Introduction
In recent years, different researchers have improved their understanding of nanotechnology and focused their projects directly or indirectly on its associated topics. These studies include planning, synthesis, characterization, and evaluation of the properties obtained by modification of their size and shape. The term “nano”, the keywords in these researches, comes from the Latin nanus, meaning “dwarf”. This kind of technology is supported in physics, chemistry, materials science, biotechnology, and biosciences, among others, to achieve a multidisciplinary understanding of the phenomena that occur in the materials. A phenomenon called “nanorevolution” has been created, which aims to the manipulation and study of nanomaterials in a wide range of compositions, sizes, shapes, and morphologies (Kolahalam et al., 2019; Hamers, 2017).
Nanoparticles are an important class of nanomaterials studied by nanotechnology, as they have applications in various fields, including those described by Hernández-Morales et al. (2019) who synthesized silver nanoparticles with antimicrobial capacity using Salvia hispanica L. seed extracts. Opris et al. (2017), synthesized gold nanoparticles using extracts of Sambucus nigra L. with the ability to lower the level of glucose in blood. It also reduces levels of inflammation and oxidative stress induced by hyperglycemia. Matinise et al. (2018) created iron-zinc oxide nanoparticles aided by Moringa Oleifera extract, with excellent electrochemical performance, suggesting great potential in this area. Lebaschi et al. (2017), employed extracts of Camellia sinensis to form palladium nanoparticles showing potent catalytic application for the synthesis of biaryls, by Suzuki cross coupling reaction and also reduction of 4-nitrophenol (4-NP) by NaBH4. Samari et al. (2018), used extracts of Mangifera indica L. to synthesize silver nanoparticles capable of chemically sensing Hg2+ ions in water. Goutam et al. (2018) made use of Jatropha curcas L. for the green synthesis of titanium oxide, for the remediation of wastewater from tanneries, and Idrees et al. (2019) worked with Sida acuta extracts to develop silver nanoparticles that acted as suitable inhibitors for the corrosion of mild steel in 0.1 H2SO4 solutions. The European Union (EU) defines nanomaterials as “A natural, incidental or manufactured material containing particles, in an unbound state or as an aggregate or as an agglomerate and where, for 50% or more of the particles in the number size distribution, one or more external dimensions is in the size range of 1 nm - 100 nm” (Gehr, 2018). However, in the biotechnological area the size of the particle used varies between 10 to 500 nm and rarely exceeds 700 nm (Mody et al., 2010).
The main reasons nanomaterials showed different properties than bulk materials are due to surface effects, since atoms on the surface have fewer neighbors than bulk atoms. This is associated with less coordination and dissatisfied bonds in nanomaterials. Therefore, they are less stable. In addition, the smaller a particle, the larger fraction of atoms on the surface, and the higher the bonding energy per atom causing a high surface area and volume ratio rate, allowing them to react much faster. Also, are due to density of states, where groups of atoms tend to form bands, and the greater the number, the higher the state density; here a gap called “forbidden” is created, which is the energy that is occupied for an electron crosses a state from lower to higher energy. These vary as the density change, being able to modify the electrical, magnetic, biological properties of the materials, among others (Roduner, 2006).
The future of nanoparticles lies in synthesizing them using greenways, living beings such as plants, microorganisms, or fungi, and seeking their effectiveness in the market (Hoseinpour and Ghaemi, 2018). There are already products with nanoscale materials in their formulations, among them are Doxorubicin (Doxil), also known as Caelyx, a cancer treatment drug. The agent mixture is inside unilaminar liposomes coated with PEG (polyethylene glycol) and is called “PEGylated liposomes”. Their size varies between 80 and 90 nm (Abdellatif and Alsowinea, 2021). Additionally, Remington Nano Silver Dryer, Infinity 230 Nano Silver Tourmaline Ceramic Folding Styler by Conair, and Zazen Professional Nano-Silver Ionic Hair-dryer are hair dryers with silver nanoparticles (Taylor et al., 2017). Furthermore, Ostim is a calcium hydroxyapatite paste [Ca10(PO4)6(OH)2] that has osteoconduction skills, with crystals of 20 nm in diameter (Farjadian et al., 2019). Sea Hawk Cukote Biocide Plus Red-3541 is a paint with biocidal capacity with copper oxide nanoparticles whose average size is 220 nm (Adeleye et al., 2016). Nemozin is a burn ointment with zinc oxide nanoparticles of 200 nm (Swathi et al., 2013). There are more than 1814 products whose ingredients include nanoparticles, the most predominant being silver (Vance et al., 2015).
There are two methods for synthesizing nanoparticles: Top-Down and Bottom Up. Top-Down processes convert the bulk material into nano-sized particles by mechanical milling, laser ablation, or sputtering. For Botton Up methods, nanoparticles are formed from smaller molecules created by the union of atoms or molecules. It can be made through solid state methods (physical vapor deposition, chemical vapor deposition), liquid state methods (Sol-gel, chemical reduction, hydrothermal), gas phase methods (pyrolysis spray, laser ablation), biological methods (bacteria, fungi, plants), among others (microwaves, supercritical fluids, ultrasound) (Jamkhande et al., 2019). Unfortunately, many of these methods need a lot of energy to operate or pollute substances, making them unattractive for industrial use. Social responsibility emphasizes the search for alternatives that are economical and take care of the environment. The biological synthesis of nanomaterials has been put into practice since it uses temperature, pH, and pressures considered mild conditions. In addition, it has advantages over other synthesis methods, including higher productivity and lower costs (Kalimuthu et al., 2019; Sign et al., 2018).
