1. Introduction
Magnetic fields can induce electric fields and electric currents on biological structures causing physiological changes (Tenforde, 1995). There are reports of in vivo experimentation that uses magnetic field stimulation with benefits, such as cell regeneration in wounds (Ottani et al., 1988) or bone formation (Kotani et al., 2002). Contrarily, some adverse results have been informed after applying magnetic fields e. g., the development of some types of cancer, specifically leukemia (Savitz & Loomis, 1995; Kleckne et al., 1990).
In vitro experiments have been carried out to study the behavior of matter outside the tissue under different conditions of magnetic radiation. It has been reported an increment in neuronal differentiation, which can contribute to novel treatments for neurodegenerative diseases by applying a magnetic field of 10G and 60 Hz (Jung et al., 2014). An increment in cell survival due to apoptosis inhibition (Fanelli et al., 1999) has been noted with the application of a static MF in the range of 6 to 60G. In other cases, experimenting with MF of 10G @ 60Hz, a significant change in DNA fragmentation in primary cultures of human fibroblasts has occurred (Focke et al., 2010).
Epithelial tissues are used as a study model to observe structural and physiological disorders resulting from magnetic field exposure in vitro (Loberg et al., 1999). Alterations in the polarization of the epithelium and excessive cellular reproduction of the tissue could act as indicators of some effects due to magnetic exposure because of the direct relationship between magnetism and ions dynamics in cells and tight junctions of the epithelia. A tool that is gaining relevance to observe the integrity of the epithelium, viability, and healthy cell growth is the measurement of transepithelial electrical impedance (TEEI). This tool, which is also called impedance spectroscopy, gives the cellular monolayer behavior information through its electrical parameters (Benson et al., 2013).
The usual experimental protocols to study the effect of magnetic fields in vitro cell cultures are based on statistics. They assume that all cells should respond in the same way to treatments. The proposal of this work considers the approach that cells could suffer physiological alterations during the growth and maintenance processes. In this sense, a system has been developed to carry out these processes and expose cell cultures to magnetic fields in a controlled environment. Therefore, the possible effects of exposure to magnetic fields could be mainly attributed to the exposure.
The proposed electrical impedance measurement system must meet the requirements to operate in situ with a magnetic exposure system for cell cultures in a controlled environment of temperature, humidity, CO2, and light exposure. The measurement system is based on the impedance spectroscopy technique and was custom designed for in vitro and in situ experimentation (Dominguez, Martínez et al., 2017; Dominguez, Arias, et al., 2017).
Currently, there is only one commercial instrument called CellZscope® (nanoAnalytics, Germany), which provides the Transepithelial Electrical Impedance (TEEI) information. From this parameter, the device estimates the electrical model components of a cellular monolayer (TEER and Ccl Cell Capacitance). However, this instrument was not designed to allow in situ experimentation with epithelia inside a controlled environment, as needed for this project.
2. Materials and methods
The proposal presented in this project is based on carrying out maintenance, experimentation, and impedance measurement actions while preserving environmental conditions since the cell culture seeding. In this way, the proposal seeks to reduce the effects of these actions external to the experiment. The results of the measurements would only be due to the exposures to the magnetic field. To reinforce the reliability of the hypothesis results, the experimentation was complemented with the control-experiment pair technique.
The designed system for transepithelial electrical impedance measurement employs the electrical impedance values obtained by the impedance spectroscopy technique to approximate the electrical parameters of the simple equivalent electrical model of a cellular monolayer, Figure 1. The parallel of the ZTEER will give the resulting electrical impedance value Z (Eq. 1) (due to the transepithelial electrical resistance) and ZCcl (due to the cellular capacitance) in series with the electrical impedance external to the cell monolayer corresponding to the resistance of the medium (Rmed) and the capacitance of the electrode-electrolyte interface (CEI).
