1. Introduction
Limes in general have wide industrial and domestic uses which are not limited to: dentistry, polym pulp and paper, cement, textiles, fuel-cell layers, composites and solar energy materials, mortar and plaster, glass and ceramics, metallurgy, acidity regulator, sewage treatment, CO2 scavenger, catalyst, and food industry. For instance, while precipitated calcium carbonate (PCC), shows diverse range of functionality in paper industry in both paper coating and as paper filler, quicklime and slaked lime are used in pulp and PCC manufacturing. In sugar refining, lime causes coagulation of plant material, allowing it to be more easily separated from the sugar syrup (Devenney et al., 2018; Hwidi et al., 2018; Joya et al., 2016). Dried spent carbide is one of the main solid wastes that constitutes municipal solid waste (MSW) generated in many of metropolitan states in Nigeria. Chukwudebelu et al. (2013) mentioned that spent carbides in Nigeria is generated on daily basis an average of 30 kg per panel beater after the hydrolysis of carbides to generate acetylene gas according to Equation (1). This is a useful component for welding main panels and sub panels of minor- and major accidental vehicles (Joya et al., 2016; Tajudeen et al., 2019; Wanyou et al., 2012). However, the indiscriminate dumping of this waste may affect soil fertility and pH of hydrosphere and its inherent organisms’ survival. Spent carbide is whitish or greyish color, pH ˂ 12, with traces of contaminants such as coke, Cu, Pb, Fe, Mn, Ni and Zn ions (Chukwudebelu et al., 2013; Man, 2018; Tajudeen et al., 2019; Wanyou et al., 2012). Man (2018) reported that the spent carbide contained 80% impure slaked lime, Fe2O3, SiO2, Al2O3 metal oxide, hydroxide, a small amount organic matter and a small amount of CaCO3 that are formed due to sorption affinity of slaked lime on the surface of spent carbide owing to long-time exposure to atmospheric CO2.
Since environmental sustainability depends on sustainable soil and water ecosystems for various utilities, then, economical means must be employed to curtail the environmental pollution caused by this solid waste. Through lime cycle, it is possible to revamp and purify limes from spent carbide by processes of drying/calcination/hydration (Erdogan & Eken, 2017; Man, 2018; Tajudeen et al., 2019; Wanyou et al., 2012). Devenney et al. (2018) have developed a process for purifying highly impure calcium hydroxide in carbide sludge to high-value precipitated calcium carbonate and other calcium products. The method (solubilization) involved heavy dilution of the slurry at 0oC, and reaction of the solution with carbon dioxide to produce calcium carbonate, the precipitated product is separated from any impurities that may be dissolved in the calcium hydroxide solution. Erdogan and Eken (2017) have also produced precipitated calcium carbonate from waste marble powder by calcination-dissolution-precipitation method under various conditions of temperatures and times.
Tajudeen et al. (2019) have made use of carbide waste to produce laboratory chemicals: calcium chloride, calcium tetra-oxo sulphate (VI) and calcium tri-oxo nitrate (V) salts whose purity almost matched laboratory commercial grades. Chukwudebelu et al. (2013) have employed solubilization means to recover pure slaked lime with 88% purity from carbide sludge. Wanyou et al. (2012) have produced high purity calcium carbonate superfine powder through calcium carbide residue recycle via drying/calcination, leaching, filtration and carbonization. Habte et al. (2019) have used sol-gel method to produce nano-calcium oxide from waste eggshell found that the particle size ranges from 50 nm-198 nm compared to other methods such as calcination. Jitjamnong et al. (2019) synthesized calcium oxide for 2 h at 900℃ from eggshells with barium impregnation as catalyst. Mohadi et al. (2016) observed that the optimum temperature for preparation of quicklime, CaO from chicken eggshells is 900℃. But Prayitno et al. (2020) compared calcined calcium oxide prepared from duck eggshells calcined at 1000℃ to nano- calcium oxide via precipitation method observed the latter has uniform crystal size 262 nm.
