The genus Amaranthus is important since it produces edible seeds with high nutritional value. Amaranthus hypochondriacus and A. cruentus are native and distributed throughout Mexico and Guatemala. The taxon Amaranthus and others such as Opuntia, Myrtillocactus, Chenopodium, Phaseolus and Zea have been important in different periods of history. This group of plants was the food base for the inhabitants of the center of Mexico during the pre-Hispanic period (McClung de Tapia et al. 2014, Janssen et al. 2017).
Among the pseudocereals, amaranths are considered to have great potential to reduce malnutrition problems, especially in developing countries, and are promising crops for coming decades with global food security in mind. They are able to adapt to poor soils, tolerate drought and salinity, and have high nutritional value, functional properties and a wide diversity of uses. In the last decades the nutritive potentialities and other unique qualities of amaranths have been highlighted around the world, however, they have not regained their place as a basic and strategic crop (Das 2016, Espitia-Rangel 2016). The grain is the principal commercial product of A. hypochondriacus, thus it is important to develop information about the ontogeny of the amaranth seed and its reproductive biology.
In spite of its high nutritional value, which is particularly due to the exceptional seed protein and amino acid balance and its ability to grow in difficult environmental conditions, there are no studies describing female gametogenesis and early seed development in relation to female gametophyte and embryo development, respectively, in A. hypochondriacus. Only two articles describe poorly the structure of mature embryo sac and structure of the seed (Pal et al. 1990, Coimbra & Salema 1999). Conversely, there is extensive literature about the properties of the Amaranthaceae seeds, covering a variety of aspects including chemical characterization of proteins and agronomic studies (Pal et al. 1990, Coimbra & Salema 1994, 1997, Silva-Sánchez et al. 2008, Morales-Guerrero et al. 2009, Huerta & Barba de la Rosa 2012, Huerta et al. 2012).
The development of the seeds is not synchronized in all inflorescence parts, due to the indeterminate growth of the amaranth inflorescence. An analysis was performed to find out how the position influences the size of the ovule and ovary. The aim of the present study was to analyze the female gametophyte and early seed development of amaranth, to fill the gaps in previous reports and generate information that could be used as baseline to identify morphological changes or for additional research.
Materials and methods
The life cycle of amaranth plants grown in greenhouse conditions was completed in 70-90 days. After anthesis, the reproductive stage (from anthesis to physiological maturity) was completed in 30 days (under field conditions the life cycle is completed in 130 days).
Plant material. We studied the reproductive biology of Amaranthus hypochondriacus L ’Revancha’. Plants were grown in pots containing 1 kg of substrate (peat moss:perlite:vermiculite 2:1:1 v/v) in a greenhouse, where the minimum and maximum temperatures were 11 ± 2 and 27 ± 2 °C. Pistillate flowers (PF) were collected before the staminate flower (SF) released pollen and two weeks after anthesis. Ovules of PF were collected during two years and approximately 25 ovules of each stage were observed for morphological studies and statistical analyses.
Variations of the ovary and seed, by branch and inflorescence position. In order to correlate ovule size with its developmental stage, these were measured at different stages. Inflorescences were divided into three sections: basal (ST1), middle (ST2) and apical (ST3) according to the procedure described by Olufolaji & Odeleye (2011), also three branches were separated into basal (BR1) middle (BR2) and apical (BR3) sections (Figure 2S). Ovary length, ovule length and ovule width were measured in each of the inflorescence positions. The data were analyzed using a randomized complete block design (three inflorescence and three branch sections) using three replicates for each treatment, using the GLM procedure of the SAS; the comparisons of means used Tukey’s honestly significant difference (HSD) test (P < 0.05). Twenty-five ovules of each stage were observed for female gametophyte development with their respective ovule dimensions.