Biological synthesis uses microorganisms and plants (or their extracts). There is a particular interest in using plants because they have many phytochemicals including ketones, aldehydes, flavonoids, amides, terpenoids, carboxylic acids, phenols, and ascorbic acids. These components are known to reduce metal salts and create metal nanoparticles. However, although there are several studies on nanoparticles, the exact mechanism involved in this process remains uncertain (Singh et al., 2018). Therefore, this review aims to present a current overview of the fundamentals of the synthesis of nanoparticles using the components found in plant extracts, the effect of reaction conditions, and the biological applications of these nanoparticles.
Fundamentals of Green Synthesis of Nanoparticles using Plant Extracts
Although the nanoparticle synthesis mechanisms are still under investigation, the formation of metal complexes is present in all reactions, so it is possible to consider the theory of the effective atomic number proposed by Lewis. This rule establishes that for coordination compounds, the summary of the electrons from the metal plus those donated by the ligands must be equal to the number of electrons present in the next noble gas, according to the period of the metal involved (Tolman, 1972).
During the synthesis of nanoparticles using natural extracts, the metal ions from precursors are captured and immobilized by different biological elements that function as ligands. Reduction and sintering processes are carried out to obtain the final product. The characteristics of nanoparticles depend on reaction conditions, being able to vary morphology, dispersion, performance, and size when modifying temperature, pH, and concentrations of precursors, among others (He et al., 2017; Skandalis et al., 2017; Ahmed et al., 2017). Temperature, for example, influences nanoparticle size, reaction rate, and shape of nanoparticles. In ZnO nanoparticles synthesis using Cherry fruit extracts, studied at 25 °C, 60 °C and 90 °C, researchers observed that as the temperature increased, the nanoparticle size increased. This explains that the extract is rich in ascorbic acid, which becomes unstable with increasing temperature leading to an uncontrolled reduction process and particle aggregation (Malek-Mohammadi et al., 2018). In an alternative study for silver nanoparticles synthesis using Mangifera indica leaf extract, the temperature was varied from ambient conditions to 80 °C, the intensity of SPR from ambient conditions to 40 °C was increased. This was due to the increase in reaction speed; between 60 °C and 80 °C, an increase in the amplitude of the peak was found accompanied by a decrease in the intensity of SPR, demonstrating that the ability to act as a reducing agent of the extract decreased and caused agglomerates of nanoparticles (Samari et al., 2018). The temperature was also shown to affect the synthesis of silver nanoparticles using extracts of Piper retrofractum fruit, where the temperature was increased from 25 °C to 80 °C, and is associated with larger particle sizes due to their agglomeration. In addition, high temperatures can denature compounds in the extracts, making them unavailable to react and forming longer nanoparticles (Amaliyah et al., 2022).
The pH of the synthesis process also effects nanoparticle size. In synthesizing silver nanoparticles using Thymus algeriensies extracts, pH from 3 to 11 was tested during the synthesis process. The resulting nanoparticles had a change in absorption length from 425 nm to 418 nm and an increase in signal intensity, indicating a decrease in size and that the alkaline environment favors the reducing and stabilizing capacity of the antioxidants present in the extract (Beldjilali et al., 2020). It has been seen that pH affects the nucleation stage in Piper chava extracts used to form silver nanoparticles. As the pH increases from 5 to 9, the absorption in SPR increases and is accompanied by a shift towards blue. This is because at low pH the aggregation of nanoparticles is induced, instead of nucleation to form new ones. At more alkaline pH, the anionic functional groups in the extract are favored to bind with silver ions and increase the nucleation sites, resulting in smaller sizes (Mahuiddin et al., 2020). The same effect is observed on silver and gold nanoparticles synthesized using Opuntia dillenii extract. When varying the pH from 1 to 13, it is observed that at low pH, there is a broadening in the bands from UV-Vis spectrum, indicating the formation of large nanoparticles. While at more alkaline pH, a narrowing of the band is observed at 400 nm indicating smaller nanoparticles (Ahmed et al., 2022).
Synthesis Using Natural Plant Extracts
Plants are autotrophic organisms, biochemically additional to the primary metabolites necessary for the organisms’ life. They synthesize secondary metabolites, also known as natural products, which are not essential for plant growth, but are compounds that help with adaptive processes, defense against predators or pathogens, ecological interactions, symbiosis, metal transport, and competition, among others. Secondary metabolites are classified into four categories according to the “British Nutrition Foundation”: Terpenoids, Phenols, Nitrogen Compounds, and Sulfide Compounds (Mera et al., 2019; E. Ahmed et al., 2017). A representative structure of each category is shown in Figure 1. Secondary or phytochemical metabolites can act as reducing and stabilizing agents responsible for the synthesis of nanoparticles (Ovais et al., 2018).
In general, nanoparticle synthesis involves mixing plant extracts with metal precursor salts. They can interact under different reaction conditions (pH, temperature, concentrations, etc.). Here, the formation of nanoparticles takes place in stages. It begins with the set of species equilibrium; once dissolved, the extracts and the precursor salt coordination complexes are formed between the metal ion and the phytochemicals of the extract. The sites where the nanoparticles will grow are created at the second nucleation stage. At the third stage of growth and adsorption, small adjacent nanoparticles come together to form particles of a larger size. Finally, during termination, the final shape of the nanoparticles occurs. The general synthesis mechanism is shown in Figure 2. The following operations consist of purification by centrifugation and washing until the impurities present are removed (Naikoo et al., 2021; Behzad et al., 2021; Bouttier-Figueroa et al., 2019a). As expected, results in the synthesis of nanoparticles show that the extract and its concentration affect the size and morphology of the nanoparticles, while temperature and pH affect the agglomeration process (Erjaee et al., 2017; Koshy, 2017).