In a similar study conducted by Tangboriboon et al. (2012), the author observed that 99.06% purity calcium oxide was best achieved at calcination temperature 900℃. The authors emphasized that the higher calcination temperature and longer calcination time, the more complete phase transformation this statement is in tandem with Erdogan and Eken (2017). However, Gallala et al. (2008) and Udeh (2021) have reported on influencing process variables such as chemical composition of the raw material, the calcination temperature and decrepitation processes to manufacture quicklime and its slaking reactivity in water. Findings showed that the presence of impurities, the tendency to decrepitate, lime/water ratio, time, and over burning of the lime during calcination decrease quicklime reactivity. This study, therefore, involved reclamation of pure limes (quicklime and slaked lime) from spent carbide by means of calcination /hydration cycle. In addition, to find the effects of time and lime to water ratio during slaking of calcined lime.
2. Materials and methods
2.1. Materials
Muffle furnace, mechanical sieve shaker, pressure pot, spent carbide, drying oven, polythene sheet, analytical balance, beakers, stirrer, plastic containers etc.
2.2. Methods
2.2.1. Sample collection and preparations
Man (2018) conveyed that raw spent carbide contained 85% to 90% water when discharged after carbide hydrolysis reaction. Therefore, the raw spent carbide collected from Panel beater auto workshop around the vicinity of Yaba College of Technology in large quantity, spread on polythene sheet and dried by natural air for 1 or 2 weeks. Drying of the sludge facilitated the removal of volatile impurities present within the body of the sludge to some extent (Wanyou et al., 2012). Dried samples was blended (with Bina tone blender), sieved using mechanical sieve shaker present at the Civil Engineering Department, School of Engineering, Yaba College of Technology, Lagos, Nigeria.
2.2.2. Calcination
About 400 g and 600 g of the sieved spent carbide of 75-micron size were calcinated at 950℃ and 1000℃ respectively in muffle furnace for a duration of 8 h. The two chosen temperatures according to Erdogan and Eken (2017) mentioned that temperature is one of the critical factors for quicklime reactivity. However, Hwidi et al., (2018) highlighted that the higher the calcination temperature the purer the quicklime, thus, temperature range between 1000℃ and 1100℃ give better purity though the decomposition temperature of slaked lime is 560℃. Besides, the 1000℃ temperature was selected to ensure that the sample completely decomposed to quicklime according to the reaction equations (2a and 2b). This is because an impure slaked lime often admixed with calcite (CaCO3) due to long time exposure of sample to air. The calcined products was stored in plastic container to preserve its chemical entity since it shows strong affinity for moisturized air from the surroundings.
2.2.3. Slaking or hydration of lime
About 20 g of the calcined spent carbide was transferred quantitatively into 500 ml beaker. 200 ml distilled water was heated to boiling temperature in pressure pot, added to the content in the beaker, stirred at 900 rpm for 5 min. and later transferred into pressure pot. The mixture was heated on hot plate, allowed to simmer at pressure pot inner temperature (greater than 100℃) and inner pressure (˃ 1 atm) for 30 min. At this time, the amount of water had been used up and the residue left in the pot almost dried up with little or no moisture. The same procedure was repeated using 500 ml of water for 60 and 90 min., respectively. The excess supernatant in these last two runs was gently decanted, and the residue oven dried at set temperature of 120℃ to drive off the water molecules by evaporation for 120 min. till the constant mass of the dried samples were obtained. The whitish solid products were separately stored and labelled as sample A30, A60 and A90, respectively.
2.3. Characterization
The raw spent carbide and the prepared powders were subjected to chemical analysis using the following instruments- atomic absorption spectrometry (Agilent 200 series AA), Fourier- transform infrared spectroscopy (Agilent Cary 630 FTIR) and X-ray diffraction (ARL’XTRA X-ray XRD 197492086).
3. Results and discussion
3.1. Atomic absorption spectroscopy (AAS) analysis
The result of AAS analysis of the dried spent carbide showed that the sample contained Na, Fe, K, and Zn in high concentration (mg/kg) while Pb, Mn and Ni concentrations ranged from 23.90 - 0.99 mg/kg. Copper (Cu) was not detected (Table 1).
