Introduction
Maize (Zea mays L.) is the most important cereal with a global production of 1,162 million tons in 2020 (FAOSTAT, 2022). This cereal has a great social impact in developing countries where it is the main food staple. However, the most abundant proteins of maize (prolamins or zeins) are deficient in the essential amino acids lysine and tryptophan affecting the nutritional quality of the grain.
Mertz et al. (1964) found that the opaque-2 (o2) mutation (Figure 1) almost doubles the lysine content in maize endosperm and improves the protein quality, but the use of this mutant in breeding programs was limited due to its poor agronomic performance associated with the opaque/soft endosperm and low seed density. Some years later, Paez et al. (1969) found that some segregating S2 lines derived from opaque S1 parents showed modified o2 kernels (50% translucent or vitreous) whose lysine content was not different from the opaque kernels. Therefore, selection for hard endosperm was started. Researchers from the International Maize and Wheat Improvement Center (CIMMYT) in Mexico (Villegas et al., 1992) and the University of Natal in South Africa (Gevers and Lake, 1992), developed a modified o2 mutant or quality protein maize (QPM) (Figure 1) by recurrent backcrossing. This process required the simultaneous selection of kernels with normal texture and enhanced levels of essential amino acids. Thus, QPM combines the protein quality of o2 with a vitreous endosperm and has better agronomic characteristics.
Figure 1 Zein profiles and endosperm phenotype of vitreous (QPM) and opaque maize genotypes. (A) SDS-PAGE separation of zein proteins. The molecular weight marker (kDa) is shown on the left and the different zein sub-fractions are indicate on the right. A greater abundance of 27-kDa γ-zein can be observed in the endosperm of vitreous lines compared to the opaque ones. Adapted from Vega-Alvarez et al. (2022). (B) Cross section of K0326Y (vitreous) and W64Ao2 (opaque) maize kernels viewed under white light. (C) Whole grains of K0326Y (vitreous) and W64Ao2 (opaque) viewed under white light.
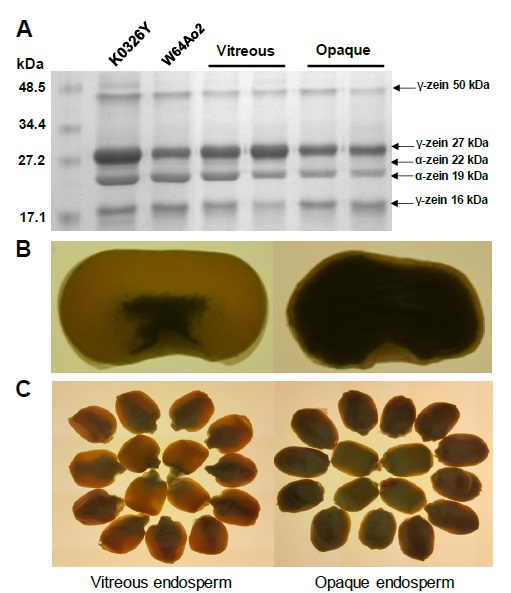
Figura 1: Perfiles de zeínas y fenotipo del endospermo en genotipos de maíz vítreos (QPM) y opacos. (A) Separación por SDS-PAGE de proteínas zeínas. El marcador de peso molecular (kDa) se muestra a la izquierda y las diferentes subfracciones de zeínas se indican a la derecha. Se puede observar una mayor abundancia de γ-zeína 27 kDa en el endospermo de líneas vítreas comparado con las opacas. Adaptado de Vega-Alvarez et al. (2022). (B) Sección transversal de granos de maíz de las líneas K0326Y (vitrea) y W64Ao2 (opaca) vistos bajo luz blanca. (C) Granos enteros de maíz de las líneas K0326Y (vitrea) y W64Ao2 (opaca) vistos bajo luz blanca.
The QPM materials developed by the CIMMYT maize breeding program have been used worldwide as donors of o2 modifiers to produce lines and hybrids adapted to each region. However, conventional QPM breeding involves the introgression of o2 into a local adapted genotype that is subsequently used to pollinate a modifier donor, a process that requires several generations of backcrossing and self-crossing and the monitoring of high levels of lysine and tryptophan, the recessive o2 mutant allele, and the modifiers. This lengthy and laborious strategy can take more than six years. The use of molecular markers has facilitated QPM breeding but this process could be more efficient if the mechanisms involved in the conversion of the soft o2 endosperm into a vitreous phenotype were understood (Gibbon and Larkins, 2005). The main mechanisms proposed for this conversion include an increase in the accumulation of small protein bodies enriched in 27 kDa γ-zein (Wu et al., 2010), the alteration of the structure and composition of starch due to the increase in the proportion of amylose and short-intermediate amylopectin chain branches (Gibbon et al., 2003; Salazar-Salas et al., 2014) and the loss of the amyloplast envelope due to a reduction in non-polar carotenoids (Wang et al., 2020). The aim of the present review is to provide the current advances about the molecular mechanisms associated with the modification of the vitreous endosperm in QPM.
