Knowledge of how aboveground biomass (AGB) and forest structure change along tropical elevational gradients is important because tropical forests account for approximately one-quarter of the terrestrial net primary production (Bonan 2008), thus playing an important role in the global carbon cycle and being key reservoirs of global biodiversity (Gibson et al. 2011). Moreover, for the 2007-2016 period, global models estimated net CO2 emissions of 5.2 Gt CO2 yr-1 from land use and land-use change (IPCC 2019). These net emissions are mostly due to deforestation, which accounted for approximately 12 % of all human-induced greenhouse gas emissions (Petrokofsky et al. 2012). Also, many tropical regions have mountains, and this fact may require a change in our view of the potential role of lowland and montane tropical forests as carbon reservoirs (Huasco et al. 2014).
Previous studies have reported four competing patterns of how AGB changes along elevational gradients: (1) a unimodal pattern, (2) a U-shaped pattern (Moser et al. 2008, Girardin et al. 2010, Marshall et al. 2012, Ensslin et al. 2015), (3) a monotonic decrease with increasing elevation (Aplet & Vitousek 1994, Dossa et al. 2013, Raich et al. 2006, Moser et al. 2008), and (4) a monotonic increase with increasing elevation (Alves et al. 2010, Rosenfield & Souza 2014). These findings suggest a high context-dependency of the relationship between AGB and elevation.
AGB of forests is affected by several factors including climate, soil properties, topography, biotic interactions, stand age, silvicultural management, species composition, and functional traits such as wood-density (Forrester et al. 2003, Moser et al. 2008, Alves et al. 2010, Slik et al. 2010, Rosenfield & Souza 2014, Ensslin et al. 2015, Mensah et al. 2016). Generally, there is an agreement that at large spatio-temporal scales climate has a principal impact on vegetation development (Woodward & Williams 1987, Moser et al. 2008, Pan et al. 2013), and it also has been identified as a critical factor for biomass accumulation (Moser et al. 2008, Slik et al. 2010, Ensslin et al. 2015). Lower temperatures at higher elevations are considered to limit photosynthesis, transpiration, and nutrient uptake, and thus AGB (Leuschner et al. 2007).
However, there is not just an effect of biophysical factors but patterns of AGB are also increasingly influenced by human activities. In general, there is an increase of AGB with successional age (Mani & Parthasarathy 2009), but estimates of AGB in different types of tropical forests vary widely between 45 and 800 t/ha (Tanner 1980, Maia-Araújo et al. 1999, Clark et al. 2001, Sarmiento et al. 2005). For example, tropical lowland old-growth rainforests in Los Tuxtlas, Veracruz, were reported to contain an AGB of 403 t/ha (Hughes et al. 1999, 2010), while in a seven-year-old secondary tropical lowland rainforest in Uxpanapa (Veracruz), Williams-Linera (1983) reported 53 t/ha. For a humid montane forest in Oaxaca State, Asbjornsen et al. (2005) estimated values ranging between 196 and 299 t/ha. Along an elevational gradient (0-1,000 m) of tropical Atlantic moist forest in Brazil, it was found that the lowland forests at sea level had on average 154 t/ha of ABG, whereas the montane forests at 500 m and 1,000 m had 239.3 t/ha and 262.7 t/ha, respectively (Alves et al. 2010).
Besides AGB, other forest structural attributes usually also change with elevation but do not necessarily follow the same patterns as AGB. For instance, the elevation is negatively correlated with tree height, and positively with stem density on tropical gradients (Aiba & Kitayama 1999, Moser et al. 2008, Culmsee et al. 2010). The basal area has also been found to decrease with elevation along tropical elevational gradients possibly due to the nutrient cycle because of the influence of low temperatures on plant metabolism (Báez et al. 2015) and reduced solar radiation due to increased cloudiness and edaphic factors that are often limiting at high elevations (Santiago et al. 2000).
