In the last two decades, tomato (Solanum lycopersicum L.) became the seventh crop in global production (Bergougnoux 2014). This crop is affected by more than 200 pests and diseases such as bacteria, fungi, viruses (Bergougnoux 2014), and weeds, which can reduce yields from 20 to 70 % (Bakht & Khan 2014, Adisa et al. 2018). The main weeds for this crop are Amaranthaceae species (Palacios et al. 2010, De Coninck et al. 2015). Farmers use conventional agrochemicals massively against pests and diseases; however, this agricultural practice has shown negative effects on the environment, like pesticide residues, development of resistant pathogen populations, and has potential risks on animal and human health (Abdel-Monaim et al. 2011, Scavo et al. 2020). Although synthetic pesticides are effective, they are not considered as GRAS (Generally Recognized as Safe) by the FDA (Food and Drug Administration, US) (de Rodríguez et al. 2011, Da Cruz Cabral et al. 2013); therefore, are not accepted by consumers looking for safe agricultural products and organic crops. For this reason, it is necessary to find effective alternatives against pests and safe for health (Talibi et al. 2012, Moshi & Matoju 2017). According to Korres et al. 2019, allelopathy is the direct or indirect effects of chemicals produced by plants or microorganisms on the growth, development, and distribution of other plants and microorganisms in natural and agricultural ecosystems. Currently, there is an increasing interest in plant extracts and secondary metabolites (allelochemicals) with bactericide, insecticide, herbicide, and fungicide activity. Several plant extracts, such as those from Mexican marigold (Tagetes minuta L.), Hairy beggarticks (Bidens pilosa L.), Black mustard (Brassica nigra L.), and others, can be an alternative for the management and control of some dominant weed species like Avena fatua L., Cyperus difformis L. (monocotiledons), and Echinochloa crus-galli L, Amaranthus spp. (dicotiledons) for wheat, cotton and rice crops (Farooq et al. 2011, Scavo et al. 2020). Plant extracts show several ecological advantages, such as being specific for target organisms, being biodegradable and have reduced risks to environment and human health (Farooq et al. 2011). These compounds have demonstrated phytotoxic activity (Vyvyan 2002), specially species of the Asteraceae (Rosas-Burgos et al. 2009, Farooq et al. 2011, Rongai et al. 2012, Talibi et al. 2012), Brassicaceae (Farooq et al. 2011) and Fabaceae (Talibi et al. 2012). Phytotoxic activity can be an alternative way to develop technology that will lead to a sustainable agriculture (Anaya et al. 1987). In the State of Tlaxcala, México, tomato crop production (4,066 ton/year) is an important economic activity with an annual increase of 4.8 % (SAGARPA 2017). In our research group, we study the potential phytotoxic activity of some local ruderal plants as an ecological alternative for the control of pests and diseases. Ruderal species are defined as those that grow along trail corridors or rural roads (Potito & Beatty 2005). We selected abundant ruderal species that grow nearby the tomato farmlands in the State of Tlaxcala, Mexico. Small-scale farmers use them to surround their fields and water channels in their traditional agroecosystems (Anaya et al. 1987). In this work, we report the phytotoxic activity of the aqueous extracts of the ruderal species Argemone mexicana (Papaveraceae), Baccharis salicifolia (Asteraceae), Lepidium virginicum (Brassicaceae), Leucaena leucocephala (Fabaceae), and Reseda luteola (Resedaceae) on three model weed species: A. hypocondriaccus L. (amaranth), L. sativa L. (lettuce) and S. lycopersicum L. (tomato).
Materials and methods
Collection of plant material. The aerial parts of five ruderal plants Argemone mexicana, Baccharis salicifolia, Lepidium virginicum, Leucaena leucocephala, and Reseda luteola (Table 1) were collected in February 2011 near Tepetitla de Lardizabal, Tlaxcala, México (19º 16´ 42” N; 98º 22´ 10” W; altitude 2,221-2,260 m asl). A voucher specimen of each species was deposited in the Casa Libertad Herbarium (Universidad Autónoma de la Ciudad de México) with numbers 1463, 1464, 1461, 14656 and 1462, respectively.
Table 1 Ruderal species and structures selected for preparing the extracts
| Species | Family | Organ |
|---|---|---|
| Argemone Mexicana L. | Papaveraceae | Leaves and stems |
| Baccharis salicifolia Pers. | Asteraceae | Leaves, stems, and flowers |
| Lepidium virginicum L. | Brassicaceae | Leaves, stems, and seeds |
| Leucaena leucocephala L. | Fabaceae | Leaves |
| Reseda luteola L. | Resedaceae | Leaves, and flowers |
All species were collected in February 2011.
Preparation of plant extracts. a) Aqueous extracts from fresh and dried plants. The aqueous extracts from fresh plants (FAE, 10 % w/v) were prepared as follows. The aerial parts collected from each species were washed with running tap water, then with sterile water, and cut into small pieces. Ten grams of fresh aerial parts were extracted by maceration with 100 mL of distilled water for 3 h. The macerated was filtered through medical gauze sheets, and then through a Wathman (No. 4) filter paper and Millipore membrane (0.45 µm) (Anaya et al. 2003). The aqueous extracts from dried plants (DAE, 1 % w/v) were prepared with the aerial parts of each species, and were dried for 10 days at room temperature, then in an oven at 40 ºC for 48 h, and grounded to fine powder in a porcelain mortar, then procedure for extraction is the same as above. b) Organic extracts from dried plants. The organic extracts (1 % w/v) were prepared as follows, powder material (50 g) was defatted with 2 L of n-hexane for 72 h with constant stirring at room temperature. The extracted biomass was decanted and placed in an oven (darkness, 30 ºC, 48 h) to remove the residual solvent. The dried biomass of each plant species was extracted with dicholoromethane-methanol 1:1 (DCM-MeOH) and next with methanol (MeOH). The extraction conditions were the same as that previously described for n-hexane. The extracts were filtered through Wathman paper (No. 4) and concentrated with a rotary vacuum evaporator (Büchi, Laboratoriums Technik, Schweiz). The yields of each extract were determined, and kept in the dark at room temperature until tested for biological assays.