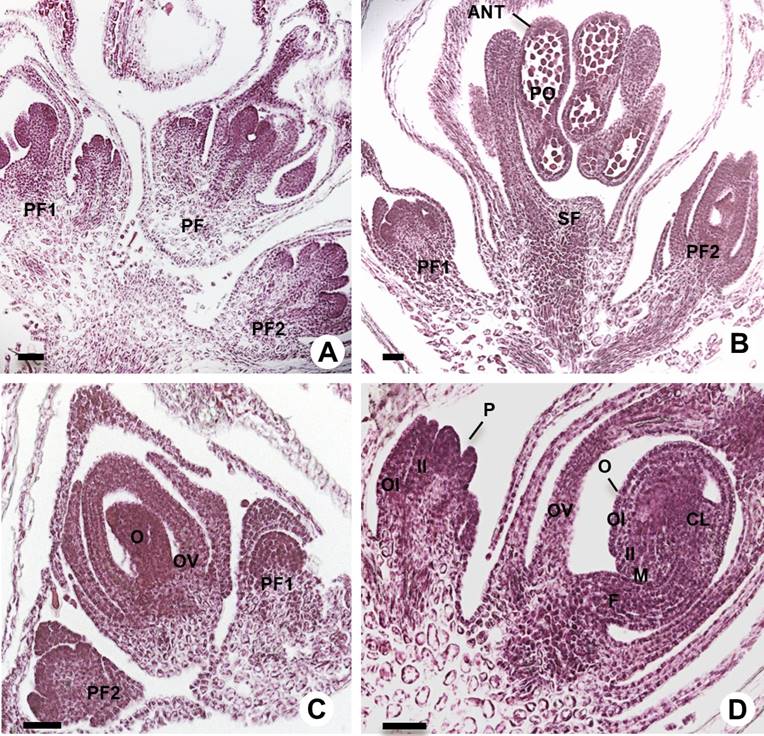
Figure 1 Longitudinal sections of glomerules and reproductive structures of amaranth. A-B: Glomerules of amaranth showing staminate (SF) and pistillate flowers (PF), SF showed anthers (ANT) with pollen (PO). C-D: Early stages of ovule (O) development inside the ovary (OV) with inner (II), outer integuments (OI), ovule primordium (P), nucellus (N), chalaza (CL), micropyle (M) and funicle (F). Scale bars = 50 µm.
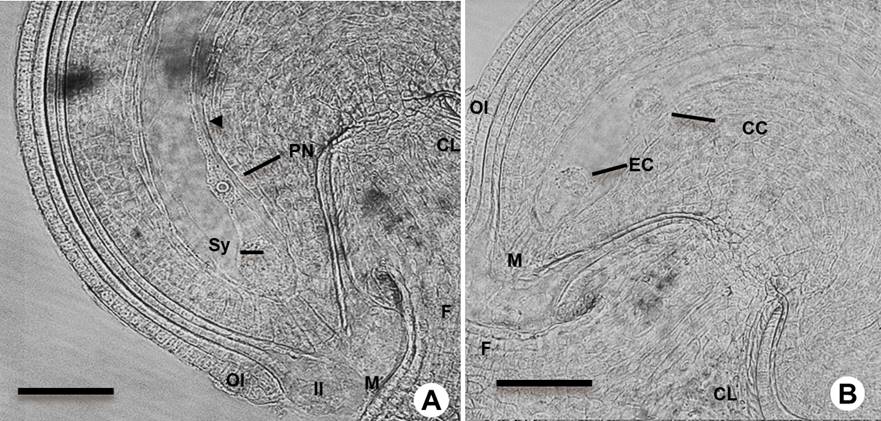
Figure 2 Mature ovules before fertilization in Amaranthus hypochondriacus. A: Mature campylotropous ovule type before fertilization under light microscopy showing the polar nuclei (PN) and the synergid cells (Sy). B: Ovule at mature female gametophyte (FG) stage showing the egg cell (EC) and central cell (CC). Outer integument (OI), inner integument (II); micropyle (M), chalaza (CL) and funiculus (F). Scale bars = 50 µm.
Histological analysis. Glomerules were fixed in FAA solution (18:1:1 ethanol: formaldehyde: acetic acid), placed under vacuum three times for 5 minutes and left in that solution for 24 h at 4 °C. After fixing, the samples were dehydrated through an ethanol series (35, 50, 75, 80, 90 and 96 % ethanol). The ethanol was gradually replaced with xylene (3:1, 1:1, 1:3 v/v) up to 100 % xylene, then the samples were embedded in Paraplast wax (Leica); samples remained in 100 % Paraplast for 24 h (Johansen 1940). Sections (8-12 µm) were cut with a microtome (Leica), mounted on glass slides and stained with hematoxylin-eosin.
Clarification. Ovules and immature seeds were fixed for 24 h in FAA solution. After fixing, the ovules and immature seeds were dehydrated in an ethanol series up to 96 % ethanol. Ovules were cleared through a series of methyl salicylate-ethanol solutions in proportions of 3:1 (for 24 h), 1:1 (for 2 h), 1:3 (for 2 h) up to 100 % methyl salicylate (Salinas-Gamboa et al. 2016). They were incubated at 4 °C from 5 to 25 days and observed with a Leica microscope (Wetzlar, Germany), under Nomarski optics.
