the highest RS amount (≈ 14 %) was obtained in the malanga starch sample when it was subjected to autoclaving.
Table 3 Enzymatic determinations (%) of total starch (TS), available starch (AS), resistant starch (RS), and retrograde resistant starch (RRS) of native, autoclaved, and lintnerized corn, from apple, and malanga starches&.
Tabla 3: Color of squash fruit peel (Cucurbita pepo L.) var. ‘Grey Zucchini’s.
| Samples | TS | AS | RS | RRS |
| NCS | 98.21 ± 0.26a | 95.15 ± 0.25a | 1.98 ± 0.03h | 0.80 ± 0.01e |
| ACS | 93.86 ± 0.32b | 84.37 ± 0.12e | 8.00 ± 0.05c | 1.10 ± 0.02b |
| LCS | 93.01 ± 0.09bc | 86.13 ± 0.04c | 5.69 ± 0.12e | 1.01 ± 0.01c |
| NMS | 91.13 ± 0.12d | 85.27 ± 0.05d | 3.24 ± 0.04g | 0.92 ± 0.01d |
| AMS | 89.33 ± 0.25e | 75.29 ± 0.11g | 14.45 ± 1.32a | 1.18 ± 0.01a |
| LMS | 88.04 ± 0.13f | 77.28 ± 0.07f | 9.27 ± 0.06b | 1.11 ± 0.01b |
| NAS | 93.56 ± 0.28b | 90.31 ± 0.15b | 2.17 ± 0.04h | 0.71 ± 0.01f |
| AAS | 92.36 ± 0.22c | 84.07 ± 0.04e | 7.28 ± 0.05d | 0.91 ± 0.01d |
| LAS | 90.96 ± 0.06d | 84.94 ± 0.04d | 4.85 ± 0.03f | 1.06 ± 0.01bc |
Determinaciones enzimáticas (%) de almidón total (AT), almidón disponible (AD), almidón resistente (AR) y almidón resistente retrogradado (ARR) de almidones nativos, autoclaveados y lintnerizados de maíz, manzana y malanga&.
Similar results in obtaining RS (by the physical treatment of autoclaving) have been reported in plantain starches, with values that increased from 1.51 % (for native starch) to 16.02 % (for autoclaved starch) (Aparicio-Saguilán et al., 2005), in corn starches (from 0.68 % for native starch to 8.47 %) (Neder-Suárez et al., 2018), and wheat starch with values that increased from 0.3 % to 4.8 % (Berry, 1986). The RS increment can be attributed to the heating and cooling cycles made by the autoclave. Autoclaving is a treatment that combing the gelatinization process (which disrupts the granular structure by heating a starch dispersion in excess water) and the retrogradation process (which produces recrystallization of starch components) upon cooling, resulting in higher production of RS and RRS (Babu et al., 2014). Similarly, in the chemical modification (lintnerization), the RS values are increased due to the depolymerization of the amylose chains and amylopectin branches; however, the lintnerization does not entirely degrade the crystalline structure (Franco et al., 2002), which can produce a starch resistant to enzymatic hydrolysis (Aparicio-Saguilán et al., 2005). These results agree with the values recently reported by Neder-Suárez et al. (2018), with values that increased from 0.68 % to 2.10 % in corn starch subjected to acid hydrolysis and in lintnerized banana starch (from 1.51% to 2.61 %) (Aparicio-Saguilán et al., 2005). Therefore, we can infer that the non-conventional sources evaluated in our study (malanga and apple) are suitable for obtaining resistant starch and that the greatest amount of resistant starch is obtained through the physical treatment of autoclaving compared to the chemical treatment of acid hydrolysis.
In vitro digestibility studies
Figure 1 shows the in vitro hydrolysis of native and modified corn, malanga, and apple starches. As can be seen, NCS presented the highest hydrolysis rate, in addition to a rapid increase in the percentage of starch hydrolysis (amylolysis) during the first 30 min and a subsequent decrease until reaching a value of ≈ 58 %. In general, the hydrolysis values in all samples increased as the in vitro digestion time increased, which has been previously reported in cereal flours and starches such as oats (Kim and White, 2012; Zamudio-Flores et al., 2015) and unconventional botanical sources such as the cowpea bean (Vigna unguiculata) (Ratnaningsih et al., 2019). The hydrothermal modification by autoclave decreased the hydrolysis rate by ≈ 41, 25, and 24 % for corn, malanga, and apple, respectively, compared to their native counterparts; no statistically significant differences were observed (p ≥ 0.05) between the autoclaved starches from the different botanical sources. This type of behavior agrees with that reported by Ratnaningsih et al. (2019), who modified cowpea bean starch by applying successive autoclaving cycles, so these modified starches showed a lower rate of hydrolysis compared to native starch, which they associated with changes in the microstructure and increase in resistant starch obtained in the autoclaved samples.