In vitro herbicide activity. The effect of plant extracts on the seed germination and the root growth were assessed as previously published (Anaya et al. 1990) using the model weed plants Amaranthus hypochondriacus L. (Amaranthaceae), Lactuca sativa L. (Asteraceae), and Solanum lycopersicum L. (Solanaceae). The FAE (10 % w/v), DAE (1 % w/v), DCM-MeOH, or MeOH extracts (1 % w/v) were mixed with agar (20 %) and poured into sterilized Petri dishes (12 mL per plate) allowing solidification. Agar with distilled water, DCM-MeOH 1:1, and MeOH were used as controls. The seeds of the three model weeds were purchased from a local market in Coyoacán, México City, Mexico. Bioassays were performed in 6 cm Petri dishes. Ten seeds of each model weed were sown directly on the agar of each Petri dish following a completely random design with four replicates. Treatments were kept in darkness at 27 ºC +1, relative humidity of 70-75 %. Seed germination and the length from the tip of the root to the beginning of the hypocotyl (stem) were measured 24 h after treatment for A. hypochondriacus, 48 h for L. sativa, and 72 h for S. lycopersicum (Anaya et al. 2003).
Toxicity to tomato seedlings. The extracts that showed the best allelopathic activity DAE from B. salicifolia and L. virginicum were tested to determine its phytotoxicity towards tomato seedlings (8 and 12 weeks old). Tomato seedlings were grown from seeds and maintained under greenhouse conditions (temperature 27 to 32 ºC, relative humidity 65 %, and photoperiod of 14:10 h light/dark). The seedlings were cultivated in plastic pots (30 cm diameter) on sterilized inert substrate (peetmoss, agrolite, and perlite, 3:1:1), and watered with hydroponic solution. Plantlets of 8 or 12 weeks old were treated with DAE aqueous extracts (1 and 2 % w/v) in doses of 50 mL every 72 h for four months until flowering. The greenhouse conditions were the same as those already described. The phytotoxic effect was measured on basis of dry biomass yields and compared with the control with a completely randomized design. The experiments were performed twice.
Statistical analysis. Data analysis was carried out by statistical analysis of variance (ANOVA) using completely random design. Comparison of means was performed by the Tukey´s honestly significant difference (HSD) test with a 0.05 probability level, using STATISTICA software, version 8.
HPLC-MS Analysis. Samples of the aqueous extracts from dried plants (DAE) of Baccharis salicifolia, and Lepidium virginicum were lyophilized, and further analyzed with an HPLC-ESI-QTOF-MS (Model G6530BA, Agilent Tech. Santa Clara, California. USA) using a column Eclipse XDB-C18 (Agilent, 150 mm × 4.6 mm × 5 μm), mobile phase, A: CH3CN/MeOH (50:50) and B: H2O with CH3COOH 0.1 % in a gradient (0-10 min 25 % A, 75 % B; 10-15 min 100 % B, 0.300 mL/min 600.00 bar; 16.5-20 min 100 % B, 1 mL/ min 600.00 bar).
Results
The aqueous extracts of fresh (FAE, 10 % w/ v) and dried (DAE, 1 % w/ v) aerial parts, and the organic extracts (1 % w/ v) of the ruderal plants Argemone mexicana, Baccharis salicifolia, Lepidium virginicum, Leucaena leucocephala, and Reseda luteola were tested for inhibition of seed germination and radicle elongation of A. hypochondriacus (amaranth), L. sativa (lettuce), and S. lycopersicum (tomato var. saladet).
Seed germination inhibition. The DAE of B. salicifolia and L. virginicum tested at 1 % w/ v completely inhibited the seed germination of all the tested model plants (amaranth, lettuce, and tomato) (Figures 1, 2). The DAE of Argemone mexicana at the same concentration also annulated seed germination of lettuce and tomato. On the other hand, the FAE extracts showed less activity than DAE extracts, except for B. salicifolia, which showed a strong inhibition (60 to 100 %) on the three model plants (Figure 1). The organic extracts DCM-MeOH (1:1) and MeOH tested at 1 % (w/ v) did not inhibited at all the germination of model plants as indicated by statistical analysis (Table S1).