Results
Effect of the position on the branch and inflorescence on ovule and ovary size. PF contains an ovary with one ovule, short style and three long stigmas (Figures 1C, D, 2S). After 71 days of planting, most gametes were fertilized and the seed stages ranged from early embryogenesis to grain filling (Figure 1S, Table 1). The ovules from the basal and middle sections were larger than the ovules from apical sections; only ovary length was not affected by the position on inflorescence (Figures 1S, 2S Table 2).
Table 1 Ovule size and female gametophyte at different development stages Amaranthus hypochondriacus. The values represent 25 replicates for each stage of development evaluated.
| Development stage | Ovule length (mm) |
SD | Ovule width (mm) |
SD |
|---|---|---|---|---|
| Megasporogenesis and megagametogenesis | < 0.123 | 0.042 | < 0.191 | 0.059 |
| Mature ovule | 0.263 | 0.044 | 0.299 | 0.055 |
| From early globular embryo to early cotyledon stage | 0.890 | 0.169 | 0.851 | 0.166 |
| Seed in plant | 1.628 | 0.089 | 1.515 | 0.058 |
| Mature seed | 1.392 | 0.053 | 1.352 | 0.050 |
Table 2 Effect of the panicle and branch position on ovule and ovary size in Amaranthus hypochondriacus.
| Parameters | Inflorescence position | Branch position | ||||
|---|---|---|---|---|---|---|
| ST1 | ST2 | ST3 | BR1 | BR2 | BR3 | |
| Ovary length (mm) (OL) | 0.947 a | 1.029 a | 1.008 a | 1.160 a | 1.035 a | 0.804 b |
| Ovule length (mm) (OvL) | 0.642 ab | 0.693 a | 0.53 b | 0.773 a | 0.649 b | 0.449 c |
| Ovule width (mm) (OvW) | 0.641 a | 0.691a | 0.496 b | 0.737 a | 0.629 a | 0.467 b |
Values with different letters in a row are significantly different. Measurements at basal (ST1 or BR1), middle (ST2 or BR2) and apical (ST3 or BR3) position.
Inflorescences. The glomerules are dichasial cymes; the first flower is a single terminal SF on the branch on either side; the second and third PF are terminal on the secondary branches (Figures 1A, B, 2S). Well-developed SF with five mature anthers containing pollen grains was observed. These flowers finished development before the PF on the same branch (Figure 1B). The development of the central PF was not synchronized with others PF, we observed PF with ovules that are developing while others already have developed ovules (Figure 1C, D).
Type of ovule. Each A. hypochondriacus ovary contains only one ovule; before pollination, when the stigmas were closed (Figures 1C, D, 2S), the nucellar tissue and the integuments were still developing and growing. The amaranth ovule is of the campylotropous type, in which the micropyle is directed toward the chalaza. The chalaza is situated at a right angle to the funiculus. The young ovule was transiently observed as anatropous (Figure 2). During its development the micropyle of this ovule is oriented towards the funicle by the curvature of the nucellus (Figure 2A, B).
Megasporogenesis and megagametogenesis. Megasporogenesis began when in the apical region of the nucellus a subepidermal cell developed as an archesporial cell (AR, Figure 3A, B), which will give rise to the megaspore mother cell (MMC) or megasporocyte (Figure 3C, D). After the MMC undergoes meiosis, it gives rise to four haploid megaspore cells (Figure 3E). At the end of the meiosis (Figure 3F), the megaspores located in the chalazal end die, while the micropylar becomes the functional megaspore (FM).
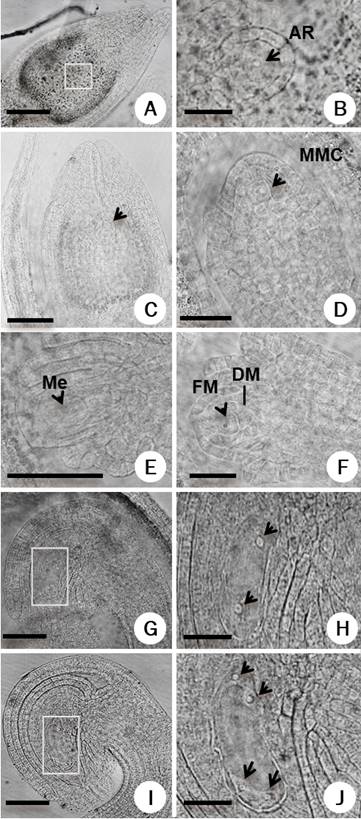
Figure 3 Megasporogenesis-megagametogenesis in Amaranthus hypochondriacus. A-B: Early stages of megasporogenesis showing archespore cell (AR), C-D: megaspore mother cell (MMC), (E) tetrad of megaspores (Me), (F) functional megaspore (FM) and degenerated megaspore cells (DM). G-H: Female gametophyte at two nuclei stage, I-J: four nuclei stage; nuclei are shown with arrows. Panels B, H and J are close-up of the rectangle in the panels A, G and I, respectively. Scale bars = 50 µm in A, C, E, G, I; 25 µm in B, H, J and 20 µm in D, F.