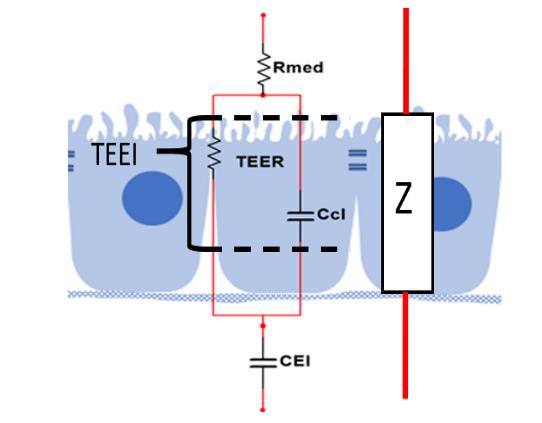
Figure 1 Simple equivalent electrical model of a cellular monolayer. Rmed: Resistance of the medium, TEER: Resistance of the tight junctions, CCl: cellular capacitance, CEl: capacitance of electrode-electrolyte interface. Z = total electrical impedance (Benson et al., 2013).
Table 1 shows the individual electrical impedances of each of the components of the electric model.
Table 1 Parameters for calculating the equivalent circuit of the transepithelial electrical impedance.
| Parameter | Component | Electrical Impedance Z | Units |
| Medium resistance |
|
|
|
| Transepithelial electrical resistance |
|
|
|
| Cell membrane capacitance |
|
|
|
| Constant phase element (CPE) |
|
|
|
The electrical impedance due to CEI (ZCel) is associated with the constant phase element (CPE), which is represented by a non-ideal capacitor that defines the characteristics of the electrical impedance of the electrode-electrolyte interface in the electric model (Macdonald & Johnson, 2005).
The module of electrical impedance
By measuring
2.1. Subjects of study
Madin-Darby Canine Kidney (MDCK) cell cultures were used as subjects of study in the experimental protocol of the present work. MDCK cells allow generating a model of the epithelium, which facilitates the analysis of cell growth regulation and the epithelium transport mechanisms (Cereijido et al., 1978; Cho et al., 1986; Irvine et al., 1999; Simmons, 1982). The cultures were seeded in inserts or porous support filters of 6.5 mm diameter (Corning 3496 Transwell®, USA), which permitted the supply of nutrients from the basolateral compartment. This configuration also allowed the flow of electrical current from the apical area of the membrane to the basolateral.
An experimental protocol based on a controlled environment in temperature, humidity, percentage of CO2, and light exposure was developed to take care of the viability and healthy cell growth. These conditions favored the physiological cell behavior in the medium and minimum exposure to electromagnetic radiation in the form of light, avoiding the influence of external parameters to the experiment (Walker, 2002).
2.2. Instrumentation
2.2.1. Four-well chamber
In order to guarantee a significant number of samples established in the experimental protocol for the measurement of electrical impedance of the monolayers sown in the inserts, it was decided to design a multi-well measuring chamber based on the arrangement proposed by Wegener et al. (2004).
The chamber consisted of four independent wells that house inserts with permeable filters to attach the cell cultures, a stainless-steel plate on the base as a reference electrode, and four recording electrodes on the upper part of the wells immersed in the culture medium on the apical side of the monolayer. Polytetrafluoroethylene (PTFE), also known as Teflon, was chosen for constructing the four-well chamber block because it is a non-polluting and electrical insulator. Besides, the wells were sealed with silicone O-Rings, as shown in Figure 3.
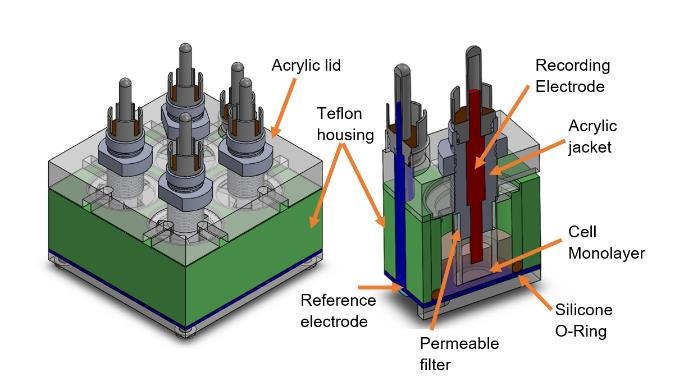
Figure 3 Four-well chamber designed to measure the electrical impedance of four cell monolayers simultaneously.