Maize kernel composition
The major components of the maize kernel are starch (64 - 78 %) and proteins (8 - 15 %). Starch is mainly found in the endosperm while proteins are more abundant in the germ. The remaining components of the grain consist of lipids (4.0 - 4.9 %), ashes (1 - 3 %) and fiber (1 - 2 %) (Serna-Saldivar, 2019). Starch and proteins influence the physicochemical and structural characteristics of the kernel, highlighting the importance of these components.
Starch biosynthesis
Starch is the main carbon reserve in cereals (70 - 80 %) and is mainly responsible for their energetic and economic value. It is formed by two homopolysaccharides: amylose, an essentially linear molecule formed by glucose units linked by α-(1,4) glycosidic bonds, and amylopectin, a molecule formed by linear portions of glucose linked by α-(1,4) glycosidic bonds and ramifications linked by α-(1,6) bonds (Pfister and Zeeman, 2016).
In cereals, starch is mainly found in endosperm cells forming granules and its biosynthesis is carried out by the coordinated action of multiple enzymes: ADP-Glucose pyrophosphorylase (AGPase), starch synthases (GBSS, SS), starch branching enzymes (SBE) and starch debranching enzymes (DBE) (Figure 2) (Comparot-Moss and Denyer, 2009). This process begins with the enzyme AGPase that produces ADP-glucose in the cytosol, which is transported into the plastid and serves as a substrate for starch synthase enzymes (Pfister and Zeeman, 2016). The granule bound starch synthase I (GBSSI) is encoded by the waxy locus in cereals and is responsible for the elongation of amylose, being essential within the granule matrix. The soluble starch synthases (SSI, SSII, SSIII and SSIV) are exclusively involved in the synthesis of amylopectin chains and are associated with the starch granules. Genetic and biochemical studies indicate that each SS isoform has different properties and a different role in amylopectin synthesis. The starch branching enzyme (SBE) generates α-(1,6) glycosidic bonds after breaking the α-(1,4) bond and transferring the chain to the C6 from a glucose residue of another chain, forming the branched structure of the amylopectin molecule (Huang et al., 2021). Two isoforms of branching enzymes are expressed in the endosperm of cereals, branching enzyme I (SBEI) and branching enzyme II (SBEII), which differ in the length of the transferred glucan chain and substrate specificity; SBEI shows greater affinity for amylose and transfers longer chains than SBEII, which transfers shorter chains and has greater affinity for amylopectin (Sawada et al., 2018).
The starch debranching enzyme (DBE) catalyzes the hydrolysis of glycosidic bonds α-(1,6). In higher plants there are two types of DBE and they are defined according to the specificity of their substrate: debranching enzymes of the isoamylase type and the pullulanase type. Isoamylase breaks down amylopectin and phytoglycogen branches, while pullulanase acts on pullulans and amylopectin but not phytoglycogen (Robyt, 2009).
Figure 2 Starch biosynthesis pathway in the endosperm of cereals. The cytosol and plastid are indicated. Enzymes are indicated in italics: Susy, sucrose synthase; UGPase, UDP glucose pyrophosphorylase; PGM, phosphoglucomutase; FK, fructokinase; PGI, phosphoglucose isomerase; PPiase, pyrophosphatase; AGPase, ADP-glucose pyrophosphorylase; GBSSI, granule bound starch synthase; SS, starch synthase; SBE, starch branching enzyme. Adapted from Comparot-Moss and Denyer (2009).
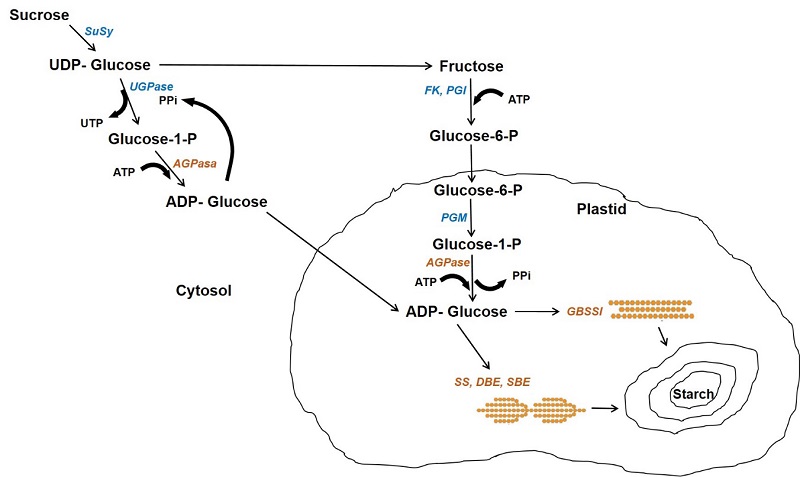
Figura 2: Ruta de síntesis de almidón en el endospermo de cereales. Se indican el citosol y el plástido. Las enzimas están indicadas en itálicas: Susy, sacarosa sintasa; UGPasa, UDP glucosa pirofosforilasa; PGM, fosfoglucomutasa; AGPasa, ADP-glucosa pirofosforilasa; GBSSI, almidón sintasa unida al gránulo; SS, almidón sintasa; SBE, enzyma ramificadora de almidón. Adaptada de Comparot-Moss and Denyer (2009).
