Introduction
The macrophyte Eichhornia crassipes (Mart.) Solms is considered an invasive species that adapts to a wide range of ecosystems and significantly affects the natural balance of lakes and lagoons (Abdel-Fattah & Abdel-Naby, 2012; Ganguly, Chatterjee, & Dey, 2012; Riaño, 2010). According to the Bogotá mayor's office, 500 t of the plant were extracted in the Juan Amarillo wetland in the western part of the Colombian capital in 2010 (Trujillo, Rial, Canon, Rojas, & Sierra, 2010).
In recent years it has been shown that E. crassipes can be treated sustainably; to this end, projects have been designed and implemented for environmental and biofuel generation purposes (Park, Anburajan, Kumar, Park, & Kim, 2017; Rahman et al., 2016; Rani, Singh, & Shimrah, 2017). Several investigations have taken advantage of E. crassipes biomass to design and set up phytoremediation processes, retaining heavy metals or other pollutants in industrial water (Bronzato, 2016; Nagarajan, Lee, Kondo, & Chang, 2017; Park et al., 2017; Rahman et al., 2016; Ri, Ren, Ding, Kim, & Guo, 2017; Riaño, 2010). Another important aspect is that E. crassipes has low lignin content (10 %) and a high amount of cellulose (28 %) and hemicellulose (33 %); the latter two compounds are easily converted into fermentable sugar, through a chemical process called hydrolysis, and therefore the production of biofuels such as bioethanol and biohydrogen with E. crassipes is totally viable (Ganguly et al., 2012; Kumar, Singh, & Ghosh, 2009; Mishima et al., 2008; Balasubramanian, Arunachalam, Das, & Arunachalam, 2012). According to Sayago (2019), phytoremediation and bioenergy production processes can be combined to obtain bioethanol with the E. crassipes biomass used in chromium (VI) removal.
The main objective of this article was to compile information on the state of the art of water treatment processes and biofuel production with E. crassipes, in order to design an integrated process for the production of biohydrogen and bioethanol with the biomass of the plant used in the phytoremediation of heavy metals.
Materials and methods
Information on the capacity of E. crassipes in phytoremediation and bioenergy was reviewed in the research available in databases such as Science Direct and Scopus. The results of such research were compared, and an integrated phytoremediation and bioenergy process was designed.
Characterization of the Eichhornia crassipes plant
The plant E. crassipes, also known as "water hyacinth", is a floating freshwater vascular macrophyte native to southern South America (Brazil and the equatorial region). The stems and leaves are made up of air-filled sacs that allow the plant to be permanently suspended on the water’s surface. Eichhornia crassipes has both sexual and asexual reproduction and is prevalent mainly in tropical and subtropical water bodies (Vasquez, 2012).
The species Eichhornia crassipes is considered invasive due to its adaptability to various ecosystems. The plant considerably affects the natural balance of aquatic systems (lagoons, lakes and wetlands) and feeds mostly on the concentration of nutrients in agro-industrial effluents and deforestation residues (Carreño, 2016a; Kumar et al., 2009).
Currently, E. crassipes biomass transformation is a promising source for the creation of truly sustainable alternative energies. Among its main attributes is the very low lignin content in contrast to the high cellulose content per unit volume of dry matter (Table 1), characteristics that make the plant easily degraded. Related research has found a high degree of cellulose (28 %) and hemicellulose (33 %) and little lignin (10 %), this being a determining factor for the development of bioprocesses that can transform these sugars into biofuels (Chuang et al., 2011; Mishima et al., 2008). Lignin is an amorphous polymer with a very complex molecular structure, similar to asphalt; it has a high molecular weight and is therefore very difficult to fractionate, and it is insoluble in acids and soluble in strong alkalis such as sodium hydroxide. On the other hand, hemicellulose and cellulose are linear or fibrous structure biopolymers tightly bound by hydrogen bonds between the hydroxyl groups of different juxtaposed sugar chains (monosaccharides), such as glucose, galactose or fructose (Zhou et al., 2009). The use of the plant as a by-product has simply consisted of direct burning to provide some energy requirement (Balasubramanian et al., 2012; Mishima et al., 2008; Tan et al., 2008).