A list of plants used for the synthesis of nanoparticles between 2017 and 2022 is presented in Table 1. It is observable that the main phytochemicals involved in the synthesis of nanoparticles are phenolic compounds, flavonoids, carboxylic acids, carbohydrates, terpenoids, alkaloids, and proteins. They contain functional groups capable of coordinating with, and reduce, metal ions. Extracts of Azadirachta indica, Hibiscus rosa-sinensis, Murraya koenigii, Moringa oleifera, Tamarindus indica, and Spinacea oleracea contain phenolic compounds and flavonoids that act as covering agents, preventing agglomeration and stabilizing the formation of copper oxide nanoparticles (Rehana et al., 2017; Al-Jawjari et al., 2022). Extracts of Aloe vera, Hibiscus sabdariffa, Atalantia monophylla, Punica granatum, Ocimum americanum, Beta vulgaris, Cinnamomum tamala, Cinnamomum verum, Brassica oleracea var. Itálica, Acalypha fruticosa L., Alchornea laxiflora, and Syzygium Cumini contain phenolic compounds, flavonoids, tannins, organic acids, and terpenoids that can trigger the reduction of zinc salts and control the size of zinc oxide nanoparticles (Mahendiran et al., 2017; Vijayakumar et al., 2017; Mohamad et al., 2019; Narendra et al., 2019; Mohanan et al., 2020; Vijayakumar et al., 2019; Ekennia et al., 2020; Rafique et al., 2022). Origanum vulgare L. contains phenolic and carbohydrate compounds that play a vital role in the reduction of Pd2+ ions and act as stabilizing agents for palladium nanoparticles (Rafi et al., 2017). Moringa oleifera leaves contain amino acids, alkaloids, flavonoids, and phenolic compounds that facilitate the synthesis of iron nanoparticles (Katata-Seru et al., 2018). The extracts of Annona muricata, Coleus aromaticus, Tasmannia lanceolata, Backhousia citriodora and Tecoma capensis have flavonoids, terpenoids and proteins responsible for the reduction of gold ions. In addition, they help in the formation and stabilization of synthesized nanoparticles (Folorunso et al., 2019; Boomi et al., 2018; Khandanlou et al., 2020; Hosny et al., 2022). Tecoma stans L. contains hydroxyl groups, alkenes, alkynes, flavonoids, carbohydrates, amines, and phosphates that act in the synthesis and formation of magnesium oxide nanoparticles (Nguyen et al., 2021). Atriplex halimus contains glycosides, terpenoids, flavonoids, and alkaloids involved in reducing and stabilizing palladium nanoparticles (Eltaweil et al., 2022). The synthesis of silver nanoparticles has been the favorite of several research groups. Several plant extracts have been used among them: Buddleja globosa hope, Lantana camara L., Viscous cleome L., Sida cordifolia, Psidium guajava L., Amaranthus cruentus, Achillea millefolium L., Phyllanthus urinaria, Pouzolzia zeylanica, Scoparia dulcis, Piper colubrinum, Phoenix dactylifera L., and Camellia sinensis. These have in common the presence of carboxylic, hydroxyl, carbonyl, and phenolic groups that are responsible for the reduction of Ag+ ions to Ag0 and act as a coating on the synthesized nanoparticles (Carmona et al., 2017; Shriniwas et al., 2017; Lakhmanan et al., 2018; Naga et al., 2017; Wang et al., 2018; Baghani et al., 2020; Yousaf et al., 2020; Nguyen et al., 2020; Santhoshkumar et al., 2021; Laouini et al., 2021).
Table 1 Characteristics of nanoparticles synthesized with natural extracts from different plants species.
Tabla 1. Características de las nanopartículas sintetizadas con extractos naturales de diferentes especies de plantas.
| Plant Species | Origin of the Extract | Nanoparticle | Nanoparticle Size (nm) | Functional Group Involved on Synthesis | Effect | Reference |
| Azadirachta indica, Hibiscus rosa-sinensis, Murraya koenigii, Moringa oleifera and Tamarindus indica | Leaves | CuO | Average size of 12 | Phenolic compounds and flavanoids | Anticancer | (Rehana et al., 2017) |
| Aloe vera and Hibiscus sabdariffa | Leaves | ZnO | 9 to 18 | Phenolic compounds and flavonoids | Antibacterial, antioxidant, and anticancer | (Mahendiran et al., 2017) |
| Buddleja globosa hope | Leaves | Ag | 1 to 53 | Phenols and carboxylic groups | - | (Carmona et al., 2017) |
| Lantana camara L. | Leaves | Ag | 410 to 450 | Carboxylic, hydroxyl, carbonyl, and phenyl groups | Antioxidant, antibacterial, and cytotoxic on Brine shrimp | (Shriniwas et al., 2017a) |
| Origanum vulgare L. | Leaves | Pd | 2 to 20 | Phenolic compounds and glycoside | Catalytic activity | (Rafi et al., 2017) |