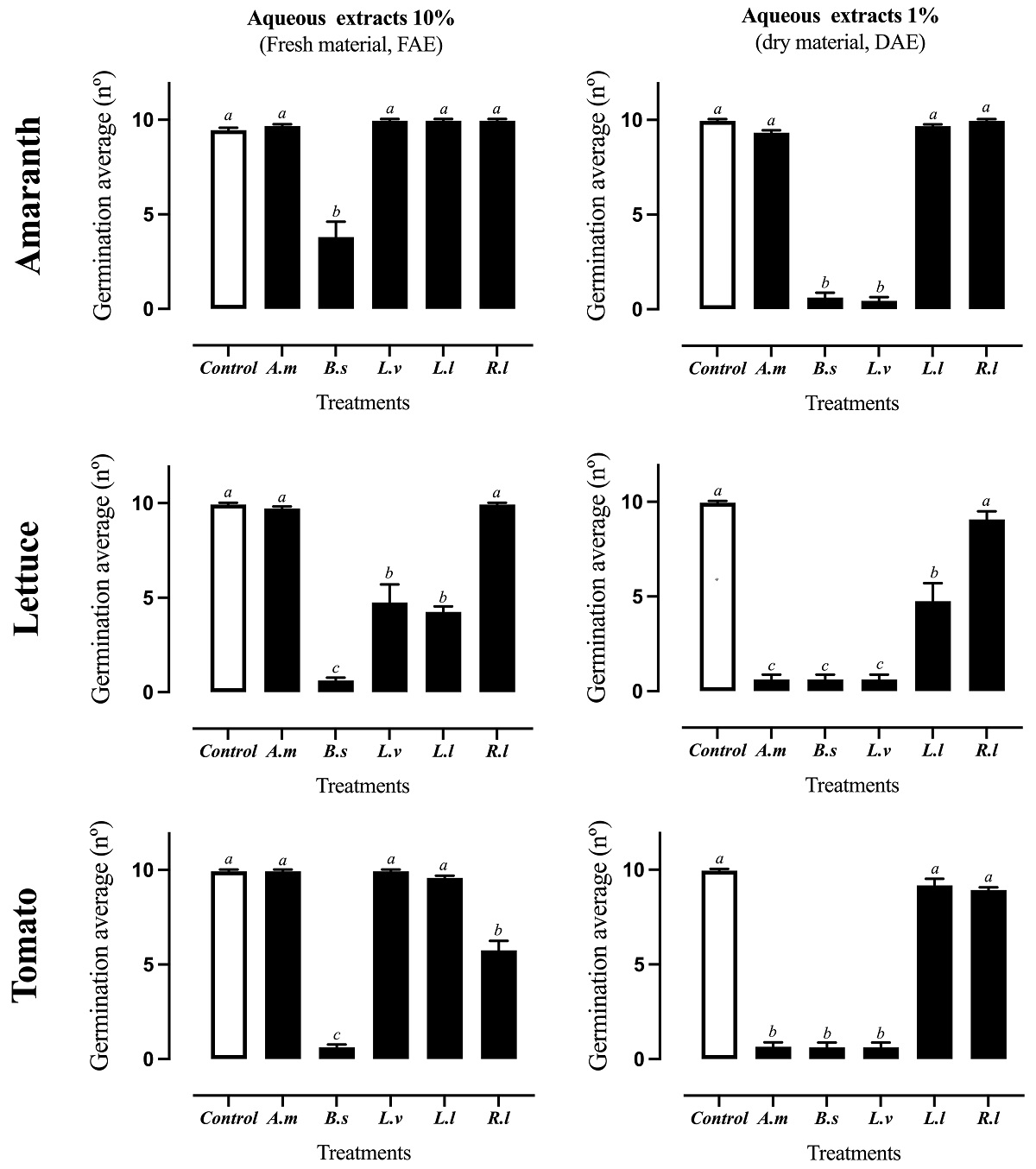
Figure 1 Assay of germination inhibition on amaranth, lettuce and tomato by FAE (10 %) and (DAE 1 %) of the ruderal plants Argemone mexicana (A.m), Baccharis salicifolia (B.s), Lepidium virginicum (L.v), Leucaena leucocephala (L.l), and Reseda luteola (R.l). Means + standard error of 4 experiments with n = 10. A different letter indicates statistical significance P < 0.05.
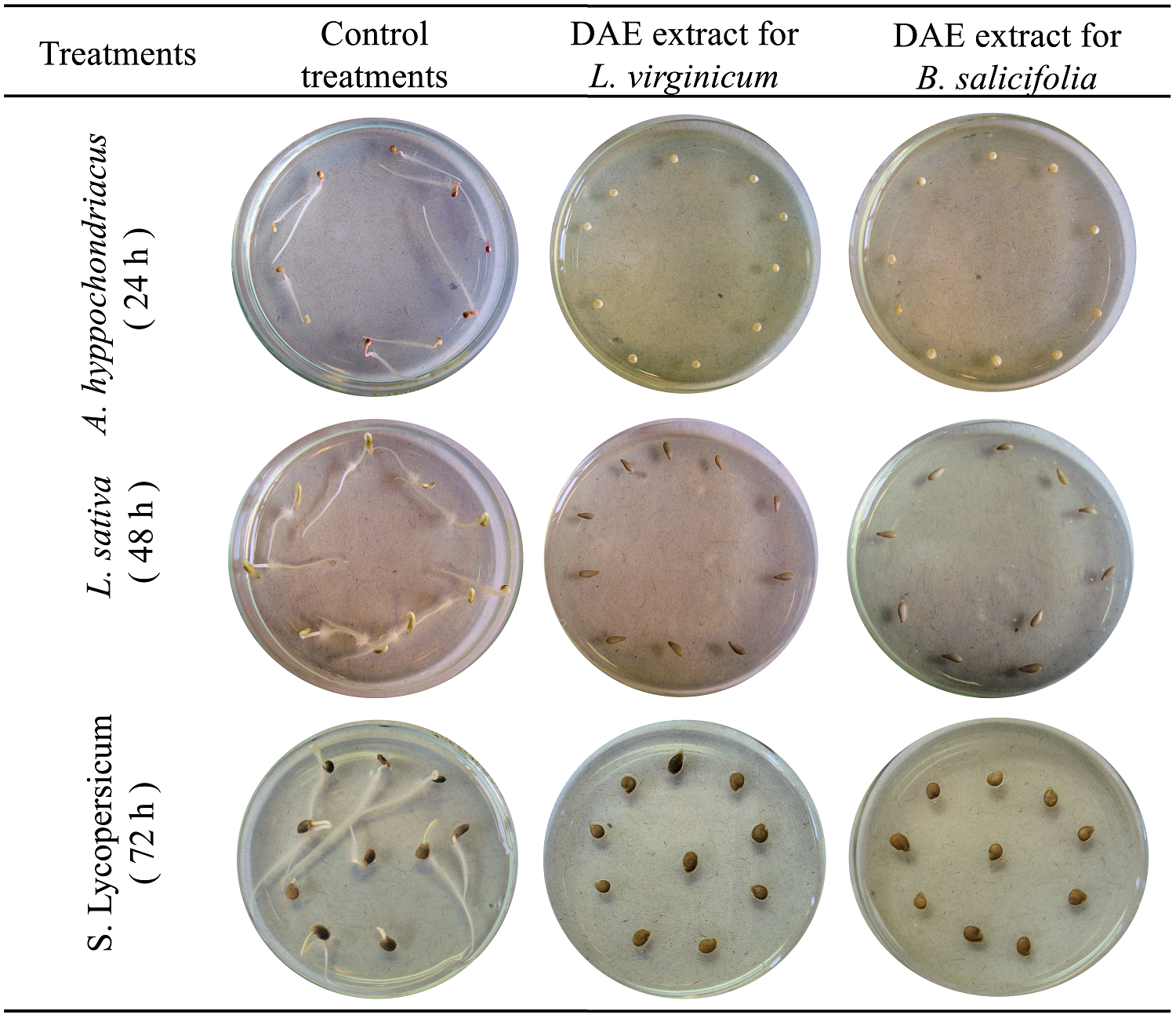
Figure 2 Inhibition on amaranth, lettuce, and tomato germination by dry aqueous extracts (DAE 1 %) of Baccharis salicifolia and Lepidium virginicum ruderal plants, recorded at 24, 48, and 72 hours, respectively.
Inhibition of radicle elongation. Aqueous extracts (DAE and FAE) from all the ruderal plants inhibited radicle elongation by 10 to 100 % on target species in comparison with control treatments. The most notable were the DAE extracts of B. salicifolia, and L. virginicum, which