Carbon storage is an important function performed by tropical forest ecosystems concerning climate change adaptation and mitigation (Marshall et al. 2012). Furthermore, there is usually a strong association between carbon storage and biodiversity conservation (Strassburg et al. 2010). Carbon-based conservation could protect biodiversity in high-value regions, while matching funds derived from their carbon content could benefit other regions (Strassburg et al. 2010). The limited knowledge on most tropical forests, however, makes it difficult to predict the effects of land-use changes on biomass and carbon storage (Ensslin et al. 2015). Rising international awareness of the importance of conserving and enhancing forest carbon stocks has, for example, led to the implementation of the program “Reducing Emissions from Deforestation and Degradation” (REDD+), with which the United Nations Framework Convention on Climate Change (UNFCCC) aims in reducing emissions from deforestation and forest degradation (Bonan 2008, Petrokofsky et al. 2012).
In the last 50 years, Mexico has lost half of its forest cover due to deforestation. The largest losses occurred between 1993 and 2000 (Velázquez et al. 2002, Barsimantov & Kendall 2012, Gómez-Díaz et al. 2018). A growing human population in combination with unclear and conflicting definitions of land rights, the extent of agricultural areas (for crop cultivation and cattle grazing), roadway construction and excessive logging for timber production were the most important drivers of deforestation (Cortina-Villar et al. 2012, Bonilla-Moheno et al. 2013, Gómez-Díaz et al. 2018). Tropical rainforests and montane pine-oak forests were most affected by high deforestation rates (Barsimantov & Kendall 2012).
While it is acknowledged that tropical forest landscapes have experienced widespread disturbance by humans, one of the key results of such disturbance is the regrowth of secondary forests, which may or may not share key structural features when compared to the original primary forest and which may show different species composition as well, while this mostly depends on successional age (Chazdon et al. 2009). Despite continuously high deforestation rates in Mexico, there is also an increase in natural succession, which means the regrowth of new secondary forests (Wright 2005), which have relevance in tropical environments because of their important role in carbon sequestration (Poorter et al. 2016).
This study aimed at analyzing the interactive effect of forest structure and aboveground tree biomass in old-growth and young secondary forests along an elevational gradient at the Cofre de Perote in Veracruz State, Mexico.
Material and methods
Study area. Our study area is located at the Cofre de Perote mountain, Veracruz, a floristically highly diverse region in the transition zone between the Neotropical and the Nearctic realms (Carvajal-Hernández et al. 2017). This region has suffered one of the highest deforestation rates in Mexico (Muñiz-Castro et al. 2015). The present forests have thus undergone substantial changes resulting in a highly fragmented landscape (Williams-Linera et al. 2007, Gómez-Díaz et al. 2018). For this region, the estimated rate for annual forest cover change was -0.44 % in the 1993-2000 period; however, this situation changed to a positive trend (+0.11 %) of forest cover gain in the following period of 2000-2014 (Gómez-Díaz et al. 2018).
The elevational gradient begins at sea level close to the Gulf of Mexico (19° 35’ N, 96° 22’ W) and ends some 81 km inland at the eastern slopes of the Cofre de Perote at 3,500 m (19° 30’ N, 97° 08’ W; Figure 1). The Cofre de Perote (4,282 m) is an extinct stratovolcano located in the Trans-Mexican Volcanic Belt in the transition zone with the Sierra Madre Oriental (Lauer 1973, Negendank et al. 1985). Due to the different eruptions of various volcanos, the region is composed of multiple basaltic deposits, with the latest lava deposition from the Holocene, approximately 10,000 years ago (Negendank et al. 1985, Castillo-Campos et al. 2008).
Sources: Esri, USGS ,NOAA.
Sources: Esri, Garmin, USGS-NPS
Figure 1 Map of the study area. Study area in Veracruz, Mexico, showing the four study sites (Palmarejo at 500 m, Los Capulines at 1,500 m, El Encinal at 2,500 m, and El Conejo at 3,500 m).