| Atalantia monophylla | Leaves | ZnO | 30 | Tannin, organic acids, terpenoids, aromatic dicarboxylic acid, and amides | Antibacterial | (Vijayakumar et al., 2018) |
| Moringa oleifera | Seeds | Fe | 2.6 to 7.4 | Amino acids, alkaloids, flavonoids, and phenolic compounds | Antibacterial and coagulant | (Katata et al., 2018) |
| Cleome viscosa L. | Fruit | Ag | 5 to 30 | Alkaloids, phenolic compounds, amino acids, carbohydrates, and tannins | Antibacterial and anticancer | (Lakshmanan et al., 2018) |
| Sida cordifolia | Whole plant | Ag | 3 to 8 | Alkaloids, carbohydrates, proteins, glycosides, flavonoids, and tannins | Antibacterial | (Naga et al., 2018) |
| Psidium guajava L. | Leaves | Ag | Average size of 25 | Flavonoids and polyphenolic groups | Antibacterial | (Wang et al., 2018) |
| Annona muricata | Leaves | Au | 27 to 32 | Flavonoids, terpenoids, and proteins | Antibacterial and antifungal | (Folorunso et al., 2019) |
| Punica granatum | Peel | ZnO | 32.98 to 81.84 | Pinucalagin, ellagic acid, caffeic acid, chlorogenic acid, and gallic acid | Antibacterial and anticancer | (Mohamad et al., 2019) |
| Coleus aromaticus | Leaves | Au | Average size of 80 | Phenolic hydroxyl, aromatic amines, and polyphenol groups | Antibacterial and anticancer | (Boomi et al., 2019) |
| Ocimum americanum | Whole plant | ZnO | Average size of 21 | Phenol, hydroxyl, and carboxyl groups | Antibacterial and anticancer | (Narendra et al., 2019) |
| Amaranthus cruentus | Flowering shoot parts | Ag | Average size of 15 | Aromatic compound, proteins, hydroxyl groups, and carboxylic acids | Antibacterial and anticancer | (Baghani et al., 2020) |
| Tasmannia lanceolata and Backhousia citriodora | Leaves | Au | Average size of 7.10 | Terpenoids, flavonoids, and phenolic compounds | Anticancer | (Khandanlou et al., 2020) |
| Achillea millefolium L. | Whole plant | Ag | 14.27 to 20.77 | Proteins, flavonoids, and phenols such as tannic acid | Antibacterial and antioxidant | (Yousaf et al., 2020) |
| Beta vulgaris, Cinnamomum tamala, Cinnamomum verum, and Brassica oleracea var. italica | Whole plant | ZnO | 20 to 47 | Hydroxil groups, proteins, aldehydes, alkanes, alkenes, ketones, and alcohol | Antibacterial and antifungal | (Mohanan et al., 2020) |
| Phyllanthus urinaria, Pouzolzia zeylanica, and Scoparia dulcis | Leaves | Ag | 4 to 52 | Tannins, flavonoids, phenolics, and terpenoids compounds | Antifungal | (Nguyen et al., 2020) |
| Acalypha fruticosa L. | Leaves | ZnO | Average size of 50 | Flavonoids | Antibacterial | (Vijayakumar et al., 2019) |
| Piper colubrinum | Leaves | Ag | 10 to 50 | Compunds with with OH and CO groups | Antibacterial | (Santhoshkumar et al., 2021) |
| Tecoma stans L. | flower, bark, and leaves | MgO | 20 to 50 | Hydroxyl/amino groups, alkene or alkyne, flavonoids or carboxylic compounds, saturated primary alcohol, carbohydrates, amines, and phosphates | Treatment of hazardous dyes from the wastewater | (Nguyen et al., 2021) |
| Alchornea laxiflora | Leaves | ZnO | Average size of 200 | Polyphenols, flavonoids, tanins, alkaloids, and saponins | Photocatalyst | (Ekennia et al., 2021) |
| Citrus limon | Fruit | Cu | 5 to 28 | Alcohols, phenols, carboxylic acids, and amino groups | Antibacterial | (Amer et al., 2021) |
| Phoenix dactylifera L. | Leaves | Ag/Ag2O | 37.71 to 28.66 | Carboxyl, carbonyls, amides, and phenols | Photocatalytic activity for azo dye degradation | (Laouini et al., 2021) |
| Syzygium Cumini | Leaves | ZnO | 64 to 78 | Hydroxyl and amide groups | Fertilizer in agriculture and catalyst for dye removal from polluted water | (Rafique et al., 2022) |
| Tecoma capensis | Leaves | Au | 20 to 25 | Alkaloids, flavonoids, glycosides, terpenoids, tannins, and phenolic compounds | Photocatalytic, anticancer and antioxidant | (Hosny et al., 2022) |
| Spinacea oleracea | Leaves | CuO | 13 to 17 | Phenols and flavonoids | Antioxidant and anticancer | (Al-Jawhari et al., 2022) |
| Atriplex halimus | Leaves | Pt | 1 to 3 | Glycosides, terpenoids, flavonoids, and alkaloids | Antibacterial, antioxidant, and catalytic | (Eltaweil et al., 2022) |
| Camellia sinensis | Leaves | Ag | 15 to 33 | Polyphenols, proteins, flavonoids, saponins, and glycosides | Antibacterial | (Widatalla et al. 2022) |
Secondary metabolites for nanoparticle synthesis
The extracts used for nanoparticle synthesis have a wide variety of secondary metabolites involved in nanoparticle reduction, formation, and stabilization. Phytochemicals in the extracts include phenols, flavonoids, alkaloids, terpenoids, proteins, carbohydrates, and amino acids.
