Introduction
The genus Schinus (Anacardiaceae) includes species producing essential oils with a pleasant spicy aroma. Some have been subjected to studies about their composition and, among them, S. terebinthifolius Raddi and S. molle L. have been of great interest (dos Santos et al., 2009) because of their insecticidal (Abdel-Sattar et al., 2010), antimicrobial (Chávez-Magdaleno et al., 2018; Scopel et al., 2013), nutraceutical (Guzzo et al., 2019) and allelopathic (Zahed et al., 2010) properties.
S. molle is a native tree found in subtropical regions and can be located in several countries (Awadh et al., 2011; Gomes et al., 2013; Huerta et al., 2010; Phiri et al., 2021; Volpini-Klein et al., 2021). In Mexico, S. molle is known as pirul tree and is a non-cultivated species that is part of the natural landscape in many regions. In several areas of the world, the essential oil of S. molle has been characterized for its composition (Gomes et al., 2013; Pereira et al., 2019; Phiri et al., 2021; Volpini-Klein et al., 2021), which can vary among plant populations (Gomes et al., 2013). In this regard, the phytochemical profile of the essential oil of leaves or fruits of S. molle from Mexico has not been studied and the generation of this knowledge may favor its exploitation.
Essential oils are complex mixtures of volatile compounds produced by some plant species (Franz & Novak, 2016), whose composition is characterized by the presence of terpenes (monoterpenes, sesquiterpenes and diterpenes), alcohols, esters, aldehydes, ketones, amines, oxides and lactones (Shankar et al., 2021). Based on their antimicrobial (Combrinck et al., 2011; Tabassum & Vidyasagar, 2013) and insecticidal (Reyes-Guzman et al., 2012) activities, essential oils have attracted much attention to be used in natural pest and disease control strategies in agricultural and food production (Combrinck et al., 2011). Specifically, there is interest in the development of agents with insect repellent properties (Islam et al., 2017), for the control of disease transmission vectors such as chikungunya and zika (Carpio-Orantes, 2016), insect control in greenhouses (da Camara et al., 2015) and grain stores (Reyes-Guzman et al., 2012), pest control in tree species (Flores-Villegas et al., 2019), control of fungi, bacteria and yeasts causing fruit diseases at postharvest (Rico et al., 2012; Valle-Ortiz et al., 2019) and even for the reduction of viral diseases (Adamski & Adamska, 2021; da Silva et al., 2020).
It has been shown that, in addition to the effect of the tree's development site, the composition of S. molle essential oil varies depending on the time of year when the plant material is collected (Pereira et al., 2019; Volpini-Klein et al., 2021), the extraction method and even the extraction time (Volpini-Klein et al., 2021). On the other hand, essential oils in general and of S. molle more specifically, have been commonly characterized with support of Clevenger apparatus (Chávez-Magdaleno et al., 2018; Gomes et al., 2013; Pereira et al., 2019; Volpini-Klein et al., 2021), but this is a laboratory-level device that contributes small amounts of product and the commercial level requires larger-scale handling. In this regard, Rayleigh batch distillation allows the extraction of essential oils with large volumes (Seader et al., 2011) and, therefore, may have greater potential for the exploitation of aromatic species, by obtaining larger quantities for commercialization. However, the effect of scaling on the chemical composition of the essential oil is unknown. Therefore, the objective of this study was to characterize the phytochemical profile of the essential oil of S. molle leaves collected in the central region of Mexico and treated under different extraction conditions, to improve the potential use of this species.
Materials and Methods
Plant material
S. molle leaves were used, which were collected at the Universidad Autónoma Chapingo (19° 29' 24" N, 98° 53' 26" W, 2 250 m elevation) located in Texcoco, Mexico, during the months of November and December 2018, characterized by average maximum temperature of 22 °C, average minimum temperature of 7 °C, cloudiness between 33 and 44 %, and precipitation between 7 and 24 mm. Leaves were cut in their first weeks of development based on the degree of coloration and free of dirt and damage by physical or biological factors. Dry matter content was determined by oven drying (Thermo Scientific 3478, USA) at 33 °C for 9 days until constant weigh.