Diverse climatic conditions occur along the elevational gradient. Mean annual temperature decreases linearly with increasing elevation and mean annual precipitation shows a unimodal trend and peaking in 2,500 mm/yr. between 1,500 and 2,500 m (Soto-Esparza & Giddings 2011, Carvajal-Hernández & Krömer 2015, Gómez-Díaz et al. 2017). The natural vegetation types include tropical sub-humid deciduous forests below 700 m, tropical oak forests between 700-1,300 m, humid montane forests between 1,300-2,300 m, pine-oak forests between 2,300-2,800 m, and coniferous (pine and fir) forests above 2,800 m (Carvajal-Hernández et al. 2020). Forests in the region are highly fragmented and the landscape consists of a mosaic of forests, extensive land-use (pastureland for cattle), sugarcane fields, mango, lime, and coffee plantations, and urban areas such as the capital city of Xalapa and surrounding cities.
Along the elevational gradient, we sampled at 500 m (Palmarejo), 1,500 m (Los Capulines), 2,500 m (El Encinal), and 3,500 m (El Conejo) above sea level (Figure 1, Table 1). In each elevational belt, we sampled old-growth and young secondary forests to analyze the impact of succession on forest structural parameters. Old-growth forests were defined as mature forests with a dominance of mature trees in which shrubs cover less than 30 % of the area, with a long time of forest ageing without human disturbance or forest utilization (50 years). We looked for young secondary forests (locally known as acahuales) that were not used since clear-cut, controlling a regenerating age between 15 to 25 years after abandonment (based on interviews with local landowners). These secondary forests are common in the study area and characterized by low-statured trees and an open canopy dominated by shrubs and a high abundance of vines (Gómez-Díaz et al. 2017, Bautista-Bello et al. 2019). In each study site (elevation) and for each successional stage (old-growth and secondary forest), we established five non-permanent 20 × 20 m plots resulting in a total of 40 plots.
Table 1 Overview of the study sites with information on forest type, tree species composition, values by other authors on aboveground biomass in similar forest types or elevations, climate (SMN 2018), and anthropogenic disturbance.
| Variables | Palmarejo 500 m | Los Capulines 1,500 m | El Encinal 2,500 m | El Conejo 3,500 m |
|---|---|---|---|---|
| Forest type | Tropical sub-humid deciduous forest. | Humid montane forest. | Pine-oak forest. | Fir forest (Abies religiosa). |
| Tree species composition | Lauraceae spp., Quercus sp.1, Q. lancifolia, Q. sp.2, Bursera simaruba, Comocladia macrophylla, and Plumeria rubra. | Q. affinis, Q. crassifolia, Q. lancifolia, Carpinus caroliniana, and Liquidambar styraciflua. | Pinus patula, P. pseudostrobus, P. teocote, P. leiophylla, P. ayacahuite, Q. affinis, Q. ocoteifolia, Q. crassipes, Q. laurina, Abies religiosa, and Cupressus lusitanica. | A. religiosa, P. patula, and P. hartwegii. |
| Aboveground biomass (t/ha) | 77 - 864 t/ha (Maia-Araújo et al. 1999, Martínez-Yrizar et al. 2009, Sarmiento et al. 2005). | 196 - 407 t/ha (Asbjornsen et al. 2005, Hughes et al. 1999, Tanner 1980). | 117 to 1,006 t/ha (Balderas-Torres et al. 2013, de Jong et al. 2000, García-Oliva et al. 2014, Ordóñez-Díaz et al. 2008). | 74 to 372 t/ha (Mendoza-Ponce & Galicia 2010, Ordóñez-Díaz et al. 2008). |
| Temperature | Mean = 23.3 °C, min = 19.7 °C, max = 26.1°C. | Mean = 23.3°C, min = 17.2 °C, max = 20.5 °C. | Mean = 12.1 °C, min = 10.3 °C, max = 13.1 °C. | Mean = 9.7 °C, min = 8.1 °C, max = 11.0 °C. |
| Precipitation | 945.8 mm/yr; rain season 150.7 - 208.0 mm/month, dry season 15.1- 25.2 mm/month. | 1,146.5 mm/yr; rain season 159.1 - 210.9 mm/month, dry season 27.8 - 46.6 mm/month. | 1,076.9 mm/yr, rain season 144.6 - 210.4 mm/month, dry season 25.1 - 47.0 mm/month. | 1,478.4 mm/yr, rain season 235.4 - 303.2 mm/month, dry season 34.4 - 48.3 mm/month. |