Synthesis of zeins
The most abundant storage proteins in maize kernels are prolamins (soluble in alcohol) or zeins (50-55%), followed by glutelins (soluble in alkaline solutions) (35-40%), while albumins (soluble in water) and globulins (soluble in saline solutions) account for less than 10% of the total proteins (Larkins, 2019; Sethi et al., 2021). Because zeins are the major proteins in the kernel, total proteins are typically divided into zeins and non-zeins. Zeins are synthesized by membrane-delimited polyribosomes and transported within the lumen of the rough endoplasmic reticulum, where they assemble into protein bodies (Lending and Larkins, 1989). Protein bodies are small spherical structures made up of a protein matrix surrounded by a simple membrane; they contain at least four types of zeins (α-, β-, γ-, δ-), which are classified based on their solubility and structural similarities as α-zeins (22 and 19 kDa), β-zeins (14 and 16 kDa), γ-zeins (16, 27 and 50 kDa) and δ-zeins (10 kDa) (Figure 1A) (Coleman and Larkins, 1999). Immunolocalization studies showed that γ- and β-zeins are generally located in the peripheral region of protein bodies, while α-zeins are located in the internal region of these structures (Lending and Larkins, 1989).
The o2 mutant has a defective transcription factor that regulates the expression of α-zeins and depending on the genetic background the content of these proteins can be reduced more than 50% (Figure 1A) (Kodrzycki et al., 1989). Zeins are deficient in some essential amino acids (lysine and tryptophan) and the reduced synthesis of these proteins in o2 results in higher levels of more nutritionally balanced non-zeins (Lopez-Valenzuela et al., 2004) and free amino acids in the endosperm (Pineda-Hidalgo et al., 2011). The use of RNA interference (RNAi) to block the expression of α-zeins has also shown to increase the levels of lysine and tryptophan in maize endosperm (Huang et al., 2006), which avoids the negative characteristics and limitations of the recessive o2 mutant. The Cas9/CRISPR technology represents another biotechnological alternative to reduce or remove zein gene expression as a strategy to increase the levels of proteins with a better balance of essential amino acids (Jiang et al., 2022).
Quality protein maize and its importance in human nutrition
Malnutrition is a problem that affects more than 828 million people worldwide, 98% of which are from developing countries, mainly Africa, Asia, Latin America and the Caribbean, and includes 150 million children (FAOSTAT, 2022). People from developing countries with a cereal-based diet have a high risk of protein and lysine deficiency (Muleya et al., 2022), although this deficiency can occur in any people with a diet based on cereals. Several investigations have documented the benefits of QPM in human nutrition, highlighting its potential to mitigate problems associated with protein-energy deficiency in children under 5 years of age, the elderly and pregnant women, considered the most vulnerable groups (Hossain et al., 2019).
The consumption of QPM instead of normal maize, increased 12 - 15% the weight growth rate in infants and young children, with mild to moderate undernutrition (Akalu et al., 2010; Gunaratna et al., 2010). QPM has also a higher content of phenylalanine and isoleucine, suggesting it can be included in the family diet to reduce the risk of inadequate protein intake (Gunaratna et al., 2019). Tortillas from nixtamalized and extruded QPM flours showed higher nutritional indicators (C-PER, protein digestibility, PER, NPR, PDCAAS) than those from normal maize, suggesting they may have a positive effect on the nutritional status of people from countries where these products are widely consumed (Gutiérrez-Dorado et al., 2008). Desalegn et al. (2015) showed that QPM based complementary foods have good sensory acceptability and can help meet the minimum recommended daily dose of energy (370 kcal) and protein (10.9 g) for children aged 6 - 36 months, as well as two thirds of the recommended iron and zinc daily dose and up to 50 % of vitamin A. The supplementation of malnourished young children (4 - 6 years old) with QPM-based biscuits reduced the percentage of anemic subjects from 63.3 % to 16.6 % and the prevalence of severe underweight from 23.3 % to 0 % (Grover et al., 2020).
In recognition of the great potential of QPM to improve human nutrition in poor countries where maize is a staple food, Dr. Surinder K. Vasal and Dr. Evangelina Villegas from CIMMYT were awarded with the World Food Prize in 2000 (Cordova, 2001).