Figure 1 In vitro starch hydrolysis. Corn ( ____ ), malanga ( __ __ .), manzana (__ __ _), circles (native starches), triangles (autoclaved starches), squares (lintnerized starches). Values represent the mean of at least three replicates (n > 3). Bars represent the standard error of the mean.
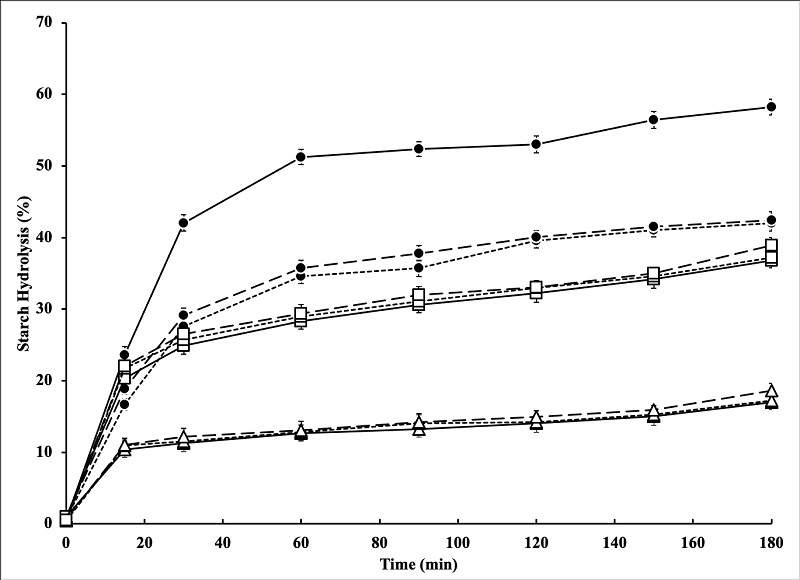
Figura 1: Hidrólisis de almidón in vitro. Maíz (____ ), malanga (__ __ .), manzana ( __ __ _), círculos (almidones nativos), triángulos (almidones autoclaveados), cuadrados (almidones lintnerizados). Los valores representan la media de al menos tres réplicas (n > 3). Las barras representan el error estándar de la media.
In relation to the lintnerized samples, it was observed that the hydrochloric acid chemical treatment decreased the starch hydrolysis rate compared to the native starches in values of ≈ 21, 5, and 3 % for the corn, malanga, and apple samples, respectively; however, this reduction was lower than the values obtained in the samples subjected to hydrothermal treatment (autoclaved). The maximum rate of hydrolysis reached by the lintnerized starches was observed in the 37 - 39% range. These values agree with those reported by Akanbi et al. (2019), who modified orange sweet potato starch by lintnerization, reporting a hydrolysis rate of ≈ 35 %.
Conclusions
Resistant starches type 3 (RS3) and 4 (RS4) were obtained by the application of successive cycles of autoclaving/cooling and lintnerization (acid hydrolysis), respectively, from native starches of corn (NCS), apple (NAS), and malanga (NMS). When comparing all analyzed samples, AMS had the highest RS content (14 %), and NCS the lowest (1.98 %). Furthermore, the autoclaved treatment decreased the enzymatic hydrolysis rate by ≈ 24 - 41 % compared to native starches, while with the lintnerization treatment, this reduction was smaller (≈ 3 - 21 %). Regarding the physicochemical properties, the autoclaved and lintnerized treatments reduced the apparent amylose content by ≈ 5 %, producing amylose with lower molecular weight (≈ 80 - 86 kDa) for autoclaved starches and ≈ 92 - 101 kDa for lintnerized starches. At the same time, the luminosity decreased by the autoclaved treatment and not by the lintnerization process. Therefore, AMS could be used in bakery applications (added in cookies or pasta) where it is desired to increase the RS content without affecting the color sensory characteristics.











 nueva página del texto (beta)
nueva página del texto (beta)