Extraction of essential oil
Two extraction systems were used. One was the Clevenger apparatus, where a hydrodistillation procedure was carried out with 100 g of plant material in 450 mL of boiling water. The second apparatus was a Rayleigh-type batch distillation column (Seader et al., 2011), with a depletion section consisting of a 50 L vessel and an enrichment phase consisting of five boiling plates (Figure 1). In this system, 10 L of water were poured into the depletion vessel and steam heating from a boiler was applied to bring the liquid to a near boiling condition. The plant material was fed in 2 kg batches placed in small organza bags.
In both systems, the essential oil was recovered through separation by density difference in a collecting vessel, weighed and stored in airtight containers at 5 °C until further analysis. In the Clevenger apparatus, extraction was performed at two times, 60 and 120 min. In the Rayleigh distillation column, process times of 20, 40, 60, 80, 100 and 120 min were evaluated. Extraction volume and oil composition were determined at each time. All routines were performed in triplicate. The operation in the Clevenger apparatus was used as a reference to evaluate the operation of the Rayleigh distiller.
Evaluating the composition of the essential oil
Gas chromatography equipment (Agilent Technologies® 7890A, USA) coupled to an Ion Tramp 240MS mass selective detector (Agilent Technologies®, USA), fitted with a 30 m long, 0.25 μm inner diameter and 0.25 mm outer diameter VF-5ms capillary column was used. The carrier gas was helium with inlet pressure of 7.91 psi and flow rate of 1 mL∙min-1. Electron impact mass detector with electron emission current of 10 μA and ionization time of 25 ms was used. The oven was programmed at initial temperature of 50 °C for 5 min, with subsequent increase of 5 °C∙min-1 until 150 °C was reached, and finally, the thermal condition was increased at the rate of 20 °C∙min-1 until 250 °C. The temperature in the injector, trap and transfer line was 250 °C. The sample was prepared by diluting 2 µL of essential oil in a final volume of 1 mL of HPLC-grade hexane. Injections were made into the equipment in triplicate. The run time was 30 min. Compounds were identified by comparing retention indices (RI) with literature data and their mass spectra with NIST (National Institute of Standards and Technology) data stored in the computer library of the gas chromatography-mass spectrometry (GC-MS) system. The C8-C22 alkanes (Sigma®) were used as reference points in the calculation of RIs, using the Kovats method (Dool & Kratz, 1963). The results were expressed as fragment ion counts on the mass selective detector.
Data analysis
According to the chromatographic analysis, five groups of terpenic compounds were identified. Yield and relative concentration data in the Clevenger and Rayleigh extracts for extraction times of 60 and 120 min were subjected to analysis of variance in a 22 factorial arrangement, using a completely randomized design, and to tests of comparison of treatment means with Tukey's statistic (P = 0.05). Furthermore, yield data and relative concentration of compounds in the extracts from the Rayleigh equipment, taken at times from 20 to 120 min, were subjected to analysis of variance in congruent form with a 6×5 factorial arrangement in a completely randomized design and to mean comparison routines with Tukey's statistic (P = 0.05), to evaluate the variation of each group of compounds depending on extraction time. All analysis was carried out with the SAS software (SAS Institute Inc., 1999).
Results and Discussion
Extraction yield
S. molle leaves had a dry matter of 35.98 ± 0.41 %. The variability in this characteristic was considered low and thus it was accepted that the plant material was homogeneous. Figure 2 shows that increasing the processing time favored obtaining higher amounts (P ≤ 0.05) of essential oil, which was an expected result that translated into higher extraction yield. The effect of time was greater in the Clevenger apparatus operation, where the yield increased at a rate of 0.0109 %∙min-1, while in the Rayleigh distiller, the change occurred at a rate of 0.0069 %∙min-1. According to Table 1, the extraction yield was similar in the two distillers after completing 60 min of operation, but with 120 min of process, the Clevenger apparatus allowed higher oil recovery efficiency than the Rayleigh distiller, with values of 1.63 and 1.03 %, respectively. For the same extraction time and using Clevenger apparatus, Volpini-Klein et al. (2021) reported 0.6 % oil extraction yield from S. molle leaves collected in Naviraí, Brazil. However, even with higher extraction yields, the Clevenger apparatus has its main application at the laboratory level, for obtaining small samples for qualitative analysis of essential oils. On the contrary, the Rayleigh distiller allows the processing of significantly larger quantities of plant material to provide higher proportions of oil, which can be useful for commercial implementation on a larger scale. If a process time of 2 h is taken as a reference, and based on the data in Table 1, it is estimated that more than 12 extraction routines with the Clevenger apparatus may be required to obtain the same amount of essential oil generated by the Rayleigh distiller.