Advances in the development of QPM
Despite the QPM nutritional value and agronomic performance, the cultivation and adoption of these materials on a large scale has not been achieved, mainly due to the low availability of genetically diverse and competitive QPM hybrids compared to non-QPM / normal hybrids, the lack of information about their health benefits and government incentives (Hossain et al., 2018; Maqbool et al., 2021). Nevertheless, breeding programs have been implemented around the world with the purpose of producing new and better QPM genotypes; they have mainly used QPM donors from CIMMYT in Mexico (Cordova, 2001; Vivek et al., 2008). Until 2015, more than 167 QPM varieties were released worldwide, 53 % in Africa, 25 % in Latin America and 22 % in Asia (Twumasi-Afriyie et al., 2016). Some of these QPM genotypes are listed in Table 1. Conventional breeding strategies such as recurrent selection were initially used for QPM development, but in the last decades a widely used strategy to develop these materials is molecular marker-assisted breeding and most of the studies have used SSR markers (e.g. phi 057, phi 112 and umc 1066) located within the o2 gene on the short arm of chromosome 7 (www.maizegdb.org) (Maqbool et al., 2021). Some examples include the QPM version of the line V25 derived from the cross V25 × CML176 (QPM), which shows an increase in tryptophan content (0.85 %) and maintains a hard endosperm (Babu et al., 2005), as well as Vivek QPM-9 (VQL 1 × VQL 2) that contains 41 % more tryptophan and 30 % more lysine than the original hybrid (Vivek Hybrid-9) (Table 1) (Gupta et al., 2013).
Tabla 1: Desarrollo de genotipos QPM.
| Name | Pedigree/Background | Gene(s) introgressed | Country | Reference |
| NB-Nutrinta (OPV) | Poza Rica 8763 | o2 | Nicaragua | Cordova (2001) |
| HQ INTA-993 (hybrid) | (CML144 × CML159) CML176 | o2 | Nicaragua | Cordova (2001) |
| HB-PROTICTA (hybrid) | (CML144 × CML159) CML176 | o2 | Guatemala | |
| HQ-61 (hybrid) | (CML144 × CML159) | o2 | El Salvador | |
| HQ-31 (hybrid) | CML176 (CML144 × CML159) | o2 | Honduras | |
| ICA- (hybrid) | CML176 (CML144 × CML159) | o2 | Colombia | |
| FONAIAP (hybrid) | CML176 (CML144 × CML159) CML176 | o2 | Venezuela | |
| BR-473, BR-451 (OPV) | o2 | Brazil | Cordova (2001) | |
| INIA- (hybrid) | CML161 × CML165 | o2 | Peru | Cordova (2001) |
| HQ-2000 (hybrid) | CML161 × CML165 | o2 | Vietnam | Cordova (2001) |
| Zhongdan 9409 (hybrid) | Pool 33 × Temp QPM | o2 | China | Cordova (2001) |
| QUIAN2609 (hybrid) | Tai 19/02 × CML171 | o2 | China | Cordova (2001) |
| Susuma (OPV) | Across 8363SR | Mozambique, Senegal | Krivanek et al. (2007) | |
| Longe-5 'Nalongo' (OPV) | Across 8363SR | Uganda | Krivanek et al. (2007) | |
| Obatanpa (OPV) | Across 8363SR | Benin, Burkina Faso, Cameroon, Cote d'Ivoire, Ghana, Guinea, Malawi, Mali, Nigeria, Senegal, South Africa, Togo | Krivanek et al. (2007) | |
| Lishe-K1(OPV) | Across 8363SR | Tanzania | Krivanek et al. (2007) | |
| EV 99 QPM (OPV) | Cote d'Ivoire, Nigeria, Senegal, Togo | Krivanek et al. (2007) | ||
| KH500Q (hybrid) | (CML144 × CML159) CML181 | o2 | Kenya | Krivanek et al. (2007) |
| BHQP542 (hybrid) | (CML144 × CML159) CML176 | o2 | Ethiopia | Krivanek et al. (2007) |
| MHQ138 (hybrid) | (CML144 × CML159) Pool15Q | o2 | Ethiopia | Jilo (2022) |
| BHQPY545 (hybrid) | CML181 × CML165 | o2 | Ethiopia | Jilo (2022) |
| QS-7705 (hybrid) | o2 | South Africa | Krivanek et al. (2007) | |
| GH-132-28 (hybrid) | P62, P63 | o2 | Ghana | Krivanek et al. (2007) |
| ZS261Q (hybrid) | (CZL01006 × CML176) × (CZL01005 × CML181) | o2 | Zimbabwe | Krivanek et al. (2007) |
| 441C (hybrid) | CML142 × CML116 | o2 | Mexico | Cordova (2001) |
| H-551C (hybrid) | CML142 × CML150 | o2 | Mexico | Cordova (2001) |