Megagametogenesis begins when the FM becomes enlarged, starting the female gametophyte development. We identified the initial coenocytic phase, in which three rounds of nuclear mitosis occur without cytokinesis. This phase starts as a mononuclear female gametophyte that undergoes a nuclear division followed by the appearance of a large vacuole. The two nuclei resulting from this division migrate to the opposite poles (Figure 3G, H).
The second round of nuclear division occurs, resulting in two nuclei at each pole (Figure 3I, J). After a third and final nuclear division, one nucleus of each pole migrates to the central zone to form the binuclear central cell. During cell formation at the chalazal end, the first cellularization event was observed for two of the nuclei (Figure 4A) and at the mature female gametophyte stage three cells were differentiated as the antipodal cells (Figure 4B). At the distal or micropylar pole the two synergids and the egg cell were formed. The three cells constitute the egg apparatus. One of the synergid cells undergoes programmed cell death before the pollen tube reaches the micropyle (Figure 4D). The central cell is the largest cell of the female gametophyte. At the early stage of development the two polar nuclei fused, giving rise to the diploid central cell (Figure 4C, D).
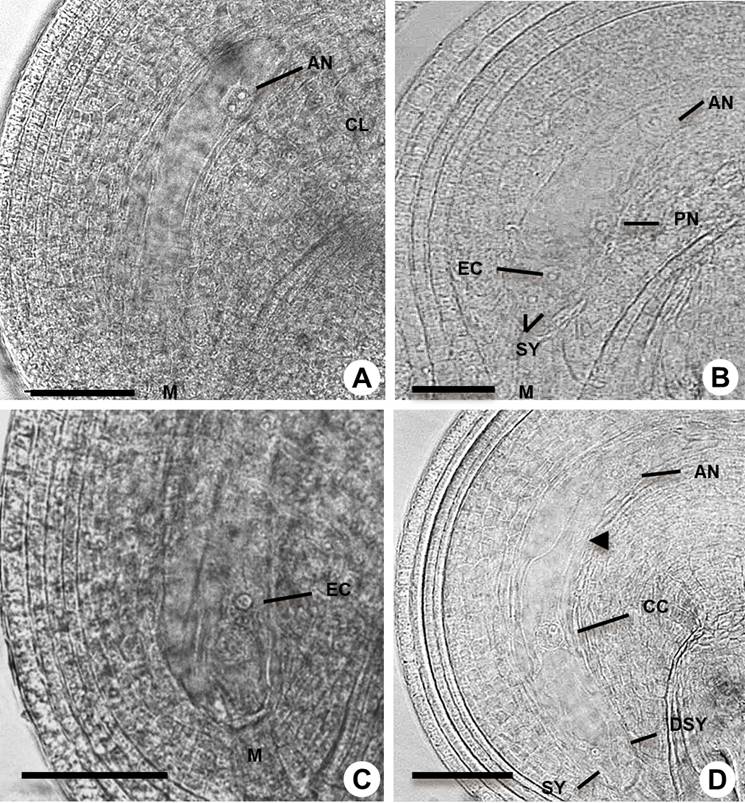
Figure 4 Megagametogenesis in Amaranthus hypochondriacus. A: Cellularization at the chalaza pole (CL). B: Detail of mature female gametophyte with antipodals (AN), egg cell (EC), synergid (SY) polar nuclei (PN) and micropyle (M). C: Cellularization at the micropyle pole. D: Female gametophyte after degeneration of one synergid (DSY) showing the central cell (CC); arrowhead shows the structure that allows free nucleus migration. Scale bars = 50 µm in A, C, D and 25 µm in B.
Embryogenesis. After the double fertilization, the initial stage is characterized by rapid cell division and the segregation of developmental programs in the descendants of the zygote (Figure 5) that form the embryo and the suspensor (Figure 6). The fertilized egg cell began to elongate and differentiate into the zygote. We observed that one of the synergids persists after fertilization (Figure 5A). The first division of the zygote was transverse (Figure 5B) and gave rise to a basal cell and an apical cell (Figures 5C, 1S), which became the suspensor and embryo, respectively. The suspensor underwent cell divisions to the four cell stage before the linear proembryo underwent its first division (Figures 5D, E). The endosperm and the perisperm were differentiated at this stage (Figures 5F, 1S, Table 1S), forming the structure that allows the growth of the embryo. The first embryo division was longitudinal, (Figures 5E, 6A, 6B, 3S), while the second division was transverse; the hypophysis was defined when the suspensor was at the seven-cell stage (Figures 6A, B, 1SA, 1SB, Table 1S).