Table 1 Composition of Eichhornia crassipes biomass.
| Lignin (%) | Cellulose (%) | Hemicellulose (%) | Others (%) | Reference |
|---|---|---|---|---|
| 1.1 | 17.3 | 24.7 | - | Chuang et al. (2011) |
| 4.1 | 19.7 | 27.1 | - | Mishima et al. (2008). |
| 3.5 | 18.2 | 48.7 | 13.3 | Magdum, More, and Nadaf (2012) |
| 1.1 | 17.3 | 24.7 | - | Lay et al. (2013) |
| 11 | 31 | 27 | 10 | Tan et al. (2008) |
| 11 12 | 27 36 | 27 42 | 10 - | Zhou et al. (2009) Balasubramanian et al. (2012) |
Eichhornia crassipes as a phytoremediation agent
Phytoremediation with E. crassipes represents an efficient and economical technology for the treatment of water contaminated with nutrients, heavy metals and high organic matter contents, since it does not require sophisticated infrastructure (Abdelraheem, Komy, & Ismail, 2017; Mahunon et al., 2018; Martínez et al., 2013).
Industrial water treatment systems with E. crassipes have been implemented, consisting of the use of the live plant together with contaminated water for the removal of heavy metals. For the removal of chromium (IV), a wetland with E. crassipes was designed and constructed, evaluating the removal of various concentrations of this contaminant. Artificial photosynthesis was carried out at 30 °C and relative humidity of 60 %, obtaining removals, on average, of 70 % in 21 days of treatment (Gupta & Balomajumder, 2015; Hadad, Maine, Mufarrege, Del Sastre, & Di Luca, 2011). For the treatment of zinc-contaminated water, the plant cultivated in water at pilot scale was used to assess initial concentrations of 5.0, 10.0, 15.0 and 20.0 mg·L-1 in two to 15 days; the removals obtained were around 90 % zinc (Borker, Mane, Saratale, & Pathade, 2013; Swain, Adhikari, & Mohanty, 2014).
Treatment systems with living plants are interesting, but they are far from being ideal treatment systems due to the large amount of time the plant needs to remove heavy metals. For this reason, the use of dried, ground E. crassipes material for the creation of biological filters has been started; the cationic exchange between the hydrogen bonds of the plant’s functional groups with zinc (II), chromium (VI), cadmium (II) and arsenic (III) favors the adsorption of these pollutants (Adanikin, Ogunwande, & Adesanwo, 2017; Martínez et al., 2013; Saraswat & Rai, 2010; Sarkar, Rahman, & Bhoumik 2017; Thi, Ong, Thi, & Ju, 2017). Sarkar et al. (2017) also designed filters with 20 g of dried, ground E. crassipes biomass for vertical downflow treatments; 5 L of polluted water from a tannery were treated, removing 75 % chromium (VI) with initial concentrations of 200 mg·L-1. The adsorption mechanism of uranium by batches from dried, crushed E. crassipes biomass has also been experimented. The results showed that uranium (VI) adsorption was highly Ph-dependent, concluding that the best pH for future removal designs is 5.5. U (VI) adsorption proceeded rapidly with an equilibrium time of 30 min and conformed to second-order kinetics (Yi et al., 2016).
Transformation of Eichhornia crassipes biomass
In order to optimize industrial wastewater treatment, a chemical or physical modification of the E. crassipes biomass can be made to achieve structural durability and efficient adsorption capacity of heavy metal ions and other pollutants (Hokkanen, Repo, & Sillanpää, 2013). An alternative is the use of iron (Fe) (III) by impregnation with iron chloride to the surface of E. crassipes. This chemical procedure has been used for the adsorption of heavy metals and dyes. Iron (III) oxyhydroxide reacts with hydratable hydroxyls of the E. crassipes cellulose forming iron hydroxides (FeOOH); the metal ions enter the interior of E. crassipes with FeOOH, exchanging with protons of hydroxyl groups. The ionic interaction is mainly responsible for the adsorption of As (III), As (VI) and Cr (IV) (Lin, Yang, Na, & Lin, 2018; Wei, Fang, Zheng, & Tsang, 2017).