| Anthropogenic disturbance | Lime plantations and settlements growing towards old-growth forests. | Replaced by Liquidambar, cattle and plantations. | Replaced by pine plantation, ongoing process of road construction, increasing human pressure, timber and charcoal production from oaks. | Replaced partly by pine plantations, firewood collection for personal needs. |
Stand structure inventory. Within each plot, we measured the following forest stand parameters: (1) stem density, (2) mean diameter at breast height (dbh, at 130 cm above-ground), (3) basal area, (4) mean tree height, and (5) AGB. Fieldwork was carried out between October and December 2015. For the determination of the species identities, specialized botanical literature was used (e.g., Flora de Veracruz) and the consultation of plant taxonomists at the Instituto de Ecología, A.C. in Xalapa. Trees that could not be identified to species level were later identified to genus level.
The number of stems with a dbh ≥ 7.5 cm was counted per plot and stem density per hectare was derived from the stem density per plot. When irregular stem shapes occurred, the diameter was measured 20 cm above or below breast height (Ensslin et al. 2015). To calculate the diameter of multiple-stemmed trees the square root of the sum of all squared trunk diameters was calculated (MacDicken et al. 1991, Grabowski & Gilman 2002, Nogueira Júnior et al. 2014).
Aboveground biomass (AGB) estimation. We estimated the AGB in tons for each tree using the Pantropical equation (1) of Chave et al. (2014):
where ρ is wood density in g cm−3, dbh is the diameter at breast height in cm, and h is the height in m. Wood density data were taken from the wood density database (Chave et al. 2009) and a collection of wood densities of Mexican tree species (Ordóñez-Díaz et al. 2015). For nearly 45 % of the tree species, wood density was available at the species level (10 species). If wood density data were not available at the species level or trees could not be identified to species level (45 % of all species), mean values of the nearest taxonomic unit (usually genus level) were used (Marshall et al. 2012). When trees could not be identified or wood density data was not available (3 species, i.e., 14 %), we used the mean values of the respective forest type. The wood density values used for the respective forest types in each study site were taken from Ordóñez-Díaz et al. (2015): i) deciduous forest at 500 m (ρ = 0.63 g cm−3); ii) humid montane forest at 1,500 m (ρ = 0.60 g cm−3); iii) pine-oak forest at 2,500 m (ρ = 0.63 g cm−3); and iv) fir forest at 3,500 m (ρ = 0.51 g cm−3).
Statistical analyses. For all forest stand parameters, we report mean values and standard deviation (SD) for each plot, for each study site, and the two successional stages. We modeled mean dbh, basal area, mean tree height, stem density, and AGB as dependent variables, and elevation and successional stages (old-growth and secondary forests) as independent variables. In the case of mean dbh, basal area and mean tree height, we fitted linear regression models with the normal distribution. Since stem density and AGB did not follow a normal distribution, we used the function descdist of the fitdistrplus R-package (Delignette-Muller & Dutang 2015) to evaluate which distribution best fit the previous variables. Therefore, we used a generalized linear model with gamma distribution in both cases. Since forest stand parameters can follow a non-linear trend with elevation, we also considered non-linear (unimodal) relationships between dependent variables and elevation by adding a quadratic term (elevation2) to the models.
For the selection model, we used the Akaike information criterion (AIC; Akaike 1974). AIC is well suited to situations where the predictive capacity of the model is important. AIC evaluates the likelihood of each model in the set, it considers how well the model fits the data and penalizes for adding additional parameters (Burnham & Anderson 2002).