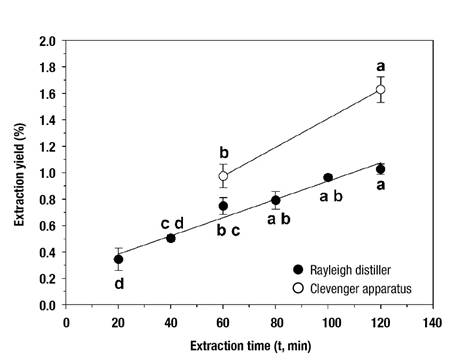
Figure 2 Variation of Schinus molle essential oil extraction yield in Clevenger apparatus and Rayleigh distiller depending on process operation time. Symbols correspond to mean values (n = 3) and the variation bars to standard errors. Different letters indicate significant difference (Tukey, P = 0.05) within the operation of each equipment.
Table 1 Comparison of means of the amount of essential oil extracted and the extraction yield from samples of 100 g and 2.0 kg of Schinus molle leaves subjected to hydrodistillation in Clevenger apparatus and Rayleigh distiller, respectively.
| Time | Devices | HSD | CV (%) | |
|---|---|---|---|---|
| Clevenger | Rayleigh | |||
| Extracted essential oil (g) | ||||
| 60 min | 0.350 ± 0.032 B b | 5.374 ± 0.486 B a | 1.352 | 20.841 |
| 120 min | 0.585 ± 0.034 A b | 7.381 ± 0.603 A a | 1.677 | 18.574 |
| HSD | 0.131 | 1.854 | ||
| CV (%) | 12.411 | 14.874 | ||
| Extraction yield (%) | ||||
| 60 min | 0.974 ± 0.089 B a | 0.746 ± 0.067 B a | 0.311 | 15.951 |
| 120 min | 1.628 ± 0.096 A a | 1.025 ± 0.083 A b | 0.355 | 11.824 |
| HSD | 0.366 | 0.268 | ||
| CV (%) | 12.411 | 14.874 | ||
Based on Tukey's honest significant difference (HSD) (P = 0.05), different capital letters in a column indicate significant difference depending on time and different small letters in a row indicate significant difference between teams. CV: coefficient of variation. ± standard error of the mean (n = 3).
Essential oil composition
The essential oils were analyzed qualitatively by GC-MS. Figure 3 shows one of the chromatograms corresponding to a sample of the extract obtained in the Clevenger apparatus with 120 min of processing. The relative percentages of the components identified in the essential oil of S. molle leaves are shown in Table 2 for the two devices used and for different extraction times. A total of 37 compounds were found, of which 31 were present in the oil obtained with the Rayleigh distiller. Four of the compounds found only in the Clevenger extract, labeled with Roman numerals in Figure 3 and Table 2, were obtained at both extraction times (60 and 120 min); however, their identity was not determined, and they were compounds present in low proportion. The other two compounds were also obtained only with the Clevenger distiller with extraction time of 120 min. One of them was labeled with the letter a and identified as γ-elemene. The other, labeled b, was not precisely identified and was categorized as an oxygenated sesquiterpene. Thus, in terms of number of compounds, the phytochemical profile of the essential oil with Rayleigh distiller, operated for 60 min, was 88.6 % similar to that obtained with Clevenger distiller, which constitutes the reference system, and 83.8 % similar with an operation time of 120 min. On the other hand, the similarity was 96. 2 % for an extraction time of 60 min, since the compounds labeled with Roman numeral had low concentration, but it was reduced to 87.2 % with a time of 120 min, which because the compound labeled with the letter b and identified as an oxygenated sesquiterpene, had a participation of 10.25 % in the oil. In this sense, if the Rayleigh equipment constitutes a modality that could be used in areas of S. molle development, due to the potential for extraction of larger quantities of essential oil, it will be necessary to address three aspects: first, verify the identity of the compounds that were only obtained with the Clevenger distiller; second, evaluate the biological activity of these compounds to determine the importance of their presence in the extracted oil; and, third, improve the operation of the Rayleigh distiller to increase the similarity with the oil obtained with the Clevenger distiller.
