Introduction
In tropical regions, rural communities have established actions to conserve and manage their natural resources, creating ecological community reserves that function as reservoirs of local biodiversity. They include strategies and practices that have been carried out in many regions worldwide since ancient times as part of a vision of rustic use-conservation (Bezaury-Creel & Gutiérrez-Carbonell, 2009; Boege, 2008; Halffter, 2005; Toledo & Barrera-Bassols, 2008). For instance, rural communities can plan the management of their lands, plan their future use, maintain their economic potential, and at the same time promote the conservation of natural resources. Moreover, some activities can be developed in ecological community reserves, such as forest exploitation, UMA (i.e., Units for Conservation, Management, and Sustainable Use of Wildlife), nurseries, ecotourism, coffee plantations, aquaculture, agriculture, and livestock, if the activities are focused on sustainability and trying to minimize the environmental impacts that could be generated. However, converting tropical forest into different land uses generates environmental variations that affect spatial and temporal biodiversity patterns and species distributions (Foley et al., 2005; Tilman, 2017) in different ways (Foley et al., 2005; Gibson et al., 2011; Reiners et al., 1994; Tilman, 2017).
In general, conversion and degradation of forests reduce the probability of population persistence by modifying the capacity of habitats to maintain their biodiversity (Barlow et al., 2007; Fahrig, 1997, 2003; Gibson et al., 2011; Nichols et al., 2007; Regolin et al., 2020). Although there is evidence of the negative impacts of different land uses on biodiversity (McGill, 2015; Newbold et al., 2015), it is necessary to consider the study of temporal changes in species richness and diversity in human-modified systems as seasonal fluctuations in resource availability, which are related to human activities, may also alter species-habitat relationships (Yabuhara et al., 2019; Yeiser et al., 2021). In fact, understanding temporal dynamics is important because it is highly related to species phenological, physiological, and behavioral changes (Leong et al., 2016). Therefore, knowing the magnitude of spatio-temporal variation of species diversity could improve our understanding of the dynamics of species assemblages and their role in maintaining biodiversity.
In ecological community reserves, spatio-temporal patterns of biodiversity could be at risk due to land use changes and other local activities. The use of bioindicator species can help us evaluate the importance and quality of rural nature reserves for the conservation of native flora and fauna (Adrián, 2021; de la Vega et al., 2014; León-Espinosa & Mujica, 2018; Vidaurre et al., 2008). In this sense, dung beetles (Coleoptera: Scarabaeidae) are excellent focal organisms for studying the effects of anthropogenic disturbances on diversity and ecosystem function in tropical regions (Escobar et al., 2008; Favila & Halffter, 1997; Slade et al., 2011). These organisms use decomposing organic matter for food and reproduction and provide various ecosystem services through their removal and burial of matter (e.g., incorporating nutrients into soil, bioturbation, indirect control of flies and livestock parasites, and secondary seed dispersal) (Alvarado et al., 2019; Nichols et al., 2008). Moreover, dung beetles are highly sensitive to environmental disturbances and are used as indicators of natural or anthropogenic environmental disturbances in tropical forests (Filgueiras et al., 2011, 2015; Favila & Halffter, 1997). Studies have shown that vegetation structure, elevation, atmospheric temperature, relative humidity, soil type, and habitat quality can influence the species occurrence and abundance of dung beetles (Joaqui et al., 2021; Pinto-Leite et al., 2018). Moreover, we have evidence that fragmentation and habitat loss are important processes that shape dung beetle diversity patterns and body condition (Escobar et al., 2008; Nichols et al., 2007; Salomão et al., 2018). Such studies have found a general pattern in which there is a high loss of species as habitat disturbance increases (e.g., Damborsky et al., 2015; De Juan & Hewit, 2014; Escobar et al., 2008; Filgueiras et al., 2015; Frank et al., 2017; Gómez-Cifuentes et al., 2017; Howden & Howden, 2001; Keyton et al., 2016; Quintero & Halffter, 2009). In addition, studies evaluating the temporal changes in the richness and diversity of species of dung beetles in tropical environments have found that there is high variation in species composition over time (i.e., species turnover) (Novais et al., 2016). However, this temporal variation tends to be context-dependent, since some studies considered annual changes (Noriega et al., 2021), while others considered the changes between seasons (Costa-Batista et al., 2016; Vernes et al., 2005) or carried out long-term evaluations (Escobar et al., 2008).