inhibited by 100 % the radicle elongation of the three model plants (amaranth, lettuce, and tomato) (Figure 3). Most of FAE extracts showed lower inhibition than DAE extracts, but in the case B. salicifolia DAE maintained 100 % phytotoxic activity against lettuce and tomato, but less on amaranth (64 % inhibition). DAE and FAE of R. luteola showed 100 % inhibition only against lettuce.
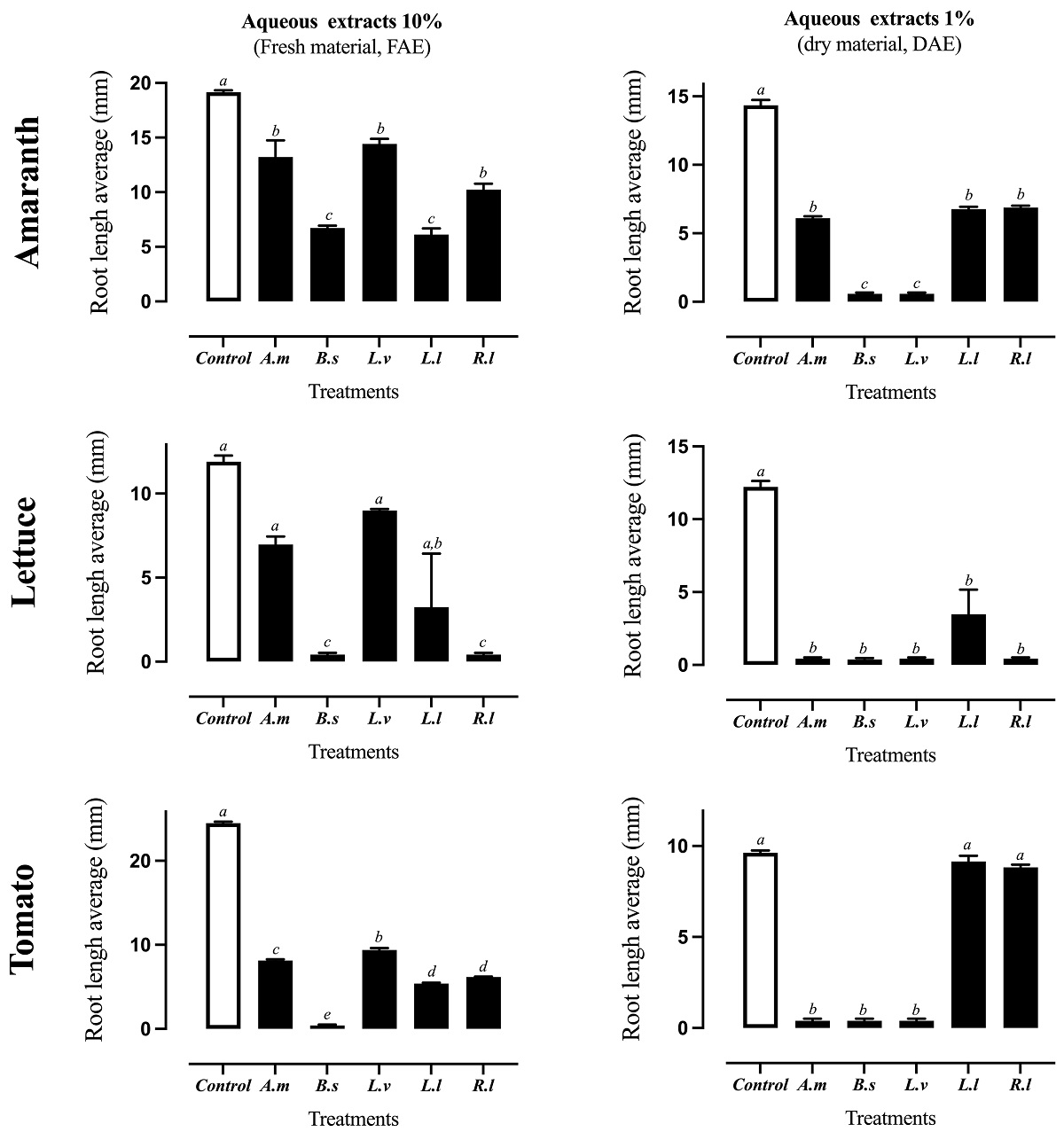
Figure 3 Assay of radicle elongation inhibition on amaranth, lettuce and tomato by FAE (10 %) and (DAE 1 %) of the ruderal plants Argemone mexicana (A.m), Baccharis salicifolia (B.s), Lepidium virginicum (L.v), Leucaena leucocephala (L.l), and Reseda luteola (R.l). Means + standard error of 4 experiments with n = 10. A different letter indicates statistical significance P < 0.05.