3.2. X- ray diffraction (XRD) analysis
The X-ray diffraction spectra (Figure 1) of the prepared samples identified as A30, A60, and A90 were investigated alongside the reference Sample Y for easy comparison of the spectra data. Figure 1 (a, b, and c) showed that the spectrum of the individual sample (a-c) possess close resemblance to sample marked ‘d’. The XRD major peaks were similar to the characteristic peaks of portlandite phase with space group P-3 m 1. It showed sharp peaks around 2θ to be 18.07o, 28.6o, 34.1o, 36.6o, 47.1o, 50.9o, 54.3o, 56.2o, 59.4o, 62.6o, 64.2o, 64.3o, and 71.8o with corresponding Miller’s index (h k l) runs from 001 to 202 plane of hexagonal portlandite Ca(OH)2 phase, respectively. However, the dominant peak (011) plane occurs at 2θ equals 34.1o. This value corresponds to the main peak of Ca(OH)2 gel observed by Habte et al. (2019). The peak values at 2θ above also matched the major peaks of portlandites commercial slaked lime used in the adsorption of lead from battery waste water by Vu et al. (2019) and 2θ at 17.98o, 34.02o and 47.04o with major peak at 34.02o (Liang et al., 2018). The observed 2-theta values also compared very well with JCPDS XRD data as reported by Mohadi et al. (2016).
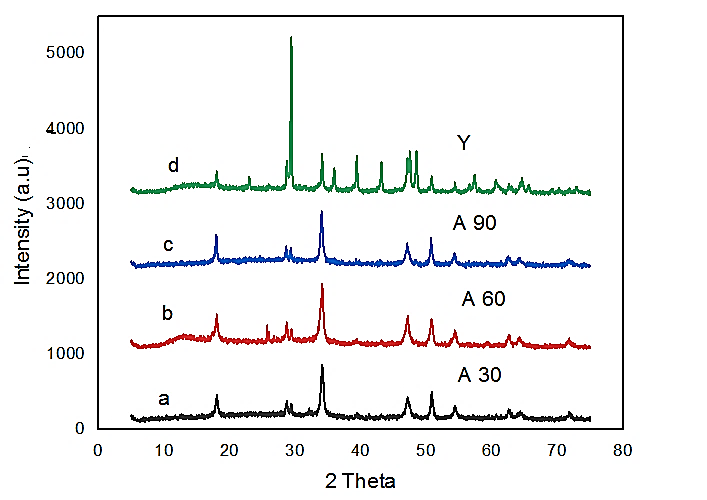
Figure 1 X-ray patterns of hydrated limes (a) 30 min., (b) 60 min., (c) 90 min., and (d) reference sample.
The XRD spectrum observed around 2θ at 26o for sample A60 but not observed in samples A30 and A90 may be traced to abstraction of CO2 by the sample due to short exposure to air to form calcite. Based on the crystal planes obtained, it is obvious that the prepared quicklime CaO was successfully produced and converted to slaked lime. The crystallite size of the powders was determined using Scherrer’s law given by.
Where,
d |
is the mean crystal size |
λ |
is wavelength of X-rays used (0.15406 nm) |
k |
is Scherrer’s constant (0.9) |
θ |
is Bragg’s angle or peak position (radians) and, |
β |
is structural broadening or full width at half maximum (FWHM) |
The crystallite size for reference sample Y, sample A90, and sample A60 were found to be 41.92 nm, 56.55 nm, and 56.56 nm respectively, except sample A30 with crystallite size of 42.42 nm. The reduction in size may be due to the amount of water to sample used and time took for slaking (this may require further investigations). However, Samanta et al. (2016) reported 44.40 nm crystallite size of nano calcium hydroxide synthesized by aqueous medium. This suggests that the grain size of calcium hydroxide depends on method of preparation and factor such as ratio of lime/water and slaking time.