Specifically for Mexico, studies dealing with dung beetles in the state of Chiapas have increased considerably in the last 15 years (Rodríguez-López et al., 2019). Most of the studies analyzed the composition and structure of the assemblages in federally protected natural areas and their zones of influence, mainly from the Lacandon rainforest region (Navarrete & Halffter, 2008a; Sánchez-de Jesús et al., 2016; Santos-Heredia et al., 2018), and the Zoque rainforest region (Arellano et al., 2008, 2013; Blas-López & Gómez, 2009; Gómez et al., 2017; Sánchez-Hernández et al., 2018), while others provided new geographic distribution data or descriptions of new species (Chamé-Vázquez & Gómez, 2005; Gómez & Chamé-Vázquez, 2003; Halffter & Halffter, 2009; Morales et al., 2004; Navarrete & Halffter, 2008b; Sánchez-Hernández & Gómez, 2018; Sánchez-Hernández et al., 2017). Nevertheless, there are still extensive areas of the Chiapas territory that are little explored, among which are most of the state-protected natural areas (Rodríguez-López et al., 2019) and community ecological reserves stand out. For these reasons, evaluating spatio-temporal patterns can be decisive for the development of more efficient management and conservation strategies (Correa et al., 2021).
In this study, we analyzed the spatial and temporal changes in the diversity of dung beetle species in 4 different environments (primary forest, secondary forest, riparian forest, and pasture for livestock use) in the Bajlum Pakal Community Ecological Reserve, Chiapas, southeastern Mexico. We postulated that changes in the original vegetation (i.e., humid tropical forest) to other land uses (i.e., secondary vegetation, remnants of disturbed riparian vegetation, and grasslands) would negatively influence the richness and species composition. Specifically, we expected that there would be higher species diversity in more conserved environments (i.e., primary forest) compared to environments with more habitat disturbance (i.e., secondary forest, riparian forest, and pasture). This should occur because primary forest presents a higher habitat quality and resource availability, allowing the coexistence of different trophic guilds characterized by species with high habitat specialization, while in mostly disturbed sites, there is a dominance of generalist and opportunistic beetle species in terms of habitat and resource use (Filgueiras et al., 2015, 2016; Kenyon et al., 2016). Moreover, we expected that changes in species composition would be related to monthly variation in local activities and climatic and environmental conditions throughout a sampling year, with a higher species number in the warmer and rainy months (March-October). This is because dung beetles in the region tend to be more active from the first rains until the end of summer when there is a greater availability of high nutritional quality resources compared to other colder seasons (Estrada et al., 1993; Flota-Bañuelos et al., 2012; Kaur et al., 2021; Morón-Ríos & Morón, 2016).
Materials and methods
This study was carried out in the Bajlum Pakal Community Ecological Reserve (BPCER), which is located 6 km south of the town of Nueva Betania, Municipality of Palenque, Chiapas, Mexico (17°16’48.09” N, 91°39’11.96” W), with an elevational range between 156 and 260 m asl. The predominant climate is hot and humid with rain in the summer accompanied by an annual average temperature of 26 °C and a rainfall of 2,156 mm per year. May is the warmest month (29.7 °C) and January is the coldest (22.4 °C) (García, 1988). The dominant vegetation in the BPCER was high evergreen forest; however, given deforestation and the change in land use over time, there is a mosaic of different plant associations (primary forest, secondary forest, remnants of riparian vegetation, lands under cultivation, and pastures for livestock). Evergreen forest (in different conservation states) occupies approximately 50% of the area surrounding the reserve, and the rest is occupied by cattle ranches, corn, and rural areas dedicated to ecotourism.
Currently in BPCER, evergreen forest and riparian forest have mainly been modified to open roads and rustic stops for ecotourism tours, generally preserving the tropical forest, but changing the understory and the herbs. Moreover, about 33% of the reserve has historically been used for livestock and agriculture, but some of those spaces have been abandoned and now are secondary forest.
The Nueva Betania community was founded in 1969 and is mainly inhabited by indigenous Choles people who have created an alternative tourism center based in Community Based Tourism (Palomino et al., 2007). The REBP maintains the connectivity of the tropical rainforest due to its strategic location. It is a part of one of the most important tourist corridors in Chiapas, connecting with Bonampak, Yaxchilán, and the Lacandon Jungle. The reserve was decreed to conserve the high evergreen forest in the communal lands that have not been used due to their topographic conditions and difficult access, so that the remnants of the original forest are regenerated and for the natural reintroduction of species (CDI, 2010).