| H-553C (hybrid) | (CML142 × CML150) CML176 | o2 | Mexico | Cordova (2001) |
| H-519C (hybrid) | (CML144 × CML159) CML170 | o2 | Mexico | Cordova (2001) |
| H-368EC (hybrid) | CML186 × CML149 | o2 | Mexico | Cordova (2001) |
| H-369EC (hybrid) | CML176 × CML186 | o2 | Mexico | Cordova (2001) |
| V-537C ( OPV ) | Poza Rica 8763 | o2 | Mexico | Gómez-M et al. (2003) |
| V-538C ( OPV) | Across 8762 | |||
| H-374C (hybrid) | (CML176 × CML142) CML186 | o2 | Mexico | Noriega González et al. (2011) |
| H-564C (hybrid) | (LT158 × LT159) LT160 | o2 | Mexico | Sierra Macías et al. (2011) |
| V556AC (OPV) | o2 | Mexico | Twumasi-Afriyie et al. (2016) | |
| ZAPATA 3 | o2 | Mexico | Twumasi-Afriyie et al. (2016) | |
| ZAPATA 9 | o2 | Mexico | Twumasi-Afriyie et al. (2016) | |
| V25 QPM (line) | V25 × CML176 | o2 | India | Babu et al. (2005) |
| Vivek QPM-9 (hybrid) | VQL1 (CM212 × CML180) × VQL2 (CM145 × CML170) | o2 | India | Gupta et al. (2009); Gupta et al. (2013) |
| BC2F4-1 (line) | QCL3024 (o16) × QCL5019 (wx) and QCL5008 (wx) | o16 | China | Yang et al. (2013) |
| BQPM9 (line) | (B99 × CLQ 06901) B99 | o2 | USA | Worral et al. (2015) |
| BQPM10 (line) | (B99 × CLRQ 00502) B99 | |||
| BQPM11 (line) | (B100 × CLQ 06901) B100 | |||
| BQPM12, BQPM16 (line) | (CLQ 06901 × B98) B98 | |||
| BQPM13, BQPM14 (line) | (CLQ 06901 × B97) B97 | |||
| BQPM15 (line) | (B91 × CLQ 06901) B91 | |||
| BQPM17 (line) | (CLQ 06901 × B113) B113 | |||
| ZPL 3 QPM (line) ZPL 5 QPM (line) | ZPL 3 × CML144 ZPL5 × CML144 | o2 | Serbia | Kostadinovic et al. (2016) |
| Zhao OP-6/o2o2 (line) | Zhao OP-6 × QPM CA339 (pool33) | o2 | China | Zhou et al. (2016) |
| BML-7 QPM (line) | BML-7 × CML-186 | o2 | India | Krishna et al. (2017) |
| CBML6 QPM (line) CBML7 QPM (line) DHM117 (hybrid) | BML6 × CML181 BML7 × CML181 CBML6 × CBML7 | o2 | India | Surender et al. (2017) |
| HM4 QPM (line) | HKI323 × HKI161 | o2 | India | Hossain et al. (2018) |
| HM8 QPM (line) | HKI1105 × CMLI61 | |||
| HM9 QPM (line) | HKI1128 × HKI193-1 | |||
| V238AC (OPV) | Comiteco race (yellow) × CML-172 | o2 | Mexico | Coutiño Estrada and Vázquez Carrillo (2018) |
| Quality Protein Popcorn (QPP) (lines) | CML154Q × (P2, P3, P9) Tx807 × P2 K0326Y × (P3, P7) | o2 | USA | Ren et al. (2018) |
| QCL8006-1 (line) QCL8006-2 (line) | QCL3024 (o16) × Taixi19 (o2) and QCL5019 (wx) | o2/o16 | China | Wang et al. (2019) |
| HM5-A (hybrid) | (HKI1344 × PMI-102-o2o16) × (HKI1348-6-2 × PMI-102-o2o16) | o2/o16 | India | Chand et al. (2022) |
| HM12-B (hybrid) | (HKI1344 × PMI-102-o2o16) × HKI1378 × PMI-102-o2o16) | |||
| V56AC (OPV) | Oloton race (yellow) × CML-172 | o2 | Mexico | Coutiño Estrada et al. (2022) |
OPV: Open pollinated variety.
Other researchers have focused on developing new QPM genotypes with certain agronomic and gastronomic characteristics, but always seeking to maintain the nutritional quality of o2. For instance, the Zhao OP-6 /o2o2 corn was generated by introducing the o2 allele into the Zhao OP-6 waxy corn to produce a waxy QPM line intended for the Chinese market, where waxy corn is widely used in food processing because of its high viscosity and digestibility (Zhou et al., 2016). Quality Protein Popcorn (QPP) was developed recently, which showed a higher lysine content compared to its parent elite line and maintained its bursting capacity, demonstrating the potential use of QPM for the production of grains with specific functional characteristics and good protein quality (Ren et al., 2018). Since most of the efforts to develop QPM have been based on the use of molecular markers that co-inherit with the o2 phenotype, improving the understanding of the molecular basis of endosperm modification could help to develop these materials more efficiently.