The significance level was set at α = 0.05. All statistical analyses were performed in R 3.1.1 (R Core Team 2019) using the libraries car, dataset, effects, graphics, grDevices, matrix, methods, moments, stats, utils, and vegan.
Results
All the described results in this section are the best-supported models according to AICc (Table 2). Stem density was affected by the interaction between elevation and forest type (total model D 2 = 0.64, P < 0.009; Table 3). We observed significantly higher stem density in secondary forests at higher elevations (Figure 2A). At lower elevations, stem density was similar in both successional stages; however, at higher elevations stem density in secondary forests was more than twice as high as in old-growth forests (Table 3).
Table 2 Description of the final models selected by the bias-corrected Akaike information criterion. The summaries link the measured forest stand parameters to environmental explanatory variables, along an elevational gradient in central Veracruz, Mexico. We reported the best models, according to the bias-corrected Akaike information criterion (AICc). We also give the change in AICc between the best model and the next best and worst. Finally, an R2 measuring variation explained by the model is given.
| Response | Model | AICc | ΔAICc (next best) | ΔAICc (worst) | R2* |
|---|---|---|---|---|---|
| Stem density | Elevation + Successional stage + Elevation : Successional stage | 558.246 | 5.262 | 40.652 | 0.64* |
| Diameter at breast height | Elevation + Successional stage + Elevation : Successional stage | 257.162 | 4.321 | 4.321 | 0.43 |
| Basal area | Elevation + Successional stage + Elevation : Successional stage | 335.788 | 0.373 | 0.373 | 0.57 |
| Mean tree height | Elevation + Elevation2 + Successional stage + Elevation : Successional stage + Elevation2 : Successional stage | 178.070 | 1.109 | 1.109 | 0.71 |
| Aboveground biomass | Elevation + Elevation2 + Successional stage + Elevation : Successional stage + Elevation2 : Successional stage | 521.023 | 18.010 | 37.654 | 0.59* |
*In the case of stem density and aboveground biomass it refers to the proportion of deviance explained by a GLM (D 2).
Table 3 Variation of the measured forest stand parameters along the elevational gradient per plot (n = 40).
| Elevation (m) | Habitat | Stem density | DBH † | BA ‡ | Height § | AGB ¶ |
|---|---|---|---|---|---|---|
| 500 | Old-growth | 610.0 ± 150.6 | 18.5 ± 3.2 | 21.4 ± 5.9 | 14.5 ± 1.4 | 172.7 ± 86.1 |
| Secondary | 520.0 ± 87.3 | 20.7 ± 6.2 | 24.8 ± 13.2 | 10.9 ± 1.7 | 188.7 ± 118.0 | |
| All | 565.0 ± 125.4 | 19.6 ± 4.8 | 23.1 ± 9.8 | 12.7 ± 2.4 | 180.7 ± 97.8 | |
| 1,500 | Old-growth | 605.0 ± 119.1 | 21.4 ± 3.3 | 32.6 ± 13.6 | 16.6 ± 2.0 | 397.1 ± 228.9 |
| Secondary | 830.0 ± 185.7 | 20.9 ± 3.7 | 49.3 ± 14.1 | 13.5 ± 1.3 | 398.4 ± 107.7 | |
| All | 717.5 ± 189.0 | 21.1 ± 3.3 | 41.0 ± 15.7 | 15.1 ± 2.3 | 397.8 ± 168.7 | |
| 2,500 | Old-growth | 650.0 ± 271.0 | 29.4 ± 9.4 | 68.9 ± 21.8 | 17.5 ± 2.7 | 799.4 ± 451.7 |
| Secondary | 1,015.0 ± 307.5 | 23.4 ± 5.0 | 51.9 ± 14.2 | 13.8 ± 2.4 | 353.5 ± 156.3 | |
| All | 832.5 ± 334.2 | 26.4 ± 7.8 | 60.4 ± 19.5 | 15.6 ± 3.1 | 576.4 ± 396.0 | |
| 3,500 | Old-growth | 735.0 ± 217.7 | 33.8 ± 6.9 | 76.3 ± 10.1 | 21.3 ± 2.7 | 513.6 ± 88.8 |
| Secondary | 1,800.0 ± 948.2 | 18.3 ± 3.8 | 48.2 ± 9.3 | 12.3 ± 1.1 | 175.6 ± 35.7 | |
| All | 1,267.5 ± 857.7 | 26.0 ± 9.7 | 62.2 ± 17.4 | 16.8 ± 5.1 | 344.6 ± 189.2 |
Mean values and standard deviation (SD) of the successional stage (habitat) in four elevational belts (each group contains five plots).