Beetle samplings were carried out every month from March 2015 to February 2016. Within the reserve, 4 types of environments and 3 sampling points were used: primary forest (represents the native forest cover), secondary forest (forest in an advanced regeneration stage enriched with fruit trees), remnants of riparian vegetation (cover constituted by arboreal vegetation located on the margins of water courses), and pasture (areas destined for cattle ranching). At each sampling point we established 2 transects with 6 baited fall traps (Halffter & Arellano, 2002), each 500 m apart. We placed the traps 50 m away from each other (Larsen & Forsyth, 2005), and they were alternately baited with 60 g of human excreta, bovine manure, and decomposing fish to record a greater number of copronecrophagous beetles. The use of multiple attractants allowed us to determine the largest number of species and trophic guilds associated with the use of the resource (Cajaiba et al., 2017). We checked the traps after 48 h of exposure and the organisms within them were collected. This methodology was repeated every month for a year. Organisms were identified at the species level, and the material was deposited in the Red de Ecoetología of Instituto de Ecología, A.C. (Mexico). We included all species of recorded copronecrophagous beetles.
We estimated the completeness of the beetle species inventory as the sample coverage for each type of environment. The sample coverage represents the proportion (with respect to all individuals in the community) that constitutes the individuals of the species collected in the sample (Hsieh et al., 2016). Subsequently, we evaluated the diversity of beetles in each environment using the method proposed by Jost (2006) , which recognizes “true diversity” through the effective number of species. Specifically, this index measures the diversity that a community composed of i equally common species would have. The effective number of species is calculated using the equation:
where qD is diversity, pi is the proportion of individuals in the total sample that belongs to species i, and q is a constant that determines the influence of common and rare species on true diversity. The exponent q = 0 is completely insensitive to the species abundances, and therefore it is the number of species present, while values of q < 1 overestimate rare species and q = 1 indicates that all species are included with a weight exactly proportional to their abundance in the community. The values of q > 1 consider the most common species (Moreno et al., 2011). All indices were obtained through the “vegan” package (Oksanen et al., 2017) in the R statistical program (R Core Team, 2021).
The graphical representation of the species composition by each environment and the sites (3 sampling units by environment) was carried out using a non-metric multidimensional scaling analysis (NMDS). This type of ordering analysis is one of the most robust and often summarizes more information in fewer axes than other techniques (Legendre & Legendre, 1998). A distance matrix (4 sites [12 rows] × species [37 columns]) was used with the Bray-Curtis dissimilarity index as a measure. This index is a measure of dissimilarity between communities that allows for the evaluation of differences in terms of abundance and species composition. This analysis was carried out with the R program (R Core Team, 2021) using the “vegan” package (“metaMDS” function) (Oksanen et al., 2017). To evaluate whether the groupings observed in the NMDS present differences in community structure, we performed a multivariate analysis of variance with 999 permutations (PERMANOVA) using the “vegan” package (“adonis” function) (Oksanen et al., 2017). Subsequently, PERMDISP analyses were performed to verify species homogeneity and comparability using the “betadisper” function in the R program (R Core Team, 2021).
We evaluated the relationship between species richness (q0) and Shannon diversity (q1) and time (months of the year) using linear mixed effects models (LMMs). In these models, we used observations each month as an independent variable and the species richness and Shannon diversity as dependent variables, with type of environment as a random factor to consider the effects of site. In this way, we were able to assess the temporal variation in species diversity more robustly regardless of the type of environment in which they occur. Due to the nature of the response variables, a Poisson error distribution was considered for species richness, and for the other indices we used a Gaussian error distribution (Guisan et al., 2002). The analyses were performed using the nlme version 3.1-152 (Pinheiro et al., 2022), and lmerTest version 3.1-3 (Kuznetsova et al., 2017) packages. LMMs were performed in R software (R Core Team, 2021).
We evaluated the diversity components to determine the spatial dynamics of the beetle assemblage between environments. To determine the contribution of each sampling point and of the environments to the total diversity (γ), we carried out an additive partition for 3 levels of diversity: average diversity between sampling points (ᾱ), diversity between traps (β1), and diversity between types of environments (β2).
The observed values were contrasted using a random distribution (n = 999 permutations) generated by null models, where the presence of the species is randomly distributed among the samples using the “adipart” function in the R package “vegan” (Oksanen et al., 2017). The p-value was obtained by comparing the random distribution generated by the null models against the values observed for each of the diversity levels (Crist et al., 2003).
Results
We registered a total of 2,555 individuals belonging to 15 genera and 37 species. Sample coverage indicated that a completeness of almost 99% was obtained for all environments (the riparian forest presented 98% completeness) (Supplementary material: Appendix 1). The most abundant genera were Canthidium (n = 500 individuals, 4 species), Onthophagus (n = 484, 8 species), and Uroxys (n = 412, 3 species). Regarding the species, the most abundant were Copris laeviceps (n = 373 individuals), found mainly in evergreen forest environments and Canthidium pseudopuncticolle (n = 296 individuals) and Onthophagus cyclographus (n = 280 individuals), both mainly associated with disturbed environments (Supplementary material: Appendix 2). There were 6 rare species (< 5 individuals): Anaides laticollis, Anomiopus cirulito, Copris lugubris (evergreen forest), Eurysternus mexicanus and Onthophagus crinitus (secondary forest), and Sisyphus mexicanus (Supplementary material: Appendix 2).