Phenols
Phenols are chemical compounds that contain a hydroxyl group (-OH) attached to an aromatic hydrocarbon. In plants, the term polyphenol is usually used for secondary metabolites from the biochemical pathways of shikimic acid. They may have more than one phenolic ring and do not contain functional groups based on nitrogen in their structure (Cirkovic et al., 2018). Below are reaction mechanisms for the synthesis of nanoparticles involving phenols. Gallic acid has been shown to interact with Au+3 ions by releasing electrons and reduce them to gold atoms. It has been proposed to form an intermediate complex during reduction that oxidizes to form quinones and gold nanoparticles. Quinones are inferred to remain on the surface of the nanoparticles, preventing their aggregation for prolonged periods (Ahmad et al., 2019). The proposed mechanism is shown in Figure 3a. In the case of silver nanoparticles, gallic acid causes the reduction of Ag ions. After reduction, it is oxidized to its quinone form and the carboxylic group (-C = O) present in the oxidized form coordinates with the surface of the nanoparticles to stabilize them (Bhutto et al., 2018). The schematic representation is shown in Figure 3b. Combining these molecules with metallic copper salts gives a polyphenolic complex with the Cu2+ ion, whose reduction generates nanoparticles of Cu0. The ions can subsequently be oxidized with oxygen to form stable CuO nanoparticles (Veisi et al., 2021). The mechanism of biogenesis is shown in Figure 3c. The general mechanism for the synthesis of nanoparticles using phenols is described by Malapermal et al. (2017), who speculate that the conversion of biomolecules with C-OH groups to C=O groups is responsible for the reduction of ions, in the case of their study Ag+ to Ag0 , leaving as a result a keto compound. The proposed mechanism is illustrated in Figure 3d.
Figure 3 Proposed mechanisms for nanoparticle synthesis from phenols compounds. Adapted from references [Cirkovic et al., 2018; Ahmad et al., 2019; Bhutto et al., 2018; Veisi et al., 2021; Malapermal et al., 2017].
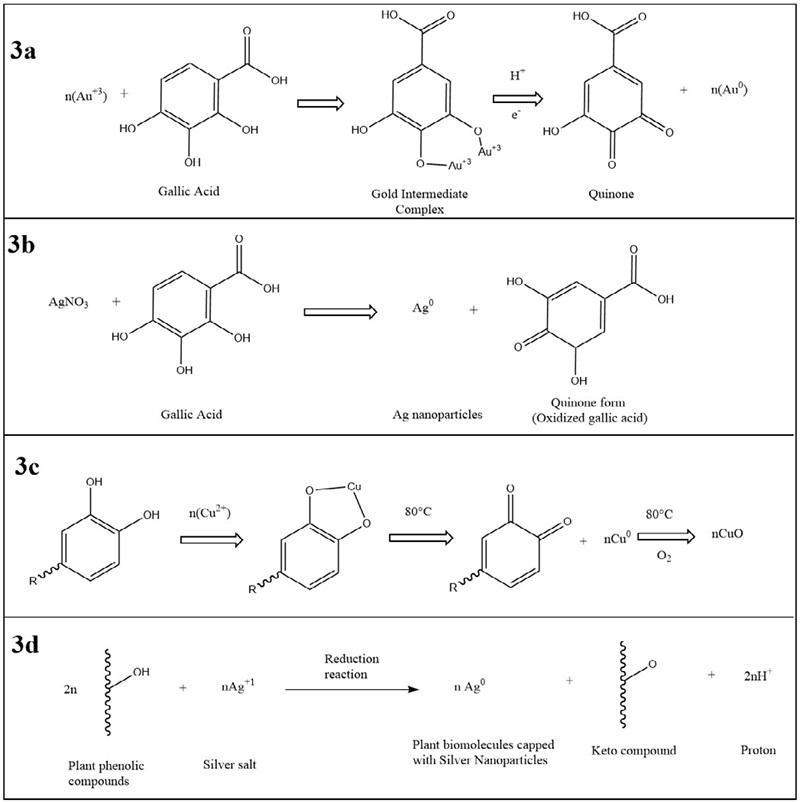
Figura 3. Mecanismos propuestos para la síntesis de nanopartículas a partir de compuestos fenólicos. Adaptado de referencias [Cirkovic et al., 2018; Ahmad et al., 2019; Bhutto et al., 2018; Veisi et al., 2021; Malapermal et al., 2017].
Flavonoids
They are hydroxylated phenolic substances synthesized by plants in response to microbial infections. The chemical structure is a propane diphenyl skeleton with fifteen carbon atoms in its primary nucleus: two six-membered rings linked with a three-carbon unit, which may or may not be a part of a third ring. In addition, two benzene rings (rings A and B) are linked through a third heterocyclic oxygen-containing pyrene ring (Karak, 2019). Flavonoids have hydroxyl and carbonyl groups that can bind metal ions through a chelating effect. This chelating ability is associated with a nucleophilic character of the aromatic ring. The antioxidant property of flavonoids is related to their ability to donate electrons and hydrogen atoms. Trivalent gold ions can form complexes in aqueous solutions with flavonoids when they oxidize to ketone groups and form the nucleus of nanoparticles (Irfan et al., 2017). The proposed mechanism is shown in Figure 4a.
Figure 4 Proposed mechanisms for nanoparticle synthesis from flavonoids compounds. Adapted from references [Irfan et al., 2017; Mayedwa et al., 2018; Kobylinska et al., 2020; Sherin et al., 2020].