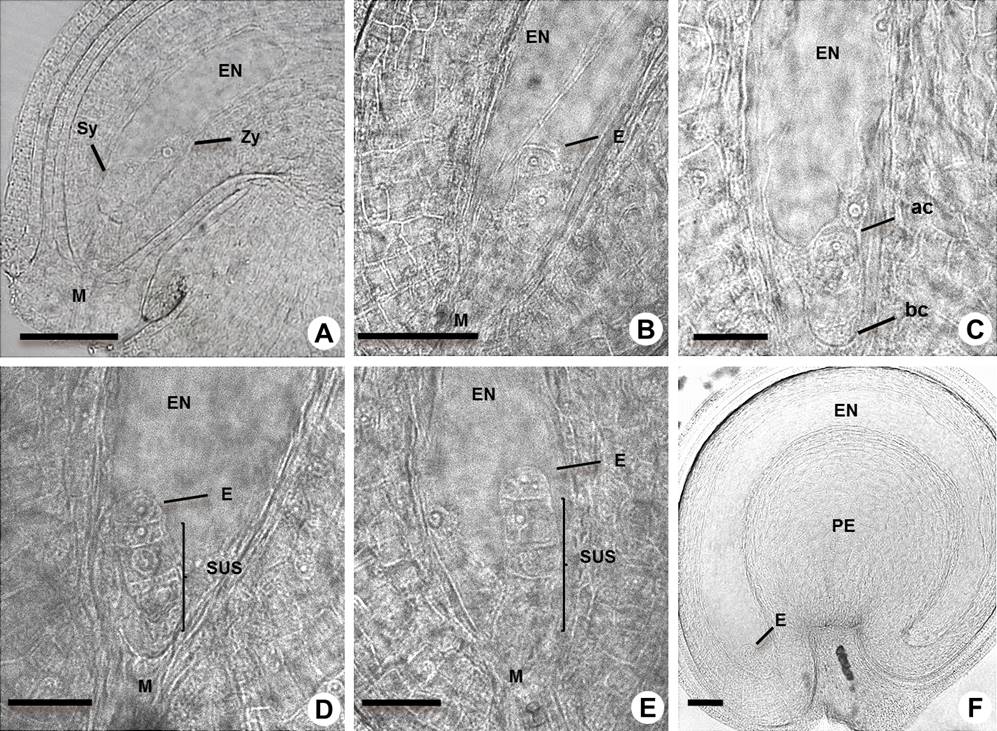
Figure 5 Early-stage embryos in Amaranthus hypochondriacus. A: Detail of elongated zygote (Zy), endosperm (EN), synergid (Sy) and micropyle (M). B-C: One cell stage and differentiation of the basal cell (bc) and apical cell (ac) of embryo (E). D: One cell stage and suspensor (SUS) at the two cell stage of embryo. E: Embryo starts the first division and the suspensor at the three-cell stage. F: Linear embryo in the seed which shows the perisperm (PE). Scale bars = 50 µm in A, B, F, and 25 µm in C, D, E.
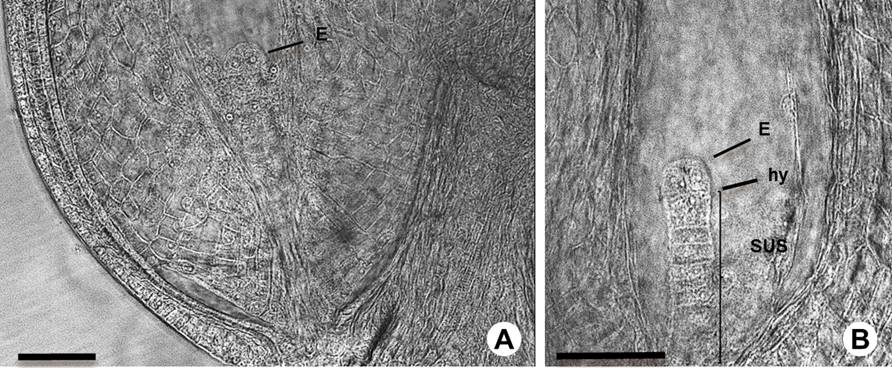
Figure 6 Early embryogenesis in Amaranthus hypochondriacus. A: Embryo (E) at two-cell stage. B: Embryo at 2-4 cell transition stage showing the suspensor (SUS) and hypophysis (hy). Scale bars = 50 µm.