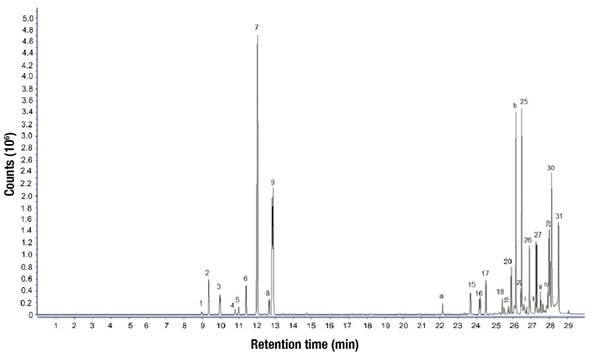
Figure 3 Chromatogram by gas chromatography-mass spectrometry (GC-MS) of Schinus molle essential oil extracted with Clevenger apparatus in a 120 min process. The Arabic numerals correspond to compounds also detected with Rayleigh distiller. Roman numerals were assigned to compounds identified only in Clevenger apparatus extract. Letters a and b indicate compounds recorded only with 120 min operation in Clevenger apparatus. The identity of the numbered compounds is specified in Table 2.
Table 2 Relative composition (%) of the essential oil of Schinus molle leaves using two distillation systems and different extraction times.
| Number | Compound | Formule | RI cal | 20 min® | 40 min® | 60 min® | 60 min© | 80 min® | 100 min® | 120 min® | 120 min© |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Tricyclene | C10H16 | 923 | 1.327 (0.356) | 0.880 (0.318) | 1.103 (0.252) | 0 (0) | 1.230 (0.309) | 1.287 (0.415) | 1.153 (0.352) | 0.170 (0.057) |
| 2 | α-pinene | C10H16 | 934 | 7.287 (0.815) | 6.120 (0.622) | 6.713 (0.552) | 3.593 (0.528) | 6.940 (0.239) | 7.357 (0.615) | 7.210 (0.516) | 2.723 (0.393) |
| 3 | Camphene | C10H16 | 951 | 7.097 (1.649) | 5.250 (1.657) | 6.393 (1.149) | 2.030 (0.573) | 7.250 (1.632) | 7.447 (2.096) | 7.130 (1.735) | 1.327 (0.453) |
| 4 | Sabinene | C10H16 | 974 | 1.453 (0.124) | 1.263 (0.092) | 1.400 (0.050) | 0.663 (0.087) | 1.410 (0.072) | 1.147 (0.240) | 1.050 (0.078) | 0.290 (0.127) |
| 5 | β-pinene | C10H16 | 979 | 2.693 (0.622) | 1.990 (0.554) | 2.390 (0.369) | 0.843 (0.201) | 2.657 (0.584) | 2.810 (0.725) | 2.710 (0.565) | 0.460 (0.211) |
| 6 | β-myrcene | C10H16 | 990 | 3.897 (0.454) | 5.320 (1.066) | 4.147 (0.620) | 3.133 (0.488) | 3.767 (0.627) | 3.707 (0.132) | 3.580 (0.555) | 2.393 (0.315) |
| 7 | α-phellandrene | C10H16 | 1 008 | 25.030 (1.898) | 22.903 (1.292) | 22.50 (0.239) | 31.293 (2.468) | 24.94 (0.408) | 22.827 (0.815) | 24.283 (1.744) | 27.320 (2.301) |
| 8 | o-cymene | C10H16 | 1 026 | 1.593 (0.139) | 1.773 (0.112) | 1.753 (0.127) | 1.587 (0.223) | 1.613 (0.256) | 1.447 (0.069) | 1.557 (0.158) | 1.303 (0.218) |
| 9 | Limonene + β-felandrene | C10H16 | 1 030 | 23.377 (1.523) | 23.037 (0.990) | 23.423 (0.311) | 6.987 (0.521) | 24.493 (1.196) | 23.690 (0.616) | 23.087 (2.541) | 8.583 (0.697) |
| 10 | γ-terpinene | C10H16 | 1 060 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.463 (0.153) | 0 (0) |
| 11 | α-terpinolene | C10H16 | 1 087 | 0 (0) | 0.273 (0.007) | 0.300 (0.015) | 0 (0) | 0.283 (0.019) | 0.303 (0.015) | 0 (0) | 0 (0) |