On the other hand, generally most organic extracts from all ruderal plants showed low or null inhibition of radicle elongation as compared to control treatments (Figure 4). The DCM-MEOH (1:1) and MeOH extracts of A. mexicana showed 48 % inhibition against tomato. The DCM-MEOH (1:1) extract of B. salicifolia inhibited in 24 and 27 % tomato and amaranth radicle elongations. Interestingly, some organic extracts stimulated the growth of the radicle. For example, both the DCM-MEOH (1:1) and MEOH extracts of B. salicifolia stimulated lettuce radicle elongation in 49 and 46 % respectively as compared against control treatments (Figure 4).
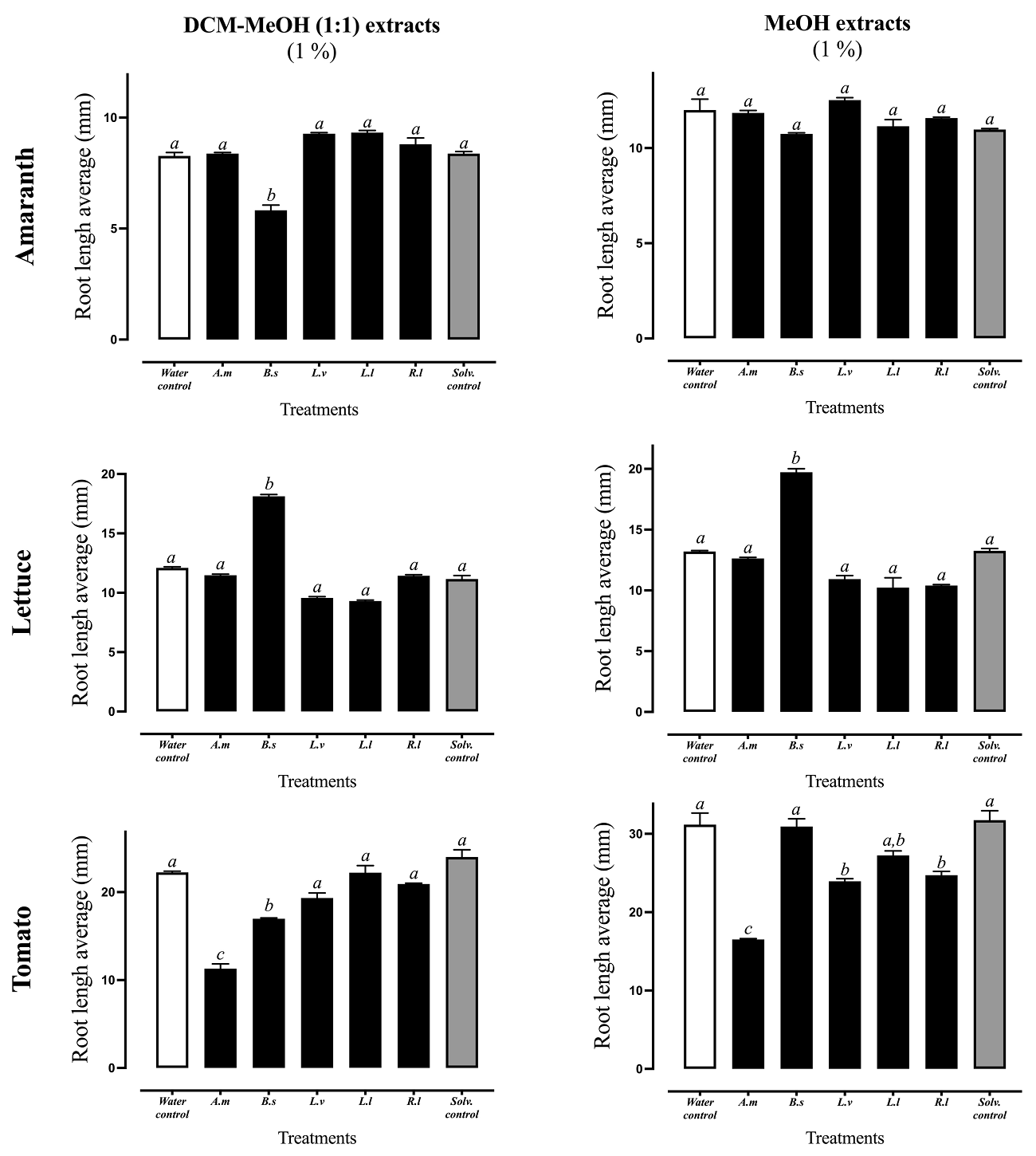
Figure 4 Assay of radicle elongation inhibition on amaranth, lettuce and tomato by DCM-MEOH and MeOH extracts (1 %) of the ruderal plants Argemone mexicana (A.m), Baccharis salicifolia (B.s), Lepidium virginicum (L.v), Leucaena leucocephala (L.l), and Reseda luteola (R.l). Means + standard error of 4 experiments with n= 10. A different letter indicates statistical significance P < 0.05.