†Trees mean diameter at breast height (cm),
‡Basal area (m2/ha),
§Mean tree height (m) and
¶Aboveground biomass (t/ha)
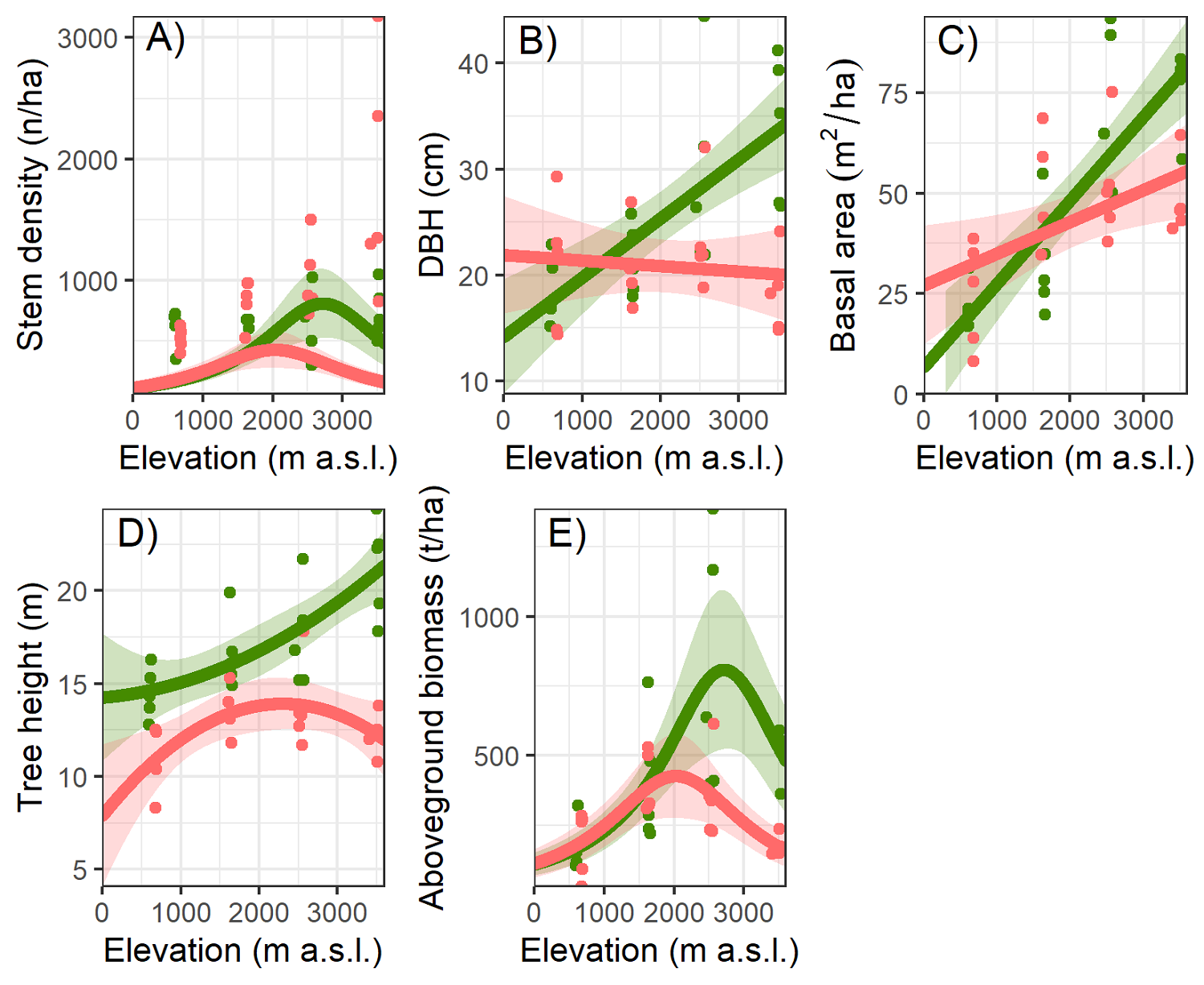
Figure 2 Forest stand parameters. Forest stand parameters along an elevational gradient and two successional stages (old-growth forest = green and secondary forest = red) at the Cofre de Perote, Veracruz, Mexico. A = stem density (n/ha), B = mean tree diameter at breast height (DBH in cm), C = basal area (m2/ha), D = mean tree height (m), and E = aboveground biomass (t/ha). We fitted the lines from a linear model (generalized linear models in the case of stem density and aboveground biomass), the shaded area marks confidence intervals (95 % confidence envelopes).
A model including elevation, successional stage, and its interaction explained less than half of the variation in mean dbh (R 2 = 0.43, F = 10.79, P < 0.001; Table 4). Elevation had a significantly positive effect on mean dbh (P < 0.001). Also, the interaction between elevation and the successional stage had a significant effect on mean dbh (P < 0.001; Figure 2B). At 500 m, higher values of mean dbh were found in secondary forests (20.7 cm ± 6.2) than in old-growth forests (18.5 cm ± 3.2). However, the contrary occurred as elevation increased with higher mean dbh in old-growth forests at the highest elevation (Table 3).
Table 4 Regression models of stand parameters of trees along the elevational gradient. Empty cells indicate terms not included in the best model for a given response variable.