Cell division and differentiation continued in the embryo proper, which developed into an early to late globular embryo (Figures 7A-E). Following the four-cell stage the embryo underwent another division to form the octant stage, and with this the early globular stage began (Figure 7A). Periclinal cell divisions were observed at the mid-globular stage, and the first division of the hypophysis occurred (Figure 7B). Then the hypophysis gave rise to two small, lens-shaped cells during the late globular stage of the embryo (Figure 7C). At the end of the globular stage the embryo has undergone periclinal and anticlinal cell divisions, and continued to the transition of the heart embryo stage (Figures 7D, E). The endosperm showed a helobial type, as we observed cellularized chalazal endosperm and the presence of a structure that perhaps functions as a scaffold to the positioning of the free nuclei (Figures 7F, 3S). There are regional specification processes that organize two main domains in the globular embryo; these domains or regions contribute to specific parts of the mature embryo.
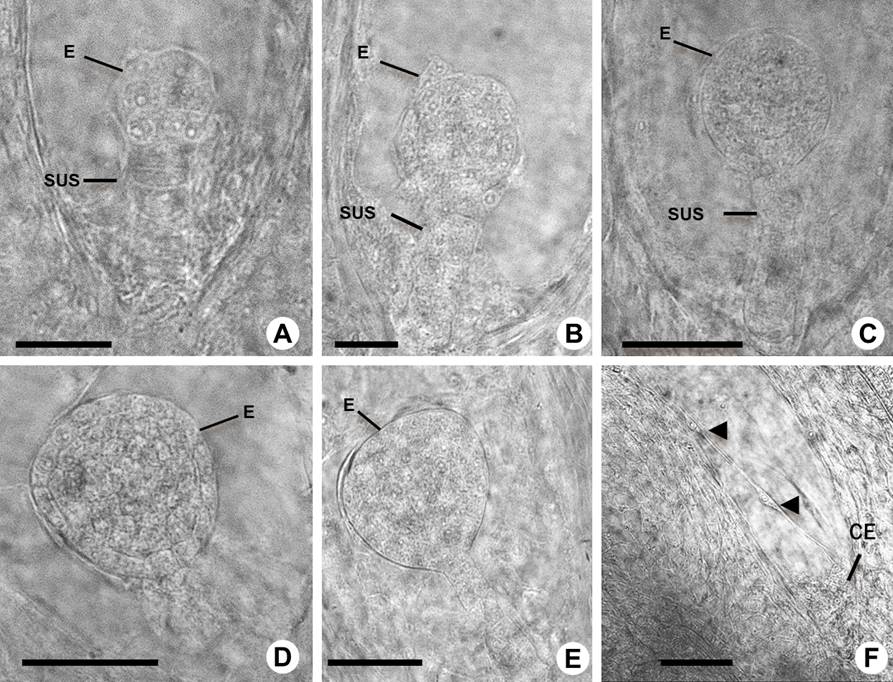
Figure 7 Mid embryogenesis in Amaranthus hypochondriacus. A-B: Early globular stage embryo (E) and suspensor (SUS). C: Mid-globular stage embryo. D: Late globular stage embryo. E: Globular-heart transition stage embryo. F: Cellularized chalazal-endosperm (CE) and free nuclear-endosperm is shown by arrowheads. Scale bars = 25 µm in A, B, and 50 µm in C-F.
At the late globular stage there was a transition from a globular to a heart-shaped polarized embryo, which is caused by the differential growth of the apical domain to form the cotyledon primordia and the shoot meristem (Figures 8A, 1S, Table 1S). At the heart stage (Figure 8A) the embryo has already differentiated most of the tissues and cell types. During the torpedo stage the endosperm has been almost consumed (Figures 8B, C, D). At the early cotyledon stage (Figure 8E) the embryo showed well-differentiated shoot and root meristems. Although in most of the suspensors the cell divisions were ordered, in some cases they were disordered (Figures 7A-E, 8B, C).