| 12 | Anethole | C10H12O | 1 300 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.850 (0.312) | 5.520 (3.475) | 0 (0) |
| 13 | Sesquiterpene (Ni) | C15H24 | 1 390 | 0.310 (0.038) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.823 (0.672) | 5.573 (1.156) | 0 (0) |
| a | γ-elemene | C15H24 | 1 436 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.733 (0.095) |
| 14 | γ-muurolene | C15H24 | 1 480 | 0.780 (0.460) | 0.353 (0.029) | 0.380 (0.012) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 15 | δ-guaiene | C15H24 | 1 493 | 1.380 (0.102) | 1.587 (0.168) | 1.407 (0.139) | 1.593 (0.417) | 1.443 (0.060) | 1.183 (0.156) | 0.953 (0.078) | 1.567 (0.113) |
| 16 | Viridiflorene | C15H24 | 1 514 | 2.487 (0.655) | 1.833 (0.091) | 1.773 (0.154) | 1.467 (0.079) | 1.723 (0.134) | 1.427 (0.231) | 1.107 (0.115) | 1.210 (0.103) |
| 17 | Sesquiterpene (Ni) | C15H24 | 1 529 | 1.943 (0.197) | 2.063 (0.105) | 1.987 (0.117) | 2.483 (0.269) | 2.100 (0.086) | 1.740 (0.227) | 1.573 (0.075) | 2.563 (0.185) |
| 18 | Germacrene B | C15H24 | 1 569 | 0.803 (0.049) | 0.890 (0.055) | 0.840 (0.067) | 2.397 (1.303) | 0.840 (0.025) | 0.690 (0.104) | 0.557 (0.041) | 0.917 (0.061) |
| 19 | Sesquiterpene(Ni) | C15H24 | 1 573 | 0.520 (0.025) | 0.560 (0.023) | 0.547 (0.030) | 0 (0) | 0.530 (0.035) | 0.517 (0.079) | 0.443 (0.047) | 0.530 (0.053) |
| 20 | Sesquiterpene O (Ni) | C15H26O | 1 591 | 4.903 (0.513) | 4.867 (0.927) | 4.557 (0.686) | 2.233 (1.275) | 3.473 (0.919) | 2.553 (0.154) | 0 (0) | 2.350 (0.131) |
| 21 | Sesquiterpene O (Ni) | C15H26O | 1 600 | 0 (0) | 3.173 (0.802) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 22 | Sesquiterpene O (Ni) | C15H26O | 1 655 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3.593 (0.859) | 0 (0) | 0 (0) |
| 23 | Sesquiterpene O (Ni) | C15H26O | 1 664 | 0 (0) | 0 (0) | 3.400 (0.647) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| b | Sesquiterpene O (Ni) | C15H26O | 1 672 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 10.250 (1.073) |
| 24 | Sesquiterpene O (Ni) | C15H26O | 1 719 | 0 (0) | 0.573 (0.043) | 0.650 (0.097) | 0 (0) | 0.693 (0.009) | 0.747 (0.107) | 0 (0) | 0.797 (0.081) |
| 25 | Sesquiterpene O (Ni) | C15H26O | 1 724 | 3.430 (0.248) | 4.02 (0.149) | 4.027 (0.137) | 8.097 (0.613) | 4.177 (0.118) | 4.203 (0.373) | 3.663 (0.511) | 8.343 (0.462) |
| I | Uc | 1 734 | 0 (0) | 0 (0) | 0 (0) | 0.853 (0.078) | 0 (0) | 0 (0) | 0 (0) | 0.300 (0.070) | |