The recording electrodes are stainless steel cylinders and fixed employing an acrylic jacket threaded in a lid of the same material. This arrangement allowed setting the position of the electrodes in the xy plane and adjusting their height inside the culture medium at 4.4 mm. In this way, the contact area between the electrode and the culture medium was 39.5 mm2, guarantying a medium volume of 1.65 ml in all cases. It should
be noted that the adjustment of the position of the electrode immersed in the culture medium reduces the effects of CPE on the electrode-medium interface and ensures repeatability in the measurements.
2.2.2. Electrical impedance measurement system
The system denominated MIMP2.1 and shown in Figure 4, was custom developed to measure the electrical impedance. It includes the acquisition card NI-USB 6208B (National Instruments, USA), a waveform generator, an Opamp array for signal conditioning, and other Opamp array for electrical current buffering for applying to the electrode-monolayer interface, which should not exceed 300 μA.
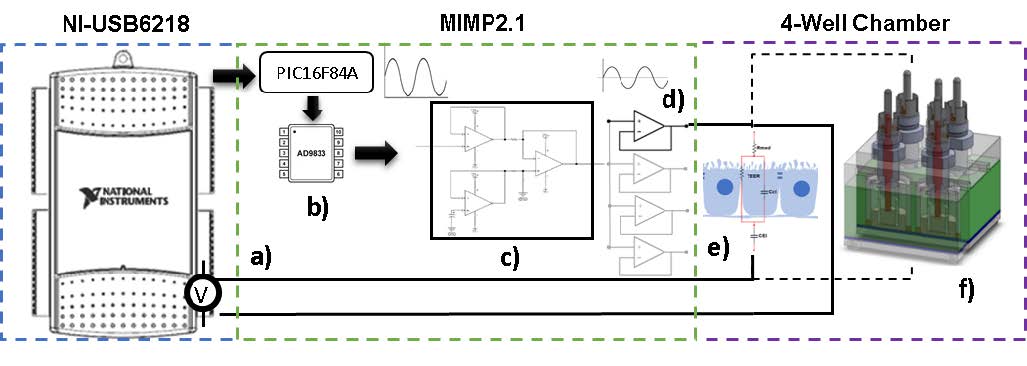
Figure 4 Diagram of the developed system for the measurement of electrical impedance in a cellular monolayer. a) Measurement of the voltage in the electrode-medium-monolayer cell, b) waveform generator, c) Opamp array for signal conditioning, d) four-buffer opamp,e) electrode-medium-monolayer cell, f) four-well chamber.
The low-power wave generator is based on the AD9833 device (Analog Devices USA) and controlled by the PIC16F84a microcontroller device (Microchip Technology Inc. USA) using the I2C communication port.
For the voltage measurements in the four wells, a data acquisition card (NI-USB6218) interfaced with the MIMP2.1 was used. Knowing the voltage and the applied current, the electrical impedance was determined by Eq. 3.
A graphical interface was developed in LabVIEW® with the NI-USB6218 card to acquire and store the electrical impedance data in * .TXT files. The front panel of this graphical user interface was designed to define the frequency range of the electrical impedance spectroscopy and the number of wells to be evaluated, indicate the correct immersion and connection of the electrodes with the culture medium, present the results of the measurements graphically and indicate the progress of the measurements process using a bar. Figure 5 shows the front panel of the graphical user interface.
2.2.3. Graphical user interface
A graphical interface was developed in LabVIEW® with the NI-USB6218 card to acquire and store the electrical impedance data in * .TXT files. The front panel of this graphical user interface was designed to define the frequency range of the electrical impedance spectroscopy and the number of wells to be evaluated, indicate the correct immersion and connection of the electrodes with the culture medium, present the results of the measurements graphically and indicate the progress of the measurements process using a bar. Figure 5 shows the front panel of the graphical user interface.
As reported in (Arndt et al., 2004; Lo et al., 1995; Sun et al., 2010; Wegener, 2010), the magnitude of the electrical impedance is presented at each of the frequencies. In this project, the frequency-dependent impedance values are presented graphically in the center area of the front panel for easy reading. In the dialogue area on the left, the parameters to be considered for the measurement are entered. Also in this area there is a button to indicate with green lights the correct contact between the electrode and the culture medium in the four wells.
2.2.4. MATLAB algorithm
A MATLAB® Script was developed using Nonlinear Least Square Method (NLSM) based on the Trust-Region Algorithm to estimate TEER, Capacitance Ccl, and Rmed values. This script uses the equation related to the electrical model of the electrical impedance (2) as well as the values of the electrical impedance previously stored, which were obtained by spectroscopy.