The environments with the highest species richness (q0) were the primary forest (S = 34) and the secondary forest (S = 29). The environments that presented the highest species diversity were the primary forest (q1 = 16.2 species) and riparian vegetation remnants (q1 = 15.2 species). We observed that in the primary forest and remnants of the riparian vegetation, the most abundant species were C. laeviceps, Deltochilum pseudoparile, and Canthon euryscelis. However, even though the most abundant species were similar in the secondary forest and in the pasture, these species change in their hierarchical order (C. pseudopuncticolle and O. cyclographus) according to their abundances (Fig. 1). Canthon indigaceus chiapas was one of the dominant species in the pastures but was not present in the secondary forest. Conversely, Uroxys deavilai was dominant in the secondary forest but not in the pasture.
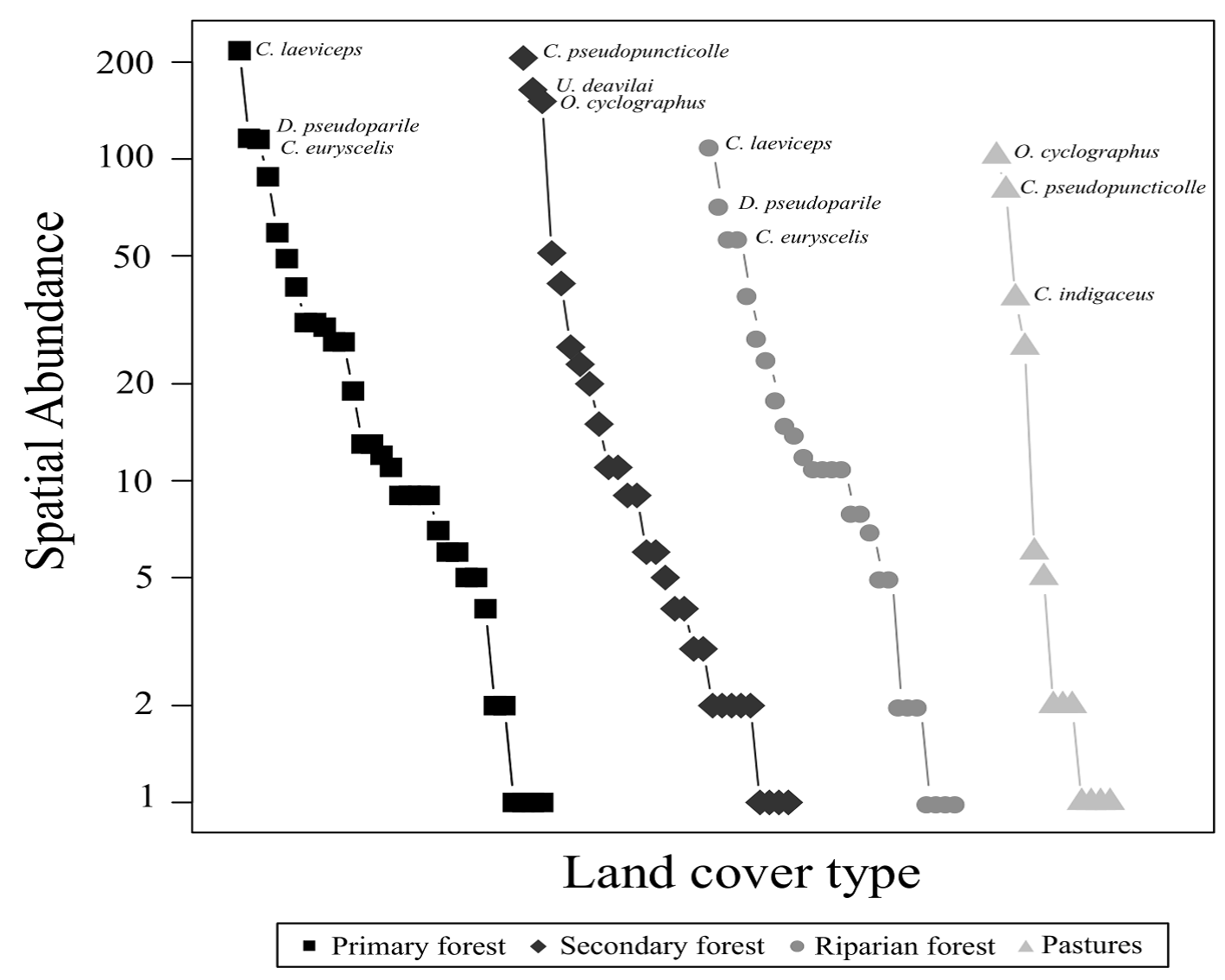
Figure 1 Rank-abundance curves of the beetle species registered in different environments (primary forest, secondary forest, riparian forest, and pasture) in the Bahlum Pakal Community Ecological Reserve, Nueva Betania, Chiapas, Mexico. The most abundant species by environment are shown.