| 26 | Uc | 1 760 | 2.550 (0.484) | 3.737 (0.179) | 3.183 (0.213) | 10.723 (0.621) | 3.387 (0.659) | 2.280 (0.467) | 3.017 (0.645) | 4.487 (0.417) | |
| II | Uc | 1 786 | 0 (0) | 0 (0) | 0 (0) | 0.437 (0.047) | 0 (0) | 0 (0) | 0 (0) | 0.377 (0.019) | |
| 27 | Uc | 1 791 | 1.897 (0.172) | 2.417 (0.382) | 2.060 (0.338) | 4.417 (0.247) | 2.097 (0.041) | 1.733 (0.181) | 1.507 (0.122) | 2.953 (0.181) | |
| 28 | Uc | 1 909 | 0 (0) | 0.337 (0.032) | 0.313 (0.028) | 1.077 (0.193) | 0.3 (0) | 0 (0) | 0 (0) | 1.013 (0.099) | |
| III | Uc | 1 921 | 0 (0) | 0 (0) | 0 (0) | 0.583 (0.050) | 0 (0) | 0 (0) | 0 (0) | 0.443 (0.047) | |
| IV | Uc | 1 950 | 0 (0) | 0 (0) | 0 (0) | 1.960 (1.041) | 0 (0) | 0 (0) | 0 (0) | 0.717 (0.090) | |
| 29 | Uc | 1 960 | 0.920 (0.253) | 0.963 (0.054) | 1.003 (0.050) | 0 (0) | 0.957 (0.103) | 1.107 (0.159) | 0.930 (0.155) | 5.250 (0.462) | |
| 30 | Uc | 1 974 | 1.270 (0.321) | 1.313 (0.089) | 1.357 (0.087) | 6.543 (0.792) | 1.270 (0.136) | 1.437 (0.207) | 1.407 (0.130) | 7.230 (0.650) | |
| 31 | Uc | 2 016 | 3.057 (0.714) | 2.513 (0.284) | 2.39 (0.336) | 5.003 (0.270) | 2.423 (0.078) | 2.087 (0.365) | 1.517 (0.123) | 3.407 (0.405) |
Values in parentheses correspond to standard error of the mean (n = 3). RI cal: calculated retention index. Sesquiterpene O: oxygenated sesquiterpene. Uc: unidentified compound. ©Clevenger apparatus. ® Rayleigh distiller
Table 3 Relative concentration (%) of terpenic groups detected in Schinus molle leaf essential oil, using two distillation equipment and different extraction times.
| Compounds | 20 min® | 40 min® | 60 min® | 60 min© | 80 min® | 100 min® | 120 min® | 120 min© |
|---|---|---|---|---|---|---|---|---|
| Monoterpenes | 73.753 (2.720) | 68.803 (1.330) | 70.120 (1.040) | 50.140 (4.249) | 74.580 (0.305) | 72.017 (3.150) | 72.230 (2.477) | 74.567 (4.319) |
| Oxygenated monoterpenes | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.850 (0.312) | 5.520 (3.475) | 7.650 (0.693) |
| Sesquiterpenos | 8.223 (1.396) | 7.290 (0.411) | 6.937 (0.50) | 7.937 (1.077) | 6.633 (0.249) | 7.387 (0.861) | 10.207 (1.127) | 0 (0) |
| Oxygenated sesquiterpenes | 8.333 (0.74) | 12.630 (0.207) | 12.633 (0.576) | 10.330 (1.61) | 8.343 (0.851) | 11.10 (1.408) | 3.663 (0.511) | 21.740 (1.667) |
| Other | 9.687 (0.772) | 11.277 (0.782) | 10.307 (0.803) | 19.880 (2.697) | 10.440 (0.716) | 8.643 (1.220) | 8.380 (0.779) | 26.177 (2.287) |
Values in parentheses correspond to standard error of the mean (n = 3). ©Clevenger apparatus. ®Rayleigh distiller.