Phytotoxicity of extracts to tomato seedlings. The extracts that presented the greatest allelopathic potential were tested on tomato seedlings of 8 and 12 weeks old. The DAE of B. salicifolia and L. virginicum at 1 and 2 % did not affect biomass yield when compared against control treatment (Figure 5), indicating that these extracts are safe for tomato seedlings.
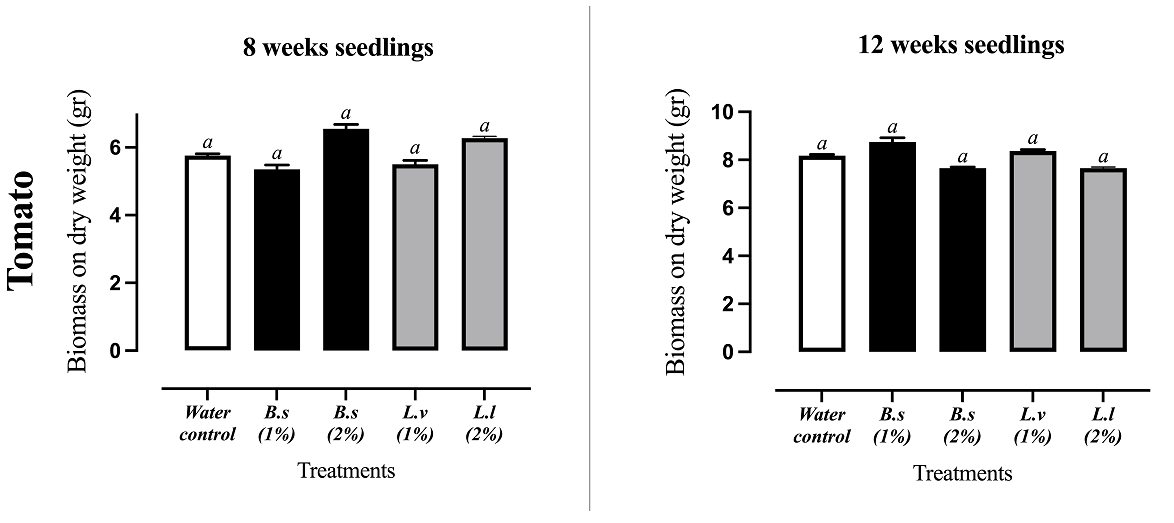
Figure 5 Effect on biomass of tomato seedlings (8 and 12 weeks old) by DAE (1 %) of the ruderal plants Baccharis salicifolia (B.s) and Lepidium virginicum (L.v). Means + standard error of 4 experiments with n = 10. A different letter indicates statistical significance P < 0.05
Positive ion polarity HPLC-ESI-QTOF-MS analysis of active extracts. The extracts with the best allopathic activity and low phytotoxicity were analyzed by High Performance Liquid Chromatography-Mass Spectrometry (HPLC-ESI- QTOF-MS). The chromatogram of B. salicifolia DAE showed two majoritarian peaks eluting at 12.77 and 15.52 minutes, with m/z 432.9852 and m/z 725.4692 respectively (Figure 6). The HPLC-ESI- QTOF-MS chromatogram of L. virginicum DAE showed also two majoritarian peaks eluting at 12.16 and 14.90 minutes with m/z 532.9326 and m/z 527.1659 respectively (Figure 7).