The months that presented the highest species richness are found in the period from June to September (Fig. 2a), while the highest diversity for most of the environments occurred between June and August (Fig. 2b). The highest abundances were found from January to April. The species that were recorded with the highest abundances in the REBP during all months of the year were Copris laeviceps (n = 373) and Deltochilum pseudoparile (n = 191), which represented mostly conserved areas. Various species had low abundances during the year (fewer than 10 individuals), such as Onthophagus crinitus and Copris luubris, which each only had 1 record in June (Supplementary material: Appendix 3). Other species had high abundances during the cold season, such as C. laeviceps, Bdelyropsis bowditchi, C. pseudoperceptibile, Onthophagus maya, Pseudocanthon perplexus, Uroxys micros, and U. deavilai (Supplementary material: Appendix 3).
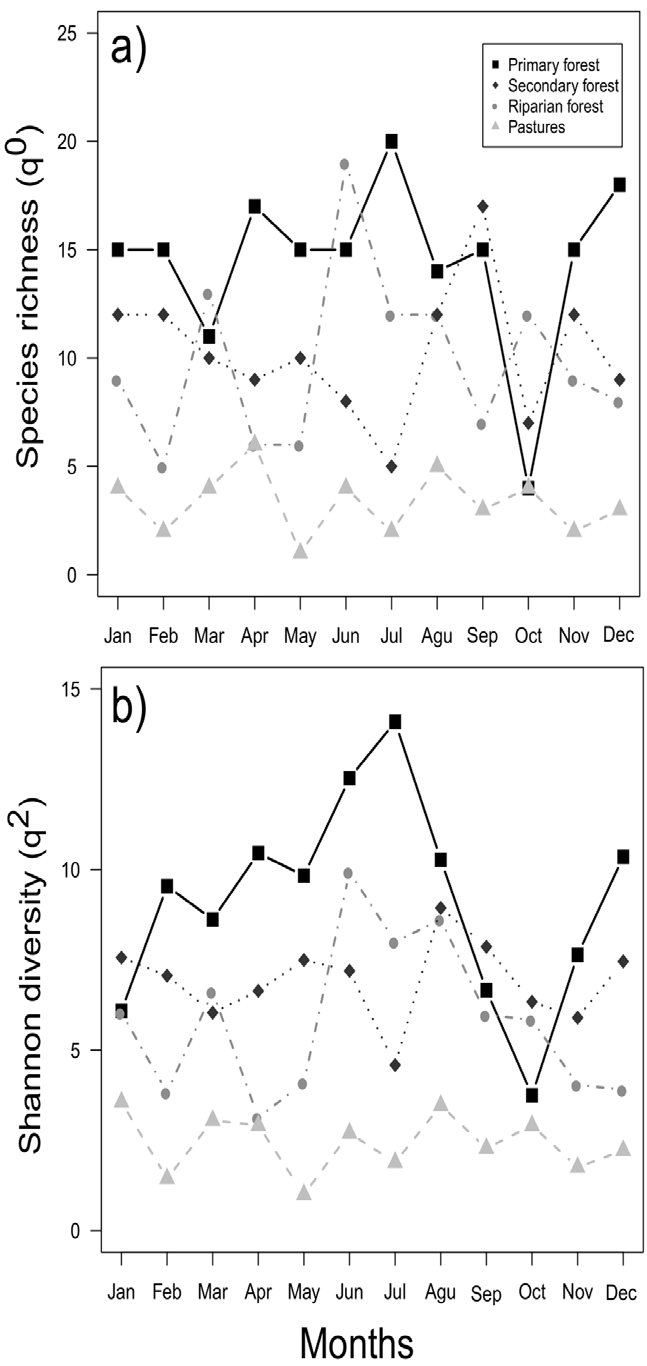
Figure 2 Monthly distribution of the richness (q0) and species diversity (q1) of dung beetles throughout a sampling year in the Bahlum Pakal Community Ecological Reserve, Nueva Betania, Chiapas, Mexico.
We observed that species composition differed among the 4 studied environments (dimensions = 2, stress value = 0.038; PERMANOVA, p = 0.002) (Fig. 3). This indicates that each environment presents a different species assemblage. In addition, no environment was heterogeneous in species composition (PERMDISP, p = 0.476). This indicates that the difference in species composition is more important than the differences within each environment.