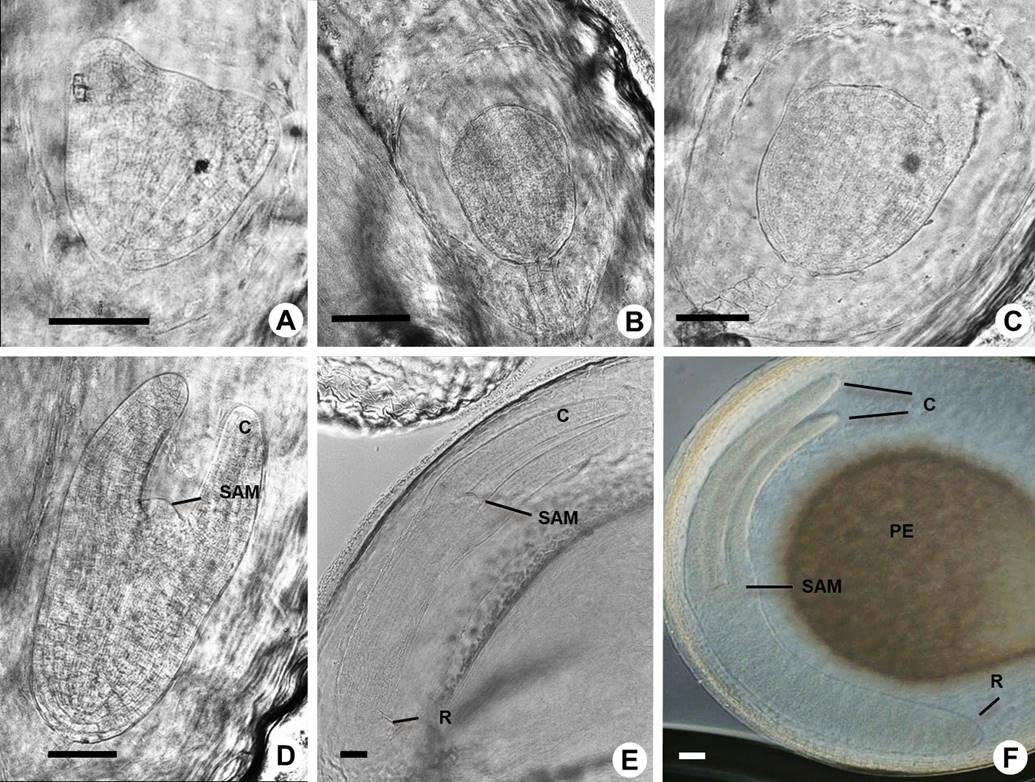
Figure 8 Late embryogenesis in Amaranthus hypochondriacus. A: Heart transition stage embryo. B-C: Heart-torpedo transition stage embryo. D: Torpedo stage embryo and general aspect of shoot apical meristem (SAM) and cotyledons (C). E: Cotyledon stage embryo with radicle (R). F: Mature embryo stage showing the main components: cotyledons, shoot apical meristem, radicle and perisperm (PE). Scale bars = 50 µm in A-F.
At the maturation stage (Figures 8F, 1S, Table 1S) the embryo increased dramatically in size due to cell expansion and the accumulation of storage products, primarily in the cotyledons. The endosperm is almost consumed; however, the perisperm is maintained, perhaps maintaining nutrition and providing appropriate space for the growth of the mature embryo.
Discussion
Ovule and ovary. Despite apparent overall simplicity in architecture, ovules are composed of a number of clearly differentiated cell types (Figure 3S). Although the size of the ovary seems not to be affected by the position on the branch or inflorescence, the ovule size is affected. Our analysis showed that ovule size is larger when it is formed at the basal or middle position of the inflorescence, suggesting a higher yield in these regions and perhaps the presence of more nutrients. The clearing analysis also showed that at first the A. hypochondriacus ovule is tenuinucellate. The MMC is originated from the L2 dermal layer and was localized at the L3 layer (Figure 3S).
The development and form of the ovule in Amaranthaceae shows variations, as reported in Celosia argentea, Amaranthus viridis, Cyathulatomentosa, Pupalialappacea and Digeria arvensis (Kajale 1935, 1940). During the early stages of ovule development their form is very similar, however, when fertilization occurs it assumes an ana-campylotropous form. In Allmania nodiflora the final form of the ovule is campylotropous, but it is derived from an amphitropous form. In Alternanthera sessilis the ovule begins to curve when the integuments are differentiated, and once the megaspores are formed it shows its typical campylotropous form (Bouman1984). The ovule type in the Amaranthaceae quinoa (Chenopodium quinoa W) has been reported as amphitropous and campylotropous (Bouman 1984, López-Fernández & Maldonado 2013a, b).
The A. hypochondriacus ovule was reported as anatropous and crassinucellate during the early stages (Coimbra & Salema 1999). The nucellus is enveloped by two integuments, leaving only a straight opening at the apex; however, our results based on histological and clearing analysis showed that at the mature female gametophyte stage the ovule changes to campylotropous; this type of ovule has a curved body but its curvature is less than the anatropous ovule.