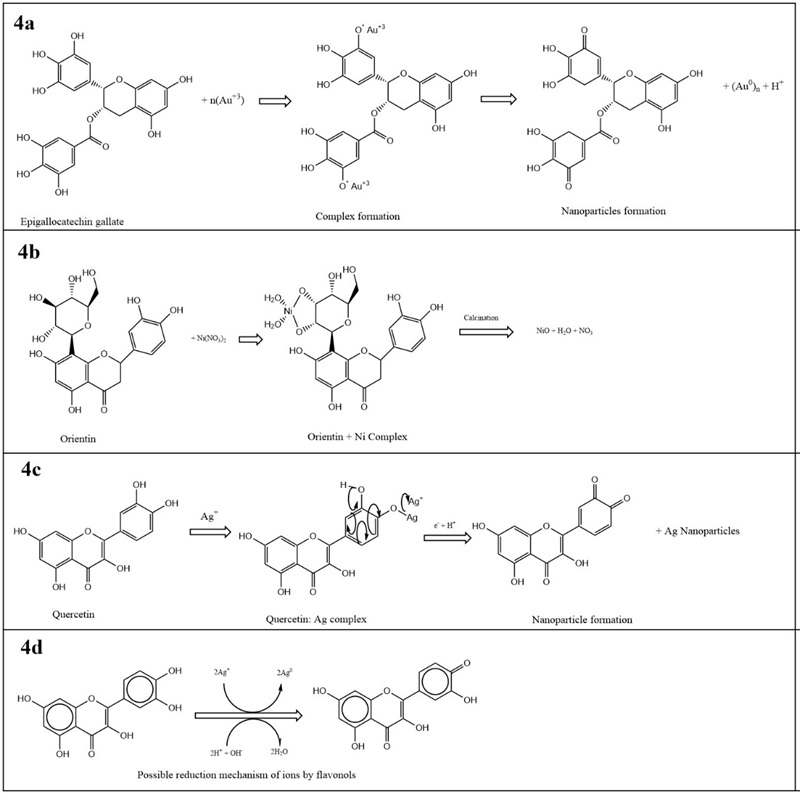
Figura 4. Mecanismos propuestos para la síntesis de nanopartículas a partir de compuestos flavonoides. Adaptado de referencias [Irfan et al., 2017; Mayedwa et al., 2018; Kobylinska et al., 2020; Sherin et al., 2020].
The flavonoid orientin contains an aromatic hydroxyl group capable of adhering to nickel ions by forming complexes with each other. Subsequently, an annealing process is applied to break down the complex and form NiO nanoparticles (Mayedwa et al., 2018). The proposed mechanism of orientin with nickel ions is shown in Figure 4b. Quercetin contains hydroxyl groups with high reductive activity. It has been used to reduce Ag+ ions by forming complex intermediates, followed by oxidation by hydrogen abstraction forming a hydrate (Kobylinska et al., 2020). The schematic is shown in Figure 4c. A general proposal for the formation of nanoparticles with flavonoids, taking silver as an example, is given by Sherin et al. (2020)). They indicated that hydroxyl groups are strong ligands that can reduce ions from Ag+ to Ag0. They suggested that if sodium hydroxide is added to the reaction medium, the reducing power of these phytomolecules is improved, and the formation can be accelerated at room temperature. The possible proposed mechanism is illustrated in Figure 4d.
Alkaloids
These are molecules present in plants with a nitrogen at any position of the molecule but must not belong to an amide or a peptide bond (Rios et al., 1989). They have demonstrated participation in the formation of platinum and palladium nanoparticles; alkaloids of Peganum harmala seeds showed rearrangement and deprotonation in their O-H and C-O groups, indicating the participation in the bioreduction of nanoparticles (Fahmy et al., 2021). In another study, this same plant was used to reduce silver nanoparticles. In this case, five main alkaloids, Harmine, Harmaline, Harmalol, Vasicine, and Vasicinone were identified, indicating the participation of the same groups in the reduction of nanoparticles (Almadiy et al., 2018). Conocarpus lancifolius presents two alkaloids, Scopolamin and Hyoscine, that can act as metal chelators and participate in silver nanoparticle formation (Raheema et al., 2020). Terminalia catappa has alkaloids involved in Nd2O3 nanoparticles formation, acting as essential sources for obtaining Nd(OH)3 complexes in hydrolysis processes necessary for the formation of nanoparticles (Lembang et al., 2018). Although it is known that alkaloids participate in the formation of nanoparticles, there are still few publications on the subject, and there is a lack of deepening in the reaction mechanism of the formation process. Hence, further research in this area is necessary.
Terpenoids
They are metabolites of plants with different atomic carbon units, where C = 5, 10, 15, 20, …, n > 40, and are thus classified as hemiterpenes (C5), monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), triterpenes (C30), tetraterpenes or carotenoids (C40), and polyterpenes (Cn, n > 40) (Proshkina et al., 2020). Extracts of only terpenes from Lantana chamber plant have been used to synthesize silver nanoparticles; these participate in reducing Ag+ ions to Ag0 ions (Shriniwas et al., 2017b). Withania coagulans has poly-oxygenated biomolecules called withanolides (C28 - steroidal lactone triterpenoids), which are involved in synthesizing silver nanoparticles (Tripathi et al., 2019). The Terpenoids present in Annona squamosa help in the synthesis of gold nanoparticles; their hydroxyl and carbonyl groups are complexed with Au3 ions and reduced to Au0 by the oxidation of carbonyl and carboxyl groups (Gangapuram et al., 2018). Pentacyclic terpenoids (lupeol and β-sitosterol) of Euclea natalensis participate in forming zirconium oxide nanoparticles, and the -OH groups are responsible for the reduction. The tautomeric transformation of enol compounds to keto is thought to release hydrogen atoms, accountable for removing the zirconium molecule ( Da Silva et al., 2019). The proposed mechanism is shown in Figure 5a. In this aspect, research has begun to offer reaction mechanisms, but it is still necessary to understand the process in detail.
Figure 5 Proposed mechanism for nanoparticle synthesis from terpenoid compounds. Adapted from Gangapuram et al. (2018).
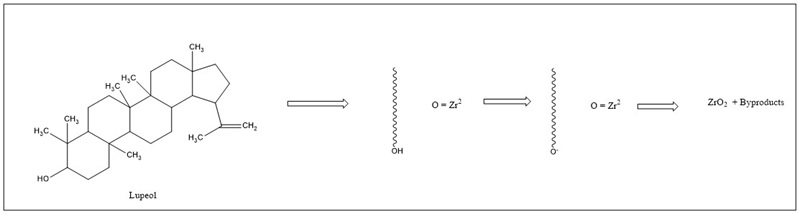
Figura 5. Mecanismo propuesto para la síntesis de nanopartículas a partir de compuestos terpenoides. Adaptado de Gangapuram et al. (2018).