A network composed of E. crassipes cellulose, chitosan and titanium oxide (TiO2) has been investigated, achieving over 90 % removal of industrial dyes as a function of pH. There is an electrostatic interaction between the negatively charged hydroxyl groups of cellulose fibers of E. crassipes together with the NH (III) anion of the chitosan; TiO2 binds to this compound and forms a chelating network, increasing the cation exchange capacity (El-Zawahry, Abdelghaffar, Abdelghaffar, & Hassabo, 2016).
A widely used process for the transformation of E. crassipes biomass is the creation of cellulose xanthogenate ((Cell-OCS2)2 Mg) developed by Tan et al. (2008) and Deng et al. (2012). This procedure consists of taking dried, ground E. crassipes biomass and adding sodium hydroxide (NaOH), creating alkaline biomass; it is then esterified with carbon disulfide (CS2) and finally treated with magnesium sulphate (MgSO4) to prepare cellulose xanthogenate. Zhou et al. (2009) characterized three types of xanthogenate biomasses: E. crassipes, rapeseed straw and cornstalk; the authors concluded that E. crassipes has more hydroxyl (OH) and carbonyl (C=O) groups than the other two plant compounds after alkalization with NaOH, increasing the adhesion of magnesium (Mg) and sulfur (S), to obtain cellulose xanthogenate. Mg and S2 are responsible for cation exchange with lead (II), removing 90 % with initial concentrations of 500 mg·L-1 (Tan et al., 2008).
E. crassipes biomass has been bound to another type of polysaccharide such as chitosan, creating microspheres with sodium tripolyphosphate (TPP) solution. The cellulose fibers of E. crassipes were embedded in the chitosan matrix. In the experimental treatment, these spheres removed about 95 % lead (II) with an initial concentration of 100 mg·L-1. The lead was adhered to the gelled cellulose compound of E. crassipes and chitosan through a chemisorption cation exchange (Ammar, Elhaes, Ibrahim, & Ibrahim, 2014; Yang, Chen, & Zhang, 2014).
An operational drawback with these treatments, both with the E. crassipes plant still alive and with the crushed biomass, is the waste, due to the high adsorption of heavy metals; for this reason, it is proposed to use this biomass in a biofuel production process.
Eichhornia crassipes as a bioethanol producing source
The production of ethanol fuel from lignocellulosic waste has become an interesting alternative that could open up new markets for its revaluation (Benítez et al., 2010). In the production of bioethanol from lignocellulosic material, various physical, chemical and biological processes take place, such as size reduction, lignin removal, acid hydrolysis, fermentation and distillation (Riaño, 2010). Eichhornia crassipes meets the criteria for bioenergy production; it is permanent because there are large quantities of plant available, it is biodegradable and it has a high cellulose content (Chuang et al., 2011). Among the main drawbacks in ethanol production is the use of food for humans and animals as raw material (Abdel & Abdel, 2012; Hossain, Chowdhury, Yeasmin, & Hoq, 2010; Ríos, 2015; Zabed, Sahu, Boyce, & Faruq, 2016).
Magdum et al. (2012) performed an acid hydrolysis with E. crassipes and sulfuric acid (H2SO4); the hydrolyzed solution showed to be rich in hexose and pentoses, which were used directly as substrate for alcohol production by means of batch fermentation using Pichia stipitis Pignal. Hydrolysis with H2SO4 is the most effective pretreatment for the E. crassipes treatment (Pattra & Sittijunda, 2015).
Saccharomyces cerevisiae (Desm.) Meyen is the most widely used yeast for the production of bioethanol from sugars, product of the hydrolysis of E. crassipes, due to its high fermentation power (Kuldiloke, Eshtiaghi, Peeploy, & Amornrattanapong, 2010; Pattra & Sittijunda, 2015). This bioethanol production, from the hydrolyzed biomass of E. crassipes, has been carried out in bioreactors designed and built with the adjusted parameters (Kuldiloke et al., 2010; Lee, Park, Cho, & Kim, 2018; Thi et al., 2017). In one year, from one hectare of E. crassipes, with a production of approximately 80 t, it is possible to produce 265 L of ethanol through a large-scale fermentation process (Bronzato, 2016).