Mechanisms associated with endosperm modification in QPM
Increased accumulation of γ-zein proteins
One of the first biochemical changes observed in QPM genotypes was an increase in γ-zein content (2 to 5 times) compared to o2 (Figure 1A) and normal maize (Wallace et al., 1990). Immunolocalization studies suggested that γ-zein initiates the formation of protein bodies (Lending and Larkins, 1989), which is supported by the fact that inhibition of the 27 kDa γ-zein encoding gene reduces significantly the number of protein bodies, while the inhibition of 19 and 22 kDa α-zeins decreases the diameter of these structures (Guo et al., 2013). The γ-zeins are highly linked with disulfide bonds and it has been hypothesized that the covalent bonds of γ-zeins and other cysteine rich proteins promote the formation of a protein network around starch granules (Lopes and Larkins, 1991). Therefore, it has been proposed that an increase in the number of protein bodies and their compaction among the starch granules is, at least partially, responsible for the modification of the endosperm (Figure 3A). A genetic analysis using recombinant inbred lines (RIL) derived from the cross of Pool 33 (QPM) and W64Ao2 (soft endosperm), identified two loci associated with endosperm modification; these loci were located on the long arm of chromosome 7, one near the centromere linked to a 27 kDa γ-zein locus and the other near the telomere (Lopes et al., 1995). Holding et al. (2008) identified 7 loci associated with o2 modifier genes (mo2) in an F2 progeny from the cross of K0326Y-QPM and W64Ao2; two major loci located on chromosomes 7 and 9 explained 40 % of the phenotypic variation and were linked to 27 kDa γ-zein and starch synthesis genes, respectively. Holding et al. (2011) developed RIL from the F2 progeny and the kernels were characterized for vitreousness, density and hardness; genetic mapping with these RIL identified loci on chromosomes 1, 7 and 9, confirming their linkage with γ-zein and starch biosynthesis genes.
Figure 3 Vitreous endosperm formation in quality protein maize. (A) Representation of the main mechanisms associated with endosperm modification: Mechanism associated with an increase in protein bodies (PB) rich in 27 kDa γ-zein that fill the spaces between starch granules (SG); Mechanism associated with changes in the composition and morphology of the starch granules that favors their compaction and interaction with PBs; Mechanism associated with the disruption of the amyloplast membrane that favors the interactions between starch granules and PBs. PL, Phospholipids; NPC, Non-polar carotenoids. Adapted from Wang et al. (2020). (B) Cross section of mature kernels from opaque-2 and QPM genotypes showing the extent of vitreous endosperm.
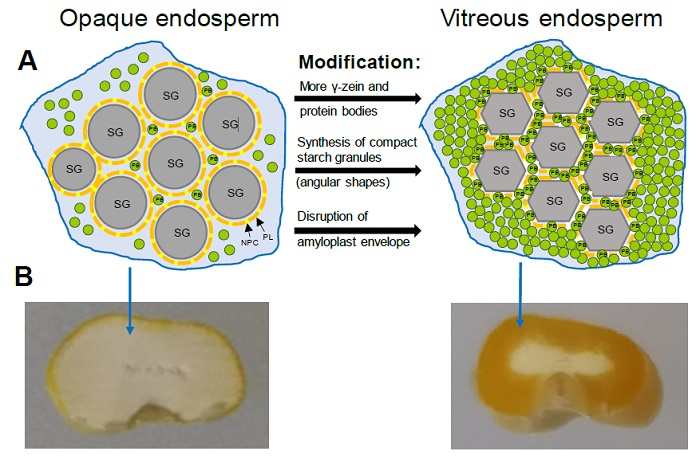
Figura 3: Formación del endospermo vítreo en el maíz de calidad de proteínica. (A) Representación de los principales mecanismos asociados con la modificación del endospermo: Mecanismo asociado con un incremento en cuerpos proteínicos (PB) ricos en γ-zeína 27 kDa que llenan los espacios entre los gránulos de almidón (SG); Mecanismos asociado con cambios en la composición y morfología de los gránulos de almidón que favorece su compactación e interacción con PBs; Mecanismo asociado con la disrupción de la membrana del amiloplasto que favorece las interacciones entre los gránulos de almidón y PBs. PL, fosfolípidos; NPC, carotenoides no polares. Adaptada de Wang et al. (2020). (B) Sección transversal de granos maduros de genotipos opaco-2 y MCP mostrando la extensión del endospermo vítreo.
Several studies have focused on the 27 kDa γ-zein locus to understand the mechanism of o2 modifier genes. There is more than one gene for 27 kDa γ-zein in this locus, which are assigned depending on the genotype and are called A and B, and together form the AB locus (Das et al., 1990). The AB locus of γ-zeins is not stable and can be rearranged to form A and B, called rA and rB, respectively (Das et al., 1990; Das et al., 1991). Because all known QPM lines contain the A and B genes, and all F2 progeny from a cross of a QPM and an o2 mutant contain the AB locus, it has been hypothesized that the AB locus is necessary for endosperm modification, although it is also emphasized that this locus by itself is not sufficient to achieve such modification (Lopes et al., 1995).