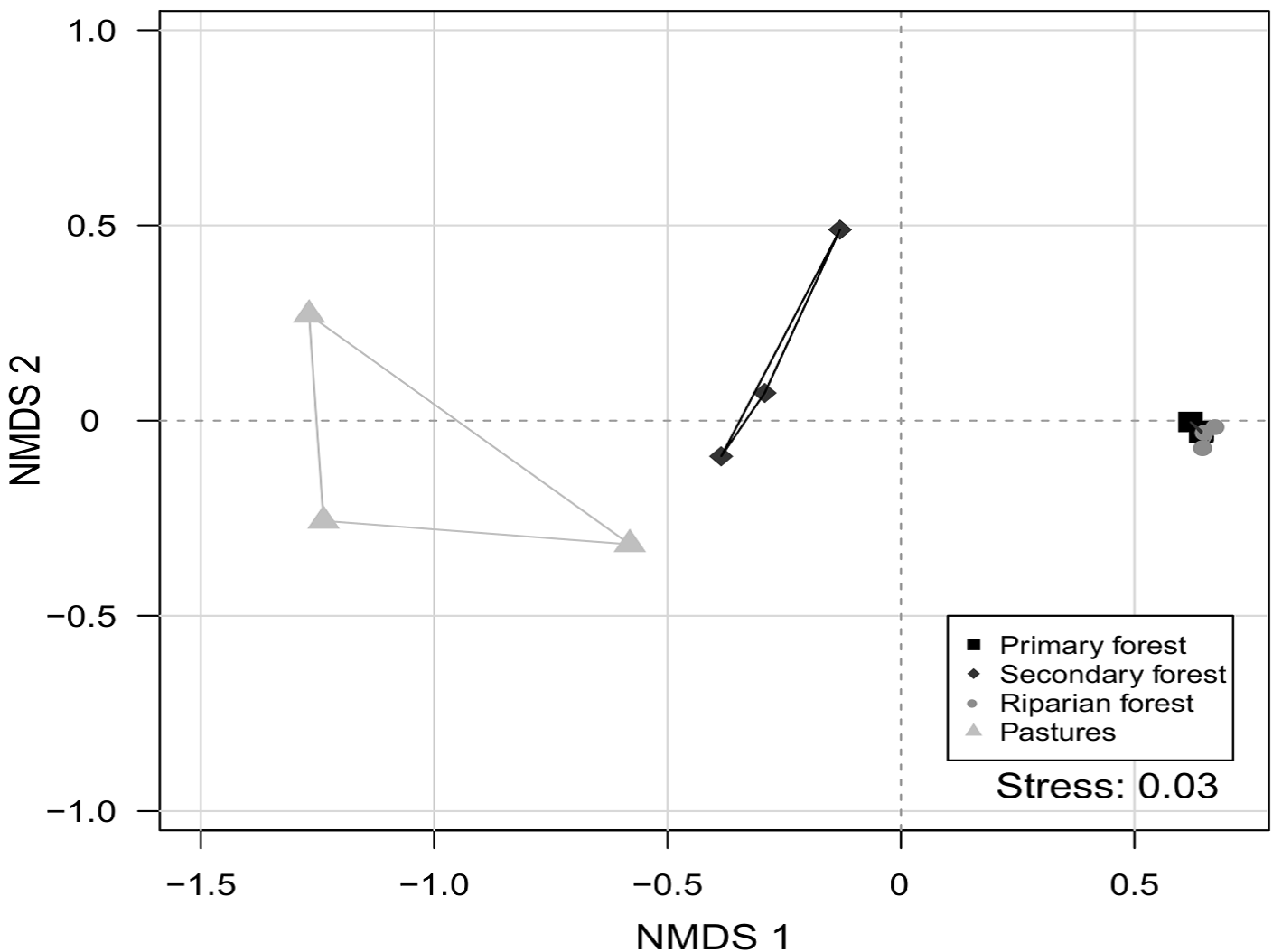
Figure 3 Non-metric multidimensional scaling analysis (NMDS) of dung beetle species composition in the Bahlum Pakal Community Ecological Reserve, Nueva Betania, Chiapas, Mexico.
When evaluating the relationship between species richness (q0) and Shannon diversity (q1) with time (months of the year), we found that neither the richness (LMM, χ 2 = 0.01, p = 0.90) nor Shannon diversity present differences (LMM, χ 2 = 0.12, p = 0.72) changes throughout the year.
Spatial dynamics were more important for explaining species richness and diversity in all environments. We found that the component of dung beetle diversity that explained the highest proportion of gamma diversity (set of total species) was β diversity (55%) (i.e., species turnover between environments). The most important component of β diversity was β2 (between environments) and it did not show differences as expected by chance (p = 0.42), which indicates that the distribution of species is random. The component that had the least importance for gamma diversity was β1 (between traps) as it was even lower than expected by chance (p < 0.05). Land use changes increased the composition dissimilarities between environments, being higher than replacement at the trap level. On the other hand, the diversity ᾱ explained 45% of the gamma diversity and was significantly higher than expected by chance (p < 0.05) (Supplementary material: Appendix 4).