Megasporogenesis and megagametogenesis in the Amaranthaceae. The differentiation of the MMC is the first step in the transition from the sporophyte to the gametophyte phase of the plant life cycle (Guo & Zheng 2013). In Digeria arvensis, although the MMC usually produces a tetrad of haploid megaspores, in some cases it gives rise to 3 megaspores due to the suppression of division in the upper dyad cell, which is normal in Alternanthera sessilis; nevertheless, the chalazal megaspore is always the functional one and the development of the female gametophyte is the normal 8-nucleate type (Kajale 1935). The antipodals are always three in number and are persistent, very often up to the early stages of embryo development. Once fertilization is completed, the female gametophyte begins to elongate along one side of the antipodals and they are left in a lateral position (Kajale 1935, Joshi 1936). We observed that the MMC divides by meiosis to produce four haploid megaspore cells. Then, as happens in quinoa (Chehregani et al. 2009), the micropylar megaspore gives rise to the functional megaspore.
On the other hand, an increase in the number of antipodal cells has been observed in several families of the Centrospermales, including the Nyctaginaceae, Chenopodiaceae and Phytolaccaceae. The Amaranthaceae do not follow a rigid sequence of development in having only three antipodals (Kajale 1954). In Pupalia lappacea several antipodals are formed as a small mass of cells. During the early stages of embryo development the antipodals degenerate. Previous reports (Kajale 1954, Coimbra & Salema 1999) observed that in A. hypochondriacus the antipodal cells degenerate before the egg apparatus is differentiated. Nevertheless, in this study we were able to observe antipodal cells in mature ovules even after the degeneration of one synergid or fertilization, suggesting that amaranth female gametophytes in some conditions require the antipodal cells for longer periods.
Embryology in the family Amaranthaceae. Embryo development stages in many plants correspond approximately to the proembryo/suspensor stage, globular stage, heart stage, maturation and dormancy, and the root apical meristem is fully constituted when the cotyledons begin to be curved (García-Campayo et al. 2011, Bartoli et al. 2016, Zhao et al. 2017). In Atriplex alimux only some stages of embryo development were described (Talamali et al. 2007). In Alternanthera sessilis the first division in the fertilized egg is transverse. The next division is again transverse and takes place in the micropyle cell, and then more transverse divisions follow. It forms a horseshoe-shaped structure in the outer part of the seed (Joshi & Kajale 1937). Similarly, in A. hypochondriacus we observed that the basal cell divides transversally, giving rise to a row of seven to nine cells (suspensor), while the apical cell (the embryo proper) undergoes a longitudinal division first. The hypophysis was differentiated when the suspensor was at the five to seven-cell stage. As the embryo develops, it becomes greatly elongated and curved. Longitudinal and asymmetric divisions in the suspensor were also observed.
The nucellus of Amaranthaceae persists even after fertilization, becoming a main source of nutrients called perisperm (Prego et al. 1998, López-Fernández & Maldonado 2013a, b, Burrieza et al. 2014).
Joshi & Kajale (1937) observed that the structure of the endosperm of Alternanthera sessilis differs from that of Digera arvensis. In D. arvensis the growth of the endosperm and the embryo destroys a part of the nucellus, but its central region remains intact. The endosperm becomes cellular in Alternanthera, but remains free nuclear at the chalazal end. In the mature seed most of the endosperm is absorbed by the growing embryo. Only a layer or two is left surrounding the radicle, as we observed in A. hypochondriacus. In A. hypochondriacus the endosperm showed a helobial development, as in the central region single nuclei were observed, but the chalazal endosperm was cellularized and at the torpedo stage the endosperm was almost consumed. Nevertheless, the perisperm is maintained, and thus acquires great importance in the nutritive value of the amaranth seed.
Finally, studies on micro and megagametogenesis in different species are an important prerequisite for developing robust hybrid seed production systems or attempting to alter reproduction (Moco & Marianth 2004, Salinas-Gamboa et al. 2016, Bartoli et al. 2017). The generation of information about reproductive development in Amaranthus species has biological and agronomic importance such as establishment of reproductive schedules, monitoring fertility problems and growth kinetics, which in turn may be the base for breeding programs and crop management. Our results show that the female reproductive development of amaranth is unique, since it can be defined as a variant of the Polygonum type considering that the micropylar megaspore is the functional one. These will also be useful to identify morphological changes during seed development and to develop new strategies to improve seed quality.











 nueva página del texto (beta)
nueva página del texto (beta)