Although the molecular mechanism of the mo2 genes is not fully understood, evidence suggests that a greater stability of the γ-zein mRNA and/or a higher transcription rate may be responsible for a larger accumulation of the 27 kDa γ-zein protein in modified o2 genotypes. Geetha et al. (1991) suggested that the role of mo2 genes is to increase the stability of γ-zein mRNA and protein synthesis. Or et al. (1993) suggested that the stability of the A gene mRNA initiates enhanced 27 kDa γ-zein protein synthesis. Burnett and Larkins (1999) found that the A:B ratio of γ-zein mRNA in mo2 endosperms was more than 40:1, compared to a 1:1 ratio for normal maize and 3:1 for o2, indicating that these relationships can result from different transcription rates of the genes A and B. These results are consistent with a model in which the two loci associated with mo2 genes influence the expression of γ-zein genes through different mechanisms: one affects the transcription of the γ-zein locus and the other influences the stability of the γ-zein RNA. Holding et al. (2011) evaluated the expression in developing endosperm (18 days after pollination, DAP) of QPM lines contrasting in vitreousness, reporting a higher expression of the 27 kDa γ-zein gene and greater accumulation of the protein in vitreous QPM lines compared to o2 lines (Figure 1A). Wu et al. (2010) used RNAi to block the expression of 27 kDa γ-zein in the CM105mo2 maize line; and found that RNAi caused the reversal of the vitreous phenotype to o2, demonstrating that 27 kDa γ-zein plays an essential role in the modification of the endosperm in QPM. Similar findings were reported by Yuan et al. (2014) who used γ-radiation mutagenesis to identify genes related to the modification of the o2 mutant; they observed a generalized decrease in α-zeins and an increase in γ-zeins (27 and 50 kDa) in the K0326Y-QPM line compared to W64A+. The authors proposed that the 27 kDa γ-zein plays an important role in the formation of protein bodies and that the 50 kDa γ-zein, despite being in a smaller proportion, could also be involved in endosperm modification.
A genetic analysis in QPM RILs identified a locus (qγ27) on chromosome 7 that results from the duplication of the 27 kDa γ-zein gene and causes an increase in gene expression and the synthesis of 27 kDa γ-zein in QPM and wild-type lines, confirming that the improved expression of 27 kDa γ-zein is critical for endosperm modification in QPM (Liu et al., 2016). The higher expression of 27 kDa γ-zein causes that QPM endosperm accumulates a greater amount of small protein bodies, which are suggested to allow the formation of a more rigid vitreous matrix that resembles a wild type of maize endosperm (Figure 3A) (Wu et al., 2010).
Alteration in starch composition and structure
The non-zein fraction in maize endosperm includes metabolic enzymes that may also play a role in QPM endosperm modification. Gibbon et al. (2003) performed a gel-based proteomic analysis of non-zeins in maize near isogenic lines contrasting in vitreousness (CM105+, CM105o2 and CM105mo2), and found that the vitreous QPM line showed an increased accumulation of the enzyme granule-bound starch synthase I (GBSSI), which is responsible for the synthesis of amylose. The authors also found that amylopectin in the vitreous endosperms showed a higher proportion of short branches compared to that of normal and o2. These alterations in starch structure may increase the proportion of amorphous regions at the surface of starch granules, which favors their compaction and the vitreous phenotype (Figure 3A). These results suggested that starch biosynthetic enzymes may play an important role in endosperm modification.
Genetic analyses of the cross between K0326Y-QPM and W64Ao2 found a locus for vitreousness on chromosome 9 near genes involved in starch biosynthesis (Holding et al., 2008; Holding et al., 2011). The biochemical characterization of this locus, using RILs derived from the same cross, showed that starch from vitreous mature endosperms had higher levels of amylose and lower crystallinity compared to starch from opaque endosperms, which was associated with lower gelatinization enthalpy (Salazar-Salas et al., 2014). This behavior was also observed by Juárez-García et al. (2013) who reported lower enthalpy values in starches from vitreous lines compared to those from opaque lines at the mature state. These results could be explained by the higher proportion of amylose and short branches of amylopectin in starch from the vitreous lines. Short branches of amylopectin can reduce crystal formation, while a higher proportion of long chains can form more organized crystals that require higher temperature and gelatinization enthalpy (Jane et al., 1999). These studies suggest that alterations in the amylopectin structure play an important role in the modification of the endosperm.
Wu et al. (2015) reported that SSSIII may affect pullulanase activity and indirectly influence the vitreousness of the kernel by altering the distribution and length of the amylopectin glucan chains. Soluble starch synthase I (SSSI) produces short chains with degrees of polymerization (GP) of 8 - 12, while SSSII and SSSIII isoforms seem to be involved in the formation of intermediate (GP 13 - 25) and long (GP > 30) chains, respectively (Nakamura et al., 2005). A higher proportion of amylopectin intermediate chains (GP 10 - 24) and a decrease in the proportion of chains with GP of 25 - 40 was observed in starch from K0326Y-QPM and vitreous RILs with respect to starch from W64Ao2 and opaque RILs, which was associated with a higher proportion of amorphous regions in the starch granules that favors their compaction adopting polygonal shapes (Gibbon et al., 2003; Salazar-Salas et al., 2014). This provides a mechanism that complements the one associated with an increase in small protein bodies rich in γ-zein (27 kDa) that fill the spaces between the starch granules creating the vitreous phenotype (Figure 3A).