Amino acids and proteins
Proteins are biomolecules constructed from the covalent polymerization of amino acids (Mora et al., 2020). Camellia sinensis extracts have been used for the synthesis of zinc oxide nanoparticles. These contain amino acids that are not directly involved in the formation of nanoparticles but stabilize the nanoparticles in suspension (Dhanemozhi et al., 2017). Ajwa and Barni were used for the synthesis of platinum nanoparticles. As a result, it was found that amino acids and proteins participate in coating the nanoparticles (Al-Radadi, 2019). Carissa carandas proteins coat and stabilize nanoparticles, as demonstrated by being employed to synthesize silver nanoparticles. Hydroxyl, carbonyl, and other functional groups in amino acids and peptides (C=O, -OCH, C-O) can bind metals, forming a coating around the metal nanoparticle. Also, the presence of the -OH group in benzenes contained in the extract participate in the formation of nanoparticles since they can release a proton, change to its anionic form, stabilize itself by its resonance structure, provide electrons that function as reducing agents, and oxidize the metallic salt (Joshi et al., 2018). Moringa oleifera extract proteins also function as coating agents of nanoparticles, in this case confirmed by Transmission Electron Microscopy (TEM) (Mateus et al., 2018). The participation of these biomolecules is not directly related to the process of nanoparticle formation but to their stabilization.
Carbohydrates
These molecules composed of carbon and hydrogen atoms, have different monomeric units and degrees of polymerization; glycosidic bonds join them and in nature, they are found together with proteins, lipids, amino acids, etc. The binding of various carbohydrates is called a polysaccharide (Mena-García et al., 2019). Carbohydrates present in Astragalus membranaceus possess weak reducing activity, and have been used for the synthesis of silver nanoparticles due to the electron-accepting nature of Ag+ ions and the electron-donating nature of hydroxyl groups in polysaccharides (Ma et al., 2017). Acacia gum and pectin have been used for the synthesis of palladium nanoparticles. They have reduced sugars in their structure, which coordinates metal ions in their carbonyl groups, allowing the synthesis of metal nanoparticles (Emam et al., 2020). A representative schematic is shown in Figure 6. Castanea mollissima has glucans with a large number of OH groups that have been used to synthesize selenium nanoparticles (Li et al., 2019). Galactomannans in Prosopis spp can function as nanoreactors to synthesize zinc oxide nanoparticles by coordinating the OH groups present in these polysaccharides (Bouttier-Figueroa et al., 2019b).
Figure 6 Proposed mechanism for nanoparticle synthesis from a polysaccharide compound. Adapted from Emam et al. (2020).
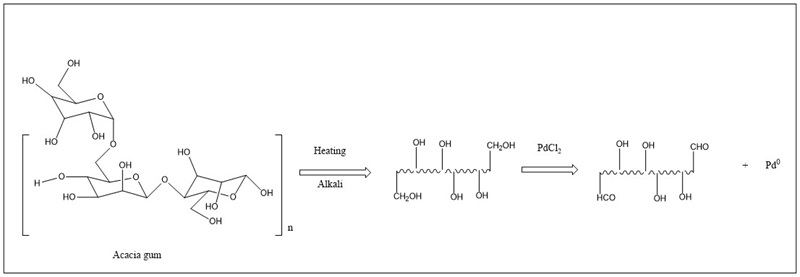
Figura 6. Mecanismo propuesto para la síntesis de nanopartículas a partir de polisacáridos. Adaptado de Emam et al. (2020).
Nanoparticles applications
Recently, nanotechnology has emerged as a science with a wide range of applications. Nanoparticles formed using nanotechnology have qualities that have allowed them to be used in different fields, such as food, agriculture, medicine, catalysis, and water treatment.
Food
Silver nanoparticles synthesized from Madhuca latifolia L. extracts can inhibit bacterial growth and be used in food packaging. Nanoparticles act as molecules that can penetrate the bacterial cell wall and affect their metabolic pathways. They can destabilize ribosomal function and DNA, causing cell death (Biswal and Misra, 2020).
Agriculture
Aqueous extract of Ulva lactuca allows the synthesizing of silver nanoparticles capable of inhibiting the proliferation of bacteria and fungi of agricultural importance by functioning as pesticides. Nanoparticles come into contact with bacteria entering their cell wall and interrupt cell permeability by interacting with enzymatic cofactors, sulfur, and phosphorous groups of DNA. In the case of fungi, the nanoparticles form pits on the cell surface, which causes changes in morphology and prevents proliferation (Amin, 2020). The extract of Cornus mas fruits has been used to synthesize Fe2O3 nanoparticles to improve the growth of barley seedlings serving as fertilizers. Nanoparticles work as an iron source; when absorbed by plants they change the concentrations of reactive oxygen species, causing a stimulus in the plant’s growth (Rostamizadeh et al., 2020).
Water treatment
Ficus benjamina leaves have been used to obtain extracts to form silver nanoparticles that can remove Cd(II) from contaminated water, functioning as ionic or elemental removers. Their results indicated that nanoparticles behave according to a Freundlich model, where Cd(II) adsorption occurs due to multiple layers of heterogeneity of the adsorbent surface (Al-Qahtani, 2017). Fumariae herba has been used to synthesize platinum nanoparticles, which can perform catalysis activities to remove organic dyes. This ability is attributed to their relatively large surface-to-volume ratios. Platinum nanoparticles showed enhanced catalytic activity for the degradation of organic dyes (Dobrucka, 2019).