Discussion
Our results indicated that the structure of dung beetle assemblages changed in different environments associated with land use changes in a tropical rainforest in southern Mexico. We found a higher richness and diversity of dung beetle species in more forested environments (i.e., primary forest and riparian forest). When evaluating the effect of the temporal factor, we did not find a relationship between species richness or Shannon diversity and monthly variation. We observed differences in species composition between environments, which could indicate that the type of environment had a greater influence on the patterns of richness and diversity of beetle species. On the other hand, the most important component of β diversity was β2 (between environments), which suggests that the environmental variation given by the high spatial heterogeneity of the different environments modulates the patterns of richness and diversity of beetle species in the Bajlum Pakal Community Ecological Reserve.
Previous research has indicated that geographic location and landscape context appeared to modify dung beetle response under habitat modification and fragmentation scenarios (Nichols et al., 2007). We showed how the spatial factor is important in modulating richness and diversity patterns of dung beetles in a rural reserve. These results are consistent with the general pattern found in some tropical environments, which indicates that species richness and abundance declined with increasing habitat modification (Nichols et al., 2007). The REBP is in an area with some of the greatest biodiversity in America (called Selva Maya), and the area where it is located has few years of occupation and exploitation (a little more than 50 years), which allows it to still preserve an important richness of dung beetles (37 species) despite being in an important tourist corridor. This species richness is slightly greater than the average species richness found in other landscapes with tropical humid forest in Mexico (31 ± 9.85 species): Sian Ka´an, Quintana Roo with 20 species (Morón et al., 1986), Yaxchilán, Chiapas with 25 species (Palacios-Ríos et al., 1990), Boca de Chajul, Chiapas with 27 (Morón et al., 1985) and 49 species (Navarrete & Halffter, 2008a), El Ocote, Chiapas with 29 species (Sánchez-Hernández et al., 2018), Los Tuxtlas, Veracruz with 30 species (Díaz et al., 2010), and Los Chimalapas, Oaxaca with 40 species (Moctezuma, 2019). This highlights the REBP as a great reservoir of local biodiversity in southern Mexico.
In this study we evaluated different measures of taxonomic diversity beyond species richness, including Shannon and Simpson diversities, to evaluate the different responses to land use change. All these biodiversity components are affected negatively by habitat modification, as we found a decline in species richness, diversity, and dominance in more modified environments (pastures). These results are consistent with Nichols et al. (2007) , who indicated that some environments such as secondary forests, selectively logged forests, and agroforests supported rich communities with many intact forest species, while cattle pastures and clear-cuts contained fewer species. Furthermore, the spatial dynamics of the dung beetle richness and diversity in the REBP showed differences between the environments, finding the greatest richness and diversity in the primary forest and in the remnants of riparian vegetation, as we expected. It is well-known that habitat conservation status influences the diversity of Scarabaeinae in Neotropical forests, since most of its species have a strong association with humid and shady areas (Halffter, 1991; Favila & Halffter, 1997; Navarrete & Halffter, 2008; Nichols et al., 2007). The most conserved environments such as the tropical rainforest and the remnants of riparian vegetation are characterized by greater forest cover and vegetation complexity (Tews et al., 2004). Increased species richness is often associated with greater habitat complexity, so the microclimatic characteristics of these sites could allow a greater number of species to coexist (Halffter & Arellano, 2002; Navarrete & Halffter, 2008). The sites with evergreen forest (i.e., primary, secondary, and riparian) are preferred by tourism and local inhabitants conserve and protect these areas because they have a higher value than open areas in the reserve.
In addition to the different spatial patterns of richness and diversity of dung beetles, the temporal patterns did not show a relationship with species richness, Shannon diversity, or Simpson diversity during the sampling period, which is contrary to other studies that found changes in the patterns of species richness and diversity over time (Correa et al., 2021; Noriega et al., 2021). We observed the highest abundance from January to April, which coincides with some of the coldest months of the region (Comisión Nacional del Agua, 2019). Species such as Copris laeviceps, Bdelyropsis bowditchi, Canthidium pseudoperceptibile, Onthophagus maya, Pseudocanthon perplexus, Uroxys micros, and U. deavilai (most being nocturnal, according to Demeza’s personal observations) were favored by cold season conditions and because these are the preferred months of tourists, who sometimes defecate in the areas they visit, representing a source of alternative resources for the species.