Regarding the compounds found in the oils extracted with the two devices, 16 were fully identified. From the rest, nine were not precisely identified, but were catalogued within the group of sesquiterpenes (three) and oxygenated sesquiterpenes (six) based on molecular weight. In general, five groups of terpene compounds were identified: monoterpenes, oxygenated monoterpenes, sesquiterpenes, oxygenated sesquiterpenes and other compounds; Table 3 shows their relative composition under the extraction conditions evaluated. According to Table 4, the statistical analysis showed differences in the overall relative participation of each group. Among the identified compounds, the group with the highest presence was monoterpenes (66.16-78.40 %), followed by oxygenated sesquiterpenes, then by sesquiterpenes and, finally, by oxygenated monoterpenes in small concentration. Similarly, Volpini-Klein et al. (2021) identified monoterpenes as the main group present, followed by sesquiterpenes, in S. molle essential oil from leaves collected in Naviraí, Brazil. However, Phiri et al. (2021) found that the sesquiterpenes group was in higher proportion than monoterpenes in S. molle leaves collected in Palapye, Botswana, confirming that the composition may vary between regions
Table 4 Mean terpenic composition of Schinus molle essential oil obtained in Rayleigh distiller.
| Compounds | Concentration (%) |
|---|---|
| Monoterpenes | 71.92 a |
| Oxigenated monoterpernes | 1.06 d |
| Sesquiterpenos | 7.78 c |
| Sesquiterpenos oxigenados | 9.45 bc |
| OT | 9.79 b |
| HSD | 1.89 |
| CV (%) | 9.99 |
Different letters associated with the means indicate significant difference between concentrations according to Tukey's honest significant difference (HSD) (P = 0.05). CV: coefficient of variation. OT: other unidentified compounds.
The operation of the Rayleigh distiller was evaluated in ranges from 20 to 120 min. This factor did not affect the relative presence of terpenic compounds, although the statistical analysis showed a significant interaction between the variation factors, given by extraction time and type of compound (Table 3). The reason for this is that monoterpenes and sesquiterpenes showed no change in their relative presence depending on the extraction time, but oxygenated monoterpenes and oxygenated sesquiterpenes did. Oxygenated monoterpenes were only identified in extracts with 100 and 120 min of processing. In the case of the oxygenated sesquiterpenes, there were differences between extraction times, but no clear trend was actually observed, although the content in the samples with 120 min of processing was noticeably lower. On the other hand, the relative presence of unidentified compounds showed no variation depending on the extraction time.
Although the monoterpenes group was the most present in all extraction times, the identification of the second and third groups had a very variable behavior. The samples corresponding to extraction times between 20 and 100 min had the oxygenated sesquiterpenes as the second most important group (6.81-13.85 %) and the hydrocarbonated sesquiterpenes as the third (5.6-10.86 %). However, in the 120 min extraction routine, the order of presence was reversed; the group with the highest presence was the hydrocarbonated sesquiterpenes (8.76-12.43 %) and the third group was the oxygenated sesquiterpenes (2.67-4.37 %). The significant presence of the oxygenated monoterpenes group was detected after 100 min of extraction, with anethole being the only identified compound of this type. The compounds with the highest presence were α-phellandrene (21.20-28.57 %), limonene + β-phellandrene (19.07-27.78 %), camphene (3.32-9.93 %), α-pinene (5.74-8.56 %), β-myrcene (2.95-6. 91 %) and β-pinene (1.31-3.55 %) which together accounted for 62.09 to 73.36 % of the total essential oil, all belonging to the group of hydrocarbon monoterpenes.