Genetic mapping of starch physicochemical properties in RIL derived from K0326Y QPM and W64Ao2 identified three loci on bins 4.05, 5.04, and 9.03 close to the starch biosynthesis genes Brittle-2 (Bt2), Amylose extender-1 (Ae1), and Waxy-1 (Wx1), respectively (Vega-Alvarez et al., 2022); the analysis of gene expression in developing endosperm (30 days after pollination, DAP) showed that the transcript levels of Wx1 were significantly higher in K0326Y QPM and vitreous RILs compared with W64Ao2 and opaque lines, which corresponded to a greater GBSSI and amylose accumulation in the vitreous lines at the same developmental stage. These results are in agreement with those reported in mature endosperm (Salazar-Salas et al., 2014) and confirms an important role for GBSSI in the modification of the QPM endosperm. Jia et al. (2013) analyzed the expression in developing endosperm (22 DAP) of W64Ao2 and its normal counterpart and found a lower expression of Wx1 in the opaque mutant. This study also revealed that the expression of genes encoding pullulanase (Zpu1) and starch branching IIb (SBEllb) enzymes was higher in W64Ao2 than W64A+. The regulation of these genes may change the proportions of amylose and the branching patterns of amylopectin in the starch granules of the o2 mutant contributing to the soft endosperm. Gonzalez-Nuñez (2022) analyzed the activity of GBSSI and SBEIIb in developing endosperms (28 DAP) of K0326Y-QPM, W64Ao2 and RIL contrasting in vitreousness; the GBSSI activity was higher in the endosperm of the vitreous lines and was associated with a higher proportion of amylose, whereas the activity of SBEIIb was higher in opaque lines that showed higher levels of amylopectin. These results support the hypothesis that endosperm modification in QPM is associated with the synthesis of starch with a higher proportion of amylose, which may facilitate the packing of the starch granules resulting in the vitreous phenotype (Figure 3A).
Modulation of carotenoid composition in amyloplast envelope
Wang et al. (2020) identified Ven1 as a major QTL influencing the vitreous phenotype in the mature maize kernel. Ven1 encodes for the enzyme β-carotene hydroxylase 3, which modulates the composition of carotenoids in the amyloplast envelope. They observed that in the opaque endosperm is a dysfunctional Ven1 allele that decreases the content of polar carotenoids and increases that of non-polar carotenoids in the amyloplast envelope, which provides greater stability to this structure that under normal circumstances disappears during kernel desiccation. The non-disruption of the amyloplast envelope results in a poor interaction between the protein bodies and the starch granules, leading to a soft endosperm (Figure 3A).
Amelioration of the stress response by increasing the energy availability
The development of the o2 endosperm involves a stress response that reduces the energy levels and affects ATP-dependent processes such as zein proteins synthesis (Li et al., 2020). This may be due to a reduction in the expression of pyruvate phosphate dikinase (PPDK1), affecting glycolysis and the energy production. Holding et al. (2008) and Holding et al. (2011) identified several differentially upregulated genes in QPM, including pyrophosphate-dependent fructose-6-phosphate 1-phosphotransferase (PFPα), a non-ATP-dependent glycolytic enzyme. It was proposed that the higher activity of PFPα in QPM compensates the reduced availability of ATP in o2 endosperm (Guo et al., 2012). A cytosolic PPDK2 was also identified by these authors as an ATP-independent glycolytic enzyme. Li et al. (2020) also found PFPα as a candidate gene for endosperm modification in QPM and identified cytosolic enolase (ENO) as another ATP-independent glycolytic enzyme. Thus, the increased synthesis of enzymes that do not require ATP for glycolysis in QPM provides energy by a mechanism that is repressed in o2 endosperm.
Conclusions
There have been important advances in the understanding of the mechanisms associated with endosperm modification in QPM. However, the application of this information for the efficient development of QPM materials is difficult due to the multiple mechanisms involved in the creation of the vitreous endosperm. So far, the increased accumulation of 27 kDa γ-zeins seems to have the major contribution to the vitreous phenotype, which is complemented with the alterations in the composition and structure of the starch granules that favor their compaction, as well as with alterations in the composition of the amyloplast envelope that result in the degradation of this structure during endosperm desiccation, allowing a better interaction between protein bodies and starch granules. These processes may not be possible without the availability of energy provided by enzymes that enhance the non-ATP-dependent glycolytic flux.











 nueva página del texto (beta)
nueva página del texto (beta)