Changes in temporal patterns have been attributed to various factors, including variation in climatic and environmental conditions and the availability of resources (Ferreira et al., 2019). However, other factors not considered in this study (e.g., different activity patterns of some species, physiological tolerances to certain environmental conditions, and more food availability in months with greater tourist activity) could also influence the presence or absence of species throughout the year in the different study environments (Slade & Roslin, 2016).
Here we showed that the spatial variation in the beetle species had a greater effect due to the change in land use and it is relatively more important than temporal richness and diversity, which suggests that the environmental variation given by the high spatial heterogeneity of the different environments modulates the patterns of richness and diversity of beetle species in the REBP. Land use change involves various processes (i.e., habitat loss and fragmentation) that directly influence the spatial patterns of the diversity of dung beetles (Alonso et al., 2020). We found that the component of beetle diversity that explained the highest proportion of gamma diversity (set of total species) was β diversity, where the most important component of β diversity was β2 (between environments). Accordingly, our results corroborate the idea that species turnover is the main component of spatial β diversity (Ferreira et al., 2019; Soininen et al., 2018). The spatial replacement of species is partially associated with environmental filters generated by the environmental variation of different classes of land use (Filgueiras et al., 2016; Scheffler, 2005). The microclimatic changes (i.e., canopy cover, vegetation structure, pH, environmental humidity, litter volume) in habitat conditions as a consequence of human activity are important factors that modulate the patterns of richness and diversity of beetles that inhabit the soil (Lassau et al., 2005). An example of this are the species with high habitat specialization that were found at more conserved sites of the REBP (i.e., D. pseudoparile), and that have preferences for certain environmental conditions (Campos & Hernández, 2013; Halffter, 1991). Many of these species cannot occupy areas with open vegetation (Almeida et al., 2011; Campos & Hernández, 2013; Spector & Ayzama, 2003), generating changes in the composition of species between environments. In contrast, in mostly disturbed sites such as pastures and secondary forest, we found more species of generalist and opportunistic beetles (Canthidium pseudopuncticolle and Onthophagus cyclographus), which were favored by the environmental conditions that allowed an increase in the abundances of these species (Roslin & Koivunen, 2001). This is a well-known pattern in areas such the REBP where the tropical rain forest dominated and where pastures are historically new environments (little more than 50 years old). However, in some cases the apparent spatial specialization of species may be due to other factors such as low dispersal ability, low availability of mates or resources, restricted distribution in time (certain month or some hours of the day), having a lower fecundity or higher mortality rate (Noriega & Costa, 2011), or simply a sampling bias where commonly used sampling methods may not provide an accurate representation of the dung beetle community (Larsen et al., 2006). Further studies at a larger number of sites are needed to explain the low density of species.
The differences in beetle compositions between the most conserved and the most disturbed environments indicate that the high environmental variation seems to modulate the presence of certain species capable of tolerating the conditions of the different environments. This information allows us to highlight the importance of primary forest sites as reservoirs for a great diversity of species (Korasaki et al., 2013). On the other hand, native species from open areas still represent a low proportion in the pool of species of the REBP since the matrix that surrounds the pastures includes fragments of forest or adjacent living fences, which could favor the presence of edge or generalist species. In addition, in the REBP, the exotic species of beetles such as Digitonthophagus gazella or Euoniticellus intermedius have not yet been found in the open areas (often associated with this type of environment), suggesting that the existing matrix surrounding the REBP does not facilitate their presence. It is important to reflect on what will happen to native beetle species in open areas in response to climate change (the main cause of the reduction of their distribution areas), and in response to livestock practices such as the use of antiparasitics. The decrease in their populations in the medium and long term due to the synergistic effects of these factors could generate a decrease in the ecosystem services they provide (Maldaner et al., 2021).
The diversity of species changes in time and space due to a great variety of factors that involve phenological patterns and life strategies, which in turn are modulated by environmental changes and variation in the availability of food resources (Pinheiro et al., 2002; Wolda, 1988). Thus, conservation strategies must consider the spatio-temporal changes that modulate species assemblages since organisms have different biological responses to land use change (Moreno & Halffter, 2001). Furthermore, changes in the spatio-temporal dynamics are reflected in the biodiversity and functioning of the ecosystem.
Finally, we highlight that our study has been developed in a community ecological reserve that provides several free benefits (scenic and cultural) and well-being for nearby and global populations, but is also a refuge for beetle species and conservation of their biodiversity. Therefore, the conservation of forest fragments and wooded areas, such as secondary vegetation or remnants of riparian vegetation, which are elements of connectivity between tropical forests, requires the active intervention of local communities and the responsible participation of all sectors of society.











 nueva página del texto (beta)
nueva página del texto (beta)