Eichhornia crassipes as a biohydrogen producer
Hydrogen has transcendental properties as a biofuel, as it is colorless, odorless, tasteless and pollutant-free; it is used in several chemical process industries as its only product is water, and it does not emit pollutants such as methane (CH4) and carbon dioxide (CO2) into the environment (Khan et al., 2017; Nagarajan et al., 2017; Rahman et al., 2016). Hydrogen production, through dark fermentation, is a promising path; it is obtained from carbohydrate-rich raw materials, such as wastewater, food waste and agricultural residues (Dessì, Lakaniemi, & Lens, 2017; Khan et al., 2017; Mechery, Biji, Thomas, & Sylas, 2017; Ri et al., 2017; Roy, Ghosh, & Sarkar, 2016), in the absence of light with the combined action of anaerobic bacteria. The generation of biohydrogen through this technique is a complex process involving microbial groups that grow in darkness, mainly of the genera Enterobacter, Bacillus and Clostridium (Chuang et al., 2011; García-Depraect, Gómez-Romero, León-Becerril, & López-López, 2017; Park et al., 2017). Biohydrogen production by anaerobic fermentation is well known to be the most suitable, due to its potential for direct utilization of wastewater and organic waste (Ganguly et al., 2012; Nagarajan et al., 2017; Rahman et al., 2016; Park et al., 2017).
Results and discussion
Design of an integrated phytoremediation and energy production system
The proposal presented is a design case study, generated from the review of the state of the art, which can be adjusted to industrial scale conditions. This design includes a phytoremediation system, a bioreactor to generate hydrolysis, a bioreactor to generate bioethanol and, finally, a bioreactor to generate hydrogen.
Set up of the experimental phytoremediation model
The dimensions of the experimental phytoremediation model are 4 m long, 1.5 m high and 1.5 m wide, where 100 L of water will be treated per day. This design allows for the placement of 20 plants that together weigh approximately 1.8 kg. For the treatment of 100 L, 20 E. crassipes plants are proposed; approximately 5 L of water are treated by each plant. The model is based on what is indicated by Chen et al. (2016), Mascarenhas and Junior (2016), Mello et al. (2017) and Wu et al. (2015). This article proposes plastic systems for the treatment of domestic and industrial wastewater.
Design of the hydrolysis process
The experimental process for the hydrolysis of the E. crassipes biomass requires 1 kg dry weight of the biomass used in the phytoremediation process. The hydrolysate bioreactor is made of glass (5 L), has a lid for gas release and sampling for pH and temperature measurement, and a heating magnetic stirrer (120 rpm at 60 °C). This design was based on and adapted from studies by Kuldiloke et al. (2010) and Lee et al. (2018); the bioreactor is simple, economical and easy to install (Thi et al., 2017).
The hydrolysate bioreactor contains 1 kg of dried E. crassipes biomass mixed with distilled water. Samples react in 1 % (w/v) NaOH at 60 °C for 12 h, then washed with tap water until the pH value of the water is reached. Subsequently, 3 % H2SO4 (v/v) is added at 60 °C for 12 h; samples are washed with tap water until they reach the pH value of the water. The content of reducing sugars is determined with the dinitro salicylic acid (DNS) method (Peña & Arango, 2009) which indirectly quantifies substrate consumption. Finally, 4 L of E. crassipes hydrolysate solution would be obtained for bioethanol and biohydrogen production.
Set up of the experimental bioethanol production model
The proposed fermentation bioreactor is made of glass (5 L), with a lid for gas release and sampling for pH and temperature measurement, and a heating magnetic stirrer (120 rpm at 60 °C). The yeast S. cerevisiae is used as a fermenting inoculum of the E. crassipes hydrolysate. In the bioreactor, 400 g of the hydrolysate are mixed with distilled water and 180 g of the inoculum of S. cerevisiae (commercial Lesaffre); the initial pH is adjusted to 5.5 with NaOH. The bioreactor is hermetically sealed with rubber septa and aluminum stoppers for 12 h. This bioreactor was adapted from Lee et al. (2018) and Thi et al. (2017). One proposal in this article is the use of recycled glass jars to reduce implementation costs. During fermentation of biomass hydrolysis, tests should be performed every two hours to determine ethanol percentages by gas chromatography.