| Term | Stem density | DBH† | BA‡ | Mean tree height§ | AGB¶ |
|---|---|---|---|---|---|
| Intercept | 1.76e-3*** | 14.19*** | 6.71 | 14.25*** | 9.04e-3*** |
| Elevation | -1.03e-7 ± 9.69e-8 | 0.01 ± 1.14e-3*** | 0.02 ± 3.04e-3*** | 3.66e-4 ± 1.92e-3 | -5.75e-6 ± 1.38e-6*** |
| Elevation2 | 4.47e-7 ± 4.55e-7 | 1.06e-9 ± 2.78e-10*** | |||
| Secondary forest | 2.94e-4 ± 3.20e-4 | 7.71 ± 3.82 | 20.37 ± 10.20 | -6.36 ± 2.53* | -2.09e-4 ± 2.63e-3 |
| Elevation: Successional stage |
-3.28e-7 ± 1.19e-7** | -0.01 ± 1.64e-3*** | -0.01 ± 4.34e-3*** | 4.88e-3 ± 2.84e-3 | -6.57e-7 ± 2.44e-6 |
| Elevation2: Successional stage |
-1.59e-6 ± 6.70e-7* | 5.22e-10 ± 5.52e-10 |
Listed are the output for the model and the parameters elevation, elevation2, successional stage (secondary forest and old-growth forest), and their interaction.
Asterisk means different significant p-values (*P < 0.05, **P < 0.01, ***P < 0.001).
†Mean tree diameter at breast height (cm)
‡Basal area (m2/ha)
§Mean tree height (m)
¶Aboveground biomass (t/ha)
The model explained more than half of the variation in basal area (R 2 = 0.57, F = 18.22, P < 0.001) and showed a significant positive influence of elevation (P < 0.001) and the interaction with successional stage (P = 0.006; Figure 2C and Table 4). At lower elevations, the larger basal area was in secondary forests compared to old-growth forests, though the opposite pattern was found at higher elevations (Table 3).