3.3. Fourier transform infrared spectroscopy (FTIR) analysis
The test was performed to check for the functional groups in the samples. According to Figure 2, the FTIR spectra bands of samples A30 and A60 which represent the hydrated lime prepared at lime to water ratio 1:10 and 1:24 for 30 and 60 min., respectively showed sharp peak at wavenumber 3637 cm-1 that matched the reference sample Y, Ca(OH)2. This wavenumber is similar to the values 3640 cm-1, 3642 cm-1, 3639.02 cm-1 and 3637 -3644 cm-1 as reported by Habte al. (2019), Liang et al. (2018) Kashyap et al. (2021) and Zakaria et al. (2018) respectively, and these values have been assigned to stretching vibration of O-H in the sample Ca(OH)2. The peak for sample A90 prepared at same ratio as A60 showed a very weak absorption O-H, this might be due to much longer time used in slaking the lime (Udeh, 2021).
The absorption bands around 1408.93 cm-1, 1110.74 cm-1, 1453.66 cm-1, 1125.65 cm-1, 1438.75 cm-1, 1103.29 cm-1 and a common band at 872.19 cm-1, all ascribed to different elongation modes of C―O of the carbonate groups
The FTIR of CaO produced via calcination of spent carbide at two different temperatures Figure 3b almost matched closely with that of reference sample X (Figure 3a). However, the absorption peak at wavenumber 3637.38 cm-1 in reference sample X is sharper than the observed peak 3645.33 cm-1 in both samples B950 and B1000. This absorption band in all samples may be due to instability nature of quicklime because of its strong affinity to absorb moisture when exposed to air even during its preparation for characterization, hence; this can cause the water molecules to be adsorbed on the surface of the calcined CaO. Prayitno et al. (2020) reported similar bands at 3619.96 cm-1 and 3678.91 cm-1 for calcined CaO sample and standard CaO and have associated the bands with OH groups of water molecules on the surface of the particle. Habte et al. (2019) and Jitjamnong et al. (2019) identified O―H absorption peak for (nano particle) CaO synthesized as well as commercial CaO at 3639.02 cm-1.
Absorption peaks at 1401.47 cm-1, 1006.38 cm-1 and 998.92 cm-1 of samples B1000 and B950 corresponded to bands of pristine sample X Figure 3a, which occurred between 1423.84 cm-1, and 1096 cm-1 are assigned to C―O bond because of carbonation of calcium oxide particles. The bands 863 cm-1 - 872.19 cm-1 alluded to the out of plane vibration modes of carbonate (
4. Conclusions
Pure limes can be obtained from spent carbides generated as wastes by artisan panel beater through the processes of drying/calcination/hydration. These processes are less tasking than solubilization process. The structural properties of the produced limes, especially hydrated quicklime upon characterization by FTIR and XRD devices revealed the possibility of revamping limes from impure state in spent carbide via lime cycle calcination/hydration. In this study, it was established that the crystallite size of the slaked lime might be influenced by the amount of water to quicklime ratio and time. The XRD data using Scherer’s equation revealed sample A30 crystallite size 42.42 nm to be close to the reference sample Y (commercial slaked lime) size of 41.92 nm. The crystallite size of sample A90, and sample A60 prepared with the same amount of water to sample ratio were found to be 56.55 nm and 56.56 nm, respectively. The XRD major peaks of both pristine and the produced slaked lime at separate times showed dominant peak (011) plane at 34.1o (2θ). FTIR spectroscopy of the pristine sample Y and prepared samples (A30 and A60) showed sharp peak better than sample A90 at wavenumber 3637 cm-1 to the presence of O―H stretching in the prepared hydrated lime. This implies that the effect, which is assigned to time in hydration of quicklime, is a critical factor. The observed FTIR peak bands of the synthesized CaO at temperatures of 950℃ (B950) and 1000℃ (B1000) were found between the range of 1401.47 cm-1and 998.92 cm-1. Absorption peak of the synthesized quicklime at mentioned temperatures was 3645.33 cm-1 indicating the presence of water molecules on the surface of the particulate powder. These prepared limes would be useful in the catalysis cracking of waste plastics to hydrocarbons in an ongoing research study.











 nueva página del texto (beta)
nueva página del texto (beta)