The presence of compound b, obtained after 120 min of extraction, stands out in the case of the essential oil extracted with a Clevenger distiller. This was catalogued as an oxygenated sesquiterpene; however, although it had a participation of 10.25 %, the confirmation of its identity requires more research work. The results partially agreed with available information from the Mograne region (northeastern Tunisia), where the main components of S. molle leaf essential oil were α-phellandrene, limonene + β-phellandrene, myrcene, and α-pinene (Zahed et al., 2010). Also, Phiri et al. (2021) found limonene, elemol, α-phellandrene and aromadendrene as the main compounds in leaves collected in Palapye, Botswana. On the other hand, Ennigrou et al. (2011) reported α-phellandrene, β-phellandrene and α-pinene as major compounds in the essential oil of S. molle leaves collected in El Ghazala, northern Tunisia. In Algeria, S. molle essential oil presented α-phellandrene, β-phellandrene, elemol and limonene (Belhamel et al., 2009). In the region of Évora, southeastern Portugal, Martins et al. (2014) reported α-phellandrene, limonene, β-phellandrene, β-myrcene and α-pinene as main compounds in S. molle essential oil. Other studies point to more notable qualitative differences; Abdel-Sattar et al. (2010) reported p-cymene, α-terpinene and β-pinene as the main volatile compounds in S. molle leaf essential oil and, in Porto Alegre, Brazil, Pawlowski et al. (2012) reported that two S. molle varieties had α-pinene as the major compound and also found limonene and β-pinene. In Tacares, Costa Rica, Díaz et al. (2008) identified β-pinene and α-pinene as majority compounds.
Therefore, in the present study, the main components of the essential oil of S. molle leaves collected in the central region of Mexico were similar to the phytochemical profiles reported in Tunisia and Portugal. The compound γ-terpinene was detected up to 120 min of extraction; α-terpinolene (0.25-0.32 %), in the range of 40 to 100 min; and γ-muurolene (0.28-1.70 %) only in the range of 20 to 60 min. The group with the greatest variation was oxygenated sesquiterpenes, where unidentified compounds (compounds 21, 22, 23; Figure 2) were only detected at one extraction time, suggesting that this factor had no influence for these compounds and such inconsistency requires further experimental evaluation for clarification. Unidentified compounds accounted for 8.38 to 11.28 % of the total oil.
Ennigrou et al. (2011) mentioned that the chemical composition of S. molle leaf essential oil varies considerably depending on the genetic origin, crop origin, season, plant part analyzed, and extraction methods. In a similar way, Volpini-Klein et al. (2021) showed that the composition depends on the time of collection of the plant material, but also on the method and time of extraction. On the other hand, it has been suggested that the differences in essential oil composition patterns are probably due to the generation of several metabolic pathways in the secondary metabolism of S. mollce (Zahed et al., 2010).
Moreover, the number of compounds from the group of oxygenated sesquiterpenes decreased for the extraction time of 120 min, because of the six possible compounds detected, only the one identified with the number 25 was recorded (Figure 2). Lucchesi et al. (2004) mentioned losses of volatile compounds and degradation of unsaturated or ester compounds due to thermal or hydrolytic effects at the time of extraction of essential oils using methods such as hydrodistillation, steam distillation and Soxhlet extraction. These authors also pointed out that the reduction in extraction time means that some compounds are not extracted, but that degradation by hydrolysis, transesterification or oxidation is minimized and, therefore, fewer degradation products are observed in the profile. In the present study, the presence of anethole was detected only in the longer extraction times of 100 and 120 min. In this regard, the 120 min times were the first to be used for extraction, so that contamination with the components of another residual essential oil in the distillation column cannot be ruled out. On the other hand, although the use of Rayleigh distillation allows obtaining an essential oil very similar to that obtained with the Clevenger apparatus, it will be necessary to complete the identification of the compounds missing in the larger scale operation to assess the impact on the biological activity of the product obtained.
Conclusions
The essential oil of Schinus molle extracted with a Rayleigh-type batch distillation column was characterized and compared with essential oil collected with a Clevenger distiller. The operating time of the equipment affected the composition of the essential oil. The scaling up of the process carried out through the Rayleigh distiller allowed obtaining a greater amount of oil, but with lower yield and lower number of compounds. The similarity of the phytochemical profile between the extracts of both equipment varied between 87.2 and 96.2 % depending on the operation time. The group with the highest presence in the essential oil was hydrocarbon monoterpenes. The compounds with the highest presence were α-phellandrene and limonene + β-phellandrene.











 texto en
texto en 