Set up of the experimental biohydrogen production model
The dark fermentation bioreactor is made of glass (5 L) and has a lid for gas release and sampling for pH and temperature measurement, and a heating magnetic stirrer (120 rpm at 60 °C). The bioreactor is hermetically sealed with rubber septa and aluminum stoppers; subsequently, the orifices are then purged with nitrogen for 5 min to ensure the anaerobic condition; this bioreactor was adapted from Ri et al. (2017), Nagarajan et al. (2017) and Rahman et al. (2016).
One proposal of this article is to take as inoculum the biosolids generated in the El Salitre wastewater treatment plant (Wtp) in the city of Bogota. These biosolids are obtained after digestion (20 days of treatment) and are used due to the suitability of their bacterial profile (Ri et al., 2017). In the bioreactor, 400 g of the hydrolysate are mixed with distilled water and 180 g of the inoculum (biosolid); the initial pH is adjusted to 5.5 with NaOH.
At two-hour intervals, the volume of biogas should be measured by plunger displacement, after which the hydrogen gas should be determined by gas chromatography using a thermal conductivity detector (TCD) in a GC-Agilent 7890 chromatograph (Chuang et al., 2011; García et al., 2017).
Figure 1 shows the proposed design to produce bioenergy from E. crassipes biomass, obtained in a phytoremediation process, and the hydrolysis and bioethanol and biohydrogen production bioreactors.
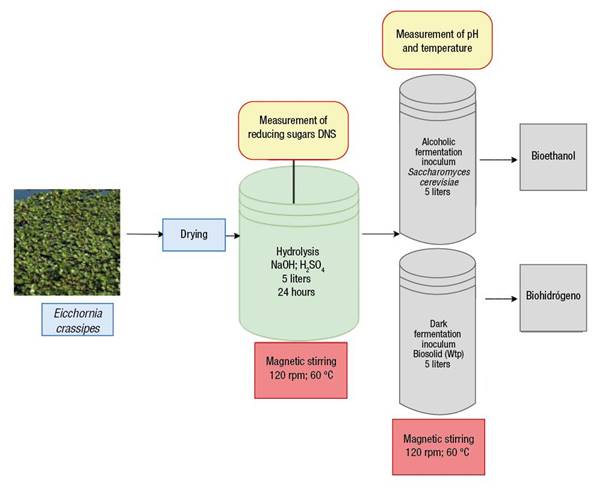
Figure 1 Design of a process for phytoremediation and production of biofuels (bioethanol and biohydrogen) from Eichhornia crassipes (adapted from Carreño, 2016b; Chen et al., 2016; Kuldiloke et al., 2010; Lee et al., 2018; Mascarenhas & Junior, 2016; Mello et al., 2017; Nagarajan et al., 2017; Rahman et al., 2016; Ri et al., 2017; Thi et al., 2017). DNS: dinitro salicylic acid; Wtp: wastewater treatment plant.
Conclusions
The integrated design presented in this paper consists of a phytoremediation process using Eichhornia crassipes biomass (living and dead), a biofuel production process composed of the hydrolysis bioreactor along with the bioreactors for bioethanol and biohydrogen production. In phytoremediation, the aquatic plant E. crassipes is an important agent that adsorbs heavy metals and nutrients due to the high cellulose content in its biomass. This biomass used in the phytoremediation process can be implemented in the production of biofuels. The bioreactors proposed for bioethanol and biohydrogen production are inexpensive and easy to implement. It is feasible to create and build a large-scale bioethanol and biohydrogen production system from E. crassipes biomass (loaded or not with heavy metals) and not waste the biomass of this plant as is currently the case.











 texto en
texto en 