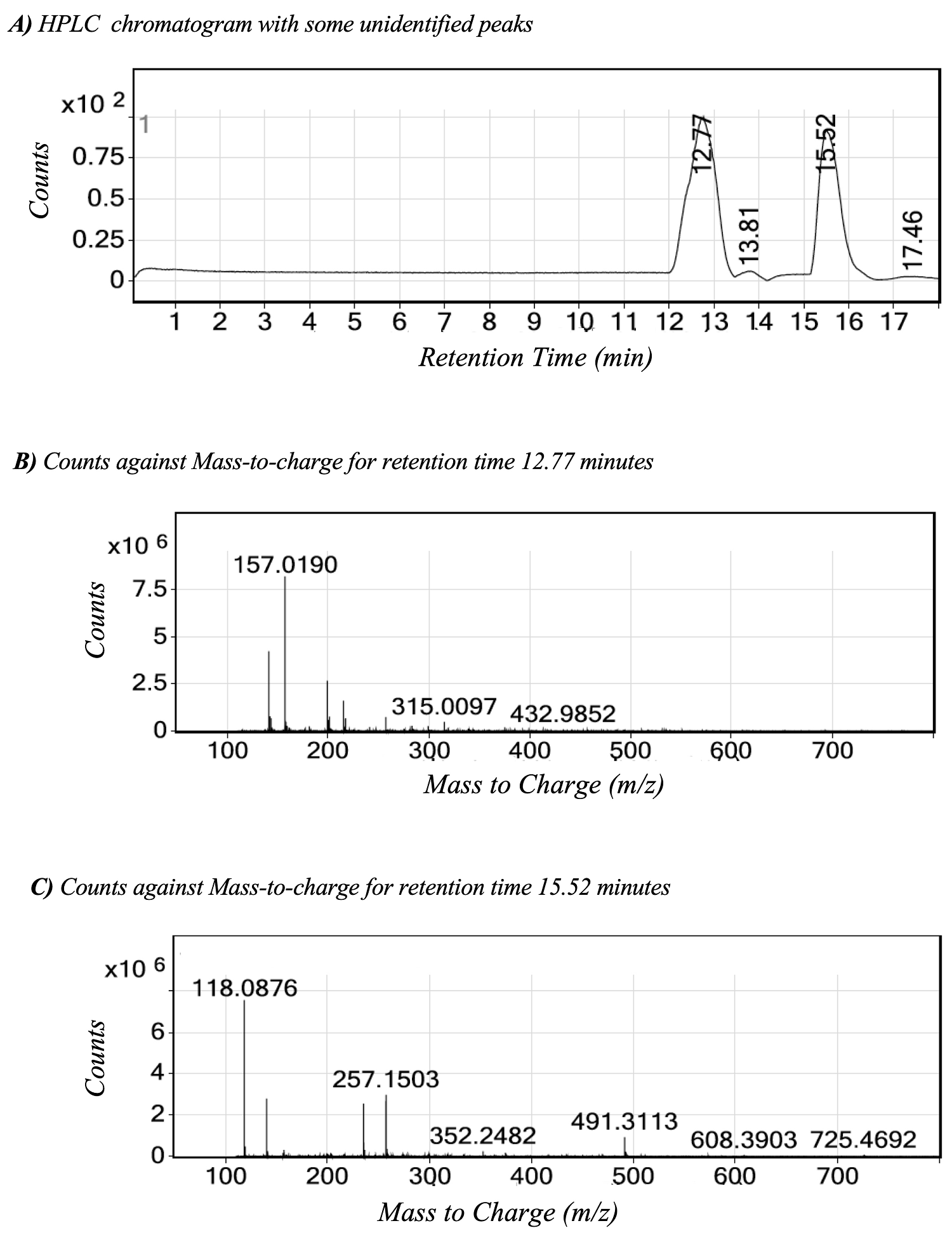
Figure 6 HPLC-ESI-QTOF-MS chromatograms of lyophilized DAE extracts. A) Baccharis salicifolia HPLC chromatogram. Retention times of major peaks: 12.77, and 15.52 minutes; B) MS spectrum of peak at 12.77 min; and C) MS spectrum of peak at 15.52 min.
Discussion
Most tomato farmers depend largely on pesticides for controlling diseases and pests (Vyvyan 2002). Among them, the herbicides glyphosate, 2,4-dichlorophenoxyacetic acid, and bromomethane (CH3Br), which pollute soils, groundwater aquifers, and air; are risky for the environment, and animal and human health (Morillo & Villaverde 2017). Some herbicides, such as CH3Br, are applied to the soil but are highly negative to the ozone layer and affect the human health. This product is severely restricted worldwide by the Vienna Convention in 1987 and the Montreal Protocol in 1988, which Mexico has signed (SEMARNAT 2015). To transform conventional agricultural production, researchers in pest control have concentrated their efforts on the search of natural products as pesticides (Vyvyan 2002, García et al. 2005). It is expected, that the allelochemicals, as pure compounds or complex mixtures, extracted from plant tissues with water, or organic solvents, can be used for pest management as ecofriendly pesticides, without polluting soil, water, air (Farooq et al. 2011).
The aim of this work was to investigate the potential phytotoxic activity of five ruderal plants for its possible application for tomato crop in Tlaxcala, Mexico. The best results were obtained for the aqueous extracts (DAE) of Baccharis salicifolia and Lepidium virginicum. Both are ruderal shrubs, moreover, some small-scale farmers use them to surround their farmland and water channels in their traditional agroecosystems (Anaya et al. 1987, del Corral et al. 2012). The aqueous extracts from dry material (DAE) of B. salicifolia and L. virginicum, tested at 1 % w/ v showed remarkable phytotoxic activity, inhibiting by 100 % the germination (Figure 1, 2), and logically the radicle elongation of amaranth and tomato seeds (Figure 3). These results are similar to treatment with 2,4-D at 1 % with these models (Anaya et al. 2003). DAE extracts did not have phytotoxic effects on biomass of tomato seedlings (8 to 12 weeks age) in greenhouse conditions (Figure 5). The next step will be to test these extracts in field conditions suggests Vyvyan (2002).