Medicine
The leaves of Coptis chinensis allow the synthesis of silver nanoparticles with anticancer activity against lung cancer cells. Nanoparticles interact with cells, affecting the metabolic pathways of mitochondria causing apoptosis and destruction of the mitochondrial membrane. Nanoparticles alter the production of Bcl2 family proteins, altering cell susceptibility to promote the production of caspase 3 and Bax proteins that cause apoptosis (Pei et al., 2019). Andrographis paniculata, Phyllanthus niruri, and Tinospora cordifolia have in their extracts agents capable of synthesizing silver nanoparticles with antiviral activity against the chikungunya virus. Nanoparticles interfere with the virus binding to the host cell, preventing it from entering, but the exact mechanism of action still needs to be studied (Sharma et al., 2019). Zinc oxide synthesized with Urtica dioica extract has been employed in rats to lower insulin levels, functioning as an antidiabetic agent. Zinc present in nanoparticles acts by mimicking the activity of insulin, the fundament being that the pancreas of a healthy person contains a greater amount of zinc than a person with diabetes (Bayrami et al., 2020).
Paeonia emodi has been used to synthesize gold nanoparticles essential for cardiovascular diseases treatment. Nanoparticles are loaded with drugs since they can enter tissues and release them directly at the site of action. They decreased the levels of alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase, and creatine phosphokinase, which cause heart damage (Ibrar et al., 2018). Zinc oxide nanoparticles have been synthesized with Aloe socotrin leaf extracts, allowing the distribution of antimicrobial agents. The nanoparticles eliminated microorganisms that cause urinary infections by destroying their membrane due to the formation of reactive oxygen species (Fahimmunisha et al., 2020). Extracts of Rose indica L petals can synthesize zinc oxide nanoparticles, which can be used in nail paint with antifungal activity. Understanding of ZnO mechanism of action in fungi needs to be better developed. It is known that H2O2 is formed, and the creation of bonds between cellulose molecules in their hydroxyl groups and oxygen atoms leads to the inhibition of fungal growth (Tiwari et al., 2017).
Electronic
Titanium oxide, synthesized from lemon peels, has optical and photocatalytic properties that allow its potential use in electronic devices. It is capable of degrading rhodamine B due to its high photocatalytic activity created by the rapid detachment capacity between the photogenerated electrons and holes (Nabi et al., 2022).
Nanoparticles Packaging
Recent research avoids mentioning the materials used to store the synthesized nanoparticles. It is essential to consider an appropriate type of material to store the nanoparticles, preserve their properties, and allow transport in safe conditions. The U.S. Food and Drug Administration (FDA) has a section that regulates materials used to store food, drugs, and cosmetics. It can be extrapolated to nanoparticle storage. Permitted materials include (Marsh et al., 2007):
Glass: It does not have aroma, is chemically inert, impermeable to gases, allows good insulation from the outside, can be produced in different ways, and being transparent, allows observation of the stored product.
Metal: Focuses on aluminum and steel. These provide excellent barriers to moisture, air, odors, light, and microorganisms; they are ductile, easily recyclable, and highly accepted by the consumers.
Plastics: They are chemically resistant and cheap to manufacture. In industrial applications, they can be heat-sealed, printed, and stored on-site. The main disadvantage is that they are permeable to light and gases.
Paper and cardboard: This material mainly stores and transports product-filled containers.
Nanoparticles characterization
After synthesizing nanoparticles, it is important to know their morphological and physicochemical properties since, depending on their size, shape, surface morphology, structure, and homogeneity, their properties can vary. The most used characterization techniques are: Uv-vis, XRD, FTIR, DLS, EDAX, SEM and TEM (Gour et al., 2019).
UV-visible: It is used to know the wavelength at which nanoparticles absorb, which is related to their Surface plasmon reverberation, providing information about their size.
XRD: It is used to know the crystalline structure of nanoparticles.
FT-IR: It is used to study the functional groups present on nanoparticles surface, which are responsible for the reduction and stabilization.
DLS: Provides information about the surface charge of nanoparticles.
EDAX: Estimates the abundance of elements present over nanoparticles surface.
SEM and TEM: They provide information about nanoparticle morphology, size, and homogeneity.
Other techniques less used, include condensation particle counter, photon correlation spectroscopy, and field emission scattering electron microscopy. Authors such as Mourdikoudis et al. (2018) have already described more details about the characterization techniques.
Conclusions
This review presented information on nanoparticle synthesis using different plant extracts with a particular interest in the reaction mechanisms involved in the formation. The synthesis using natural plant extracts has allowed the elaboration of materials greenly (friendly to the environment, cost reduction, easy obtaining of raw material, and use of non-toxic solvents). The biomolecules present in the extracts are directly related to the reduction of metal ions, participation in the capture of ions, formation of complexes, and finally, the protection of the synthesized material. The development of nanotechnology has allowed applications in different areas such as electronics, medicine, chemistry, physics, engineering, environment, etc. These materials are synthesized invitro and participate in many secondary metabolites in their formation, including alkaloids, carbohydrates, phenols, and Terpenoids. Recent research focuses on specific metabolites for their role in nanoparticle formation. Further studies on the role of selected metabolites in the formation of nanoparticles are needed. The current overview of nanoparticle storage needs to be described in the literature, and there is a research opportunity to study the effects on packaging. This review addresses the characterization techniques, emphasizing the role of each of them in order to introduce their fundamentals. In this way, it will be possible to understand their physical and chemical properties better and subsequently standardize production processes at the industrial level.











 nueva página del texto (beta)
nueva página del texto (beta)