The model for mean tree height (R 2 = 0.71, F = 19.71, P < 0.001) revealed that secondary forests had significant lower mean height (P = 0.02), as well as the interaction with elevation (P = 0.02; Figure 2D and Table 4). In general, trees in old-growth forests are taller than trees in secondary forests and this is more pronounced at higher elevations (Table 3).
The model for AGB (D 2 = 0.59, P < 0.001) revealed a significant effect only for elevation and its quadratic term (P < 0.001; Figure 2E and Table 4). The AGB of the study sites showed a unimodal pattern, with a peak at 2,500 m (576.4 t/ha) and a decrease in the extremes of the gradient (Figure 2 and Table 4). The widest range in the same elevation and the same successional stage occurred in old-growth forests at 2,500 m, where the lowest value was 399.2 t/ha and the highest 1,385.4 t/ha (Figure 2 and Table 3).
Discussion
Forest stand parameters along the elevational gradient at the Cofre de Perote mostly agree with general trends of elevational gradients and anthropogenic influences of tropical mountains worldwide. In general, these show an increase in stem density, mean tree diameter at breast height, and basal area with increasing elevation (Aiba & Kitayama 1999, Moser et al. 2008, Alves et al. 2010, Culmsee et al. 2010, Slik et al. 2010). There is also a decrease in stem density, mean tree diameter at breast height, and basal area in the early stages of succession. We did not find a decreasing pattern with elevation, but a hump-shaped pattern on the mean tree height of secondary forest and AGB of both successional stages. Therefore, biomass and other forest stand parameters are expected to change along elevational gradients due to variations of climatic conditions. The interaction between succession and elevation was important for all forest stand parameters except for ABG (Tables 2, 4).
Stem density. Stem density is higher in secondary than in old-growth forests, this is fully expected, as trees in secondary forests are usually denser and characterized by smaller and thinner stems compared to undisturbed forests (Marin-Spiotta et al. 2007, Ensslin et al. 2015). There is an increase of stem density of secondary forests with elevation, which is a pattern found in several studies across the tropics (Grubb 1977, Raich et al. 1997, Tanner et al. 1998, Waide et al. 1998, Aiba & Kitayama 1999, Givnish 1999, Kitayama & Aiba 2002, Takyu et al. 2005, Lovett et al. 2006, Moser et al. 2007, Alves et al. 2010, Slik et al. 2010). These differences in stem density are perhaps linked to light responses (secondary forests have a more open canopy with more light), higher light incidence in the understory and processes that are more related to germination and competition (Slik et al. 2010).
Mean diameter at breast height. There is an increase of mean dbh of old-growth forest with elevation, this pattern was also found in other studies (Lieberman et al. 1996, Scaranello et al. 2012). A possible explanation is that at lower elevations there is an influence of high seasonality with a prolonged drought season, creating stress factors that influence the dbh (Suwa et al. 2013, SMN 2018). Fisher et al. (2013) found that there is a co-limitation of N and P at lower elevations, and thus trees having low dbh values. Also, they found that trees at higher elevations concentrate more on dbh change than on foliar nutrient concentration, which explains that trees at higher elevations in old-growth forests have the highest dbh values (Fisher et al. 2013). Less carbon distribution to roots implies that additional carbon can be allotted away, such as to wood (i.e., dbh; Fisher et al. 2013).
We also found a pattern of high dbh values in secondary forests at lower elevations and the opposite at higher elevations. This is explained by the differences between the needle and broadleaf species along the elevational gradient. This pattern is common with lower dbh associated with needle leaf trees, in our case Pinus spp. of secondary forests at mid and high elevations (Gao & Zhang 2006). These changes are related to environmental conditions such as temperature and precipitation, as well as the ecological and biological characteristics of the species (Gao & Zhang 2006).
Basal area. Basal area increased with elevation, this pattern is called additive basal area trend, in which the basal area of mixed conifer-broadleaf diverse woods befalls