Comparable germination inhibition of 100 % has been reported with the aqueous extracts of Tetraclinis articulate (Cupressaceae) leaves at 30 g/ L for lettuce seeds, in the case of tomato was 100 % with 40 g/ L (M'barek et al. 2018). Therefore, DAE extracts of B. salicifolia and L. virginicum are 3-4 fold more potent than T. articulate.
The DCM-MeOH 1:1 (v/ v) and MeOH extracts from B salicifolia did not show phytotoxic activity; however, Palacios et al. (2010) and Domínguez (2002) reported strong germination inhibition (80 to 100 %) on amaranth and lettuce of B. salicifolia ethanol extracts at 10 %. Phytotoxic activity of the dichloromethane extract from the leaves of B. salicifolia has been reported on seeds of model plants (Lolium multiflorum, monocotyledon and Lactuca sativa, dicotyledon). This extract contains methoxylated flavanones, including sakuranetin, which showed herbicidal activity on the above-mentioned model plants (Domínguez 2002). Regarding to radicle elongation the aqueous extracts (DAE) of B. salicifolia and L. virginicum showed the highest inhibition (100 %) on amaranth.
Baccharis salicifolia has been thoroughly studied chemically, identifying 15 flavones (Dominguez et al. 1986), 7 flavanones (apigenin, quercetin, naringenin, sakuranetin) (Zdero et al. 1986, del Corral et al. 2012), several diterpenes, including 17 labdanes (Jakupovic et al. 1990, del Corral et al. 2012), three clerodanes some of them with ß-xylopyranoside and ß-fucopyranoside (Zdero et al. 1986, Jakupovic et al. 1990, Loayza et al. 1995), two triterpenes (del Corral et al. 2012), phytoesterols (Dominguez et al. 1986), monoterpenes (α-phellandrene, α-terpineol, α-thujene, camphene) (Loayza et al. 1995, García et al. 2005, Carrizo Flores et al. 2009, Sosa et al. 2012), (cis- α-copaen-8-ol, bicyclogermacrene, germacrene-D) (Loayza et al. 1995, García et al. 2005, Carrizo Flores et al. 2009, Sosa et al. 2012), cadinene derivates (cadinols) (Zdero et al. 1986, Jakupovic et al. 1990, Loayza et al. 1995), and other compounds (baccharis oxide, benzofurans, coumarins) (Dominguez et al. 1986, Zdero et al. 1986).
Several flavonoids of B. salicifolia, such as quercetin, apigenin, naringenin showed phytotoxicity against Lactuca sativa, being the most effective quercetin at 15 - 40 ppm (Céspedes et al. 2006). In addition, from this species it has been isolated the diterpenoids, 5-hydroxy-6-hydro-salicifolic and salicifolic acids, together with sakuranetin, apigenin, and scopoletin (del Corral et al. 2012). Germination inhibition mediated by 5-hydroxy-6-hydro-salicifolic and salicifolic acids was determined against Panicum miliaceum and Raphanus sativus; and the 5-hydroxy-6-hydro-salicifolic acid resulted the most active compound (del Corral et al. 2012).
Regarding Lepidium virginicum L., several glucosinolates have been isolated from the leaves, such as sinigrin and their derivatives; these compounds are involved in the defense versus herbivorous insects (Agrawal & Kurashige 2003). The powders of aerial parts from some species of Brassicaceae family containing glucosinolates with herbicidal activity (Ahmed et al. 2020, El-Masry et al. 2019). In addition, benzylglucosinolate from L. virginicum is active against Entamoeba histolytica (Calzada et al. 2003) and has antimicrobial and insecticidal properties (Agrawal & Kurashige 2003).
The HPLC-ESI-QTOF-MS analysis showed two majoritarian peaks in the range with m/z 432.9- 725.4 for the active FAE extracts of B. salicifolia and also for L. virginicum. Since phytotoxic flavonoids, including apigenin, have been reported for B. salicifolia (Céspedes et al. 2006), it can be proposed FAE could contain glycosilated flavonoids, such as thalictiin (apigenin-7-alloside) with m/z 432.10565 isolated from Cassia occidentalis (Purwar et al. 2003). This molecular ion matches to that found for the peak m/z 432.9852 with retention time of 12.77 minutes (Figure 6). In the case of L. virginicum, FAE could have phytotoxic glucosynolates. However, further detailed chemical studies are needed for its identification.
In conclusion, two local ruderal plants from Tlaxcala, Mexico have phytotoxic activity on three model weeds (amaranth, lettuce and tomato). The best treatments were the aqueous extracts (DAE, 1 %) prepared with the dry aerial parts of Baccharis salicifolia and Lepidium virginicum. Both inhibited by 100 % the germination and radicle elongation of the three model weeds; but were not phytotoxic to tomato seedlings when watering the plantlets 72 hrs for 4 months. The HPLC-ESI-QTOF-MS analysis showed two majoritarian peaks in the range m/z 432-725 for each extract and could be glycosylated flavonoids for B. salicifolia and glucosinolates for L. virginicum. These aqueous extracts are promising material as natural herbicides for tomato crop reducing the dependence on synthetic herbicides. Future biological evaluations and identification of its active components are needed to advance in the generation of ecological alternatives for weeds control in tomato crop.
Supplementary material
Supplemental data for this article can be accessed here: https://doi.org/10.17129/botsci.2727











 nueva página del texto (beta)
nueva página del texto (beta)