3. Test and results
3.1. MATLAB algorithm
As a phase in developing the electrical impedance measurement system, a test was performed to evaluate the NLSM approximation algorithm by comparing the results to those provided by the the commercial system Cellzscope® (nanoAnalytics, Germany).
Ten records of the electrical impedance were made for each cell culture of the
MDCK type in confluence, using the commercial system Cellzscope, changing the
frequency in the range of 1Hz-100kHz. The system delivered the values of TEER,
Capacitance Ccl of the monolayer, the resistance of the culture medium, and
Finally, both approximations were compared employing the Wilcoxon matched-pairs signed Rank test since normality was not assumed. The results are shown in Table 2.
3.2. Impedance measurements evaluation
The evaluation of the impedance measurements consisted of comparing ten values of three MDCK-type cell cultures with those of the commercial Cellzscope® instrument. The Mann-Whitney test was applied to determine the differences. The results are shown in Table 3.
3.3. Preliminary tests
Two preliminary system tests for evaluation were carried out, measuring the electrical impedance of MDCK monolayers in situ and in vitro. The first to monitor the growth or maturation of the cell monolayer and the second to observe the possible effects of the application of extremely low-frequency magnetic fields, variable in frequency and amplitude, on confluent cell monolayers, by analyzing the electrical impedance values.
The study of the maturation of the cell monolayers was followed by the TEER values extracted from the electrical impedance spectroscopy. The results of the experiment are shown in Figure 6.
The graph shows an increment in TEER as growth occurred in the cell monolayer, which is the typical behavior reported in the literature. It could be observed that the TEER fell drastically and recovered in about ten hours when the medium was renewed, indicating that the change of medium significantly affected the electrical impedance of the cell monolayer.
Related to the preliminary tests, when the cellular monolayers were exposed to sinusoidal magnetic radiation of 10 and 50G @ 60Hz, TEEI measurements were practiced before and after the magnetic radiation to observe possible effects on TEER and Ccl capacitance. A paired t-Student analysis was performed so that differences before and after the magnetic exposure to 10 G @ 60 Hz could be observed. The results yielded p values for both TEER and Ccl, pTEER = 0.7046 and pCcl=0.4856 for the control cells, and pTEER = 0.7410 and pCcl=0.9096 for those exposed; these values do represent a statistically non-significant difference.
In the same way, the results obtained for the values of TEER and Ccl before and after the magnetic exposure to 50 G @ 60 Hz yielded values of pTEER = 0.3943 in TEER and pCcl = 0.2213 in Ccl in the control cells, and of pTEER = 0.6089 in TEER and pCcl =0.0121 in Ccl in treated cells. The p-values show that no significant differences were observed either, and therefore there were no effects from 50 G exposure.
4. Discussion
Cell electrical impedance values can be sensitive and eventually affected by different external factors beyond those considered in the experimental protocol. Therefore, having carried out the exposure in a controlled environment of cell cultures to magnetic fields in situ and in vitro, where factors external to the experiment were eliminated, reliable results and thus a simplified experimental procedure were obtained. An evaluation test was applied to the developed system by comparing its electrical impedance measures with a widely used commercial system, which operates with the experimental-control pair scheme, implying in some cases a large sample size since it is based on statistics. This development contributes to reducing the experimental procedure and rethinking the idea that cells are the same and respond in the same way.
5. Conclusions
The multiwell chamber, designed to house the inserts with permeable filters, was a significant element of the system due to its easy assembly, small size, and versatility of dimensional adjustments for measurements. On the other hand, the graphical interface developed that interacts with MATLAB generated reliable, scalable, and friendly software.
The developed electrical impedance measurement system for experimentation with cell cultures in a controlled environment was designed to work in situ under physiological conditions, avoiding the possible influence of external variables on the experiment. This proposal altogether with the standard procedure of the control-experimental pair, plus the corresponding statistical analysis, allowed to affirm that the effects produced by radiation to magnetic fields in cell cultures were due only to the exposure and not to those produced by the experimental management.











 nueva página del texto (beta)
nueva página del texto (beta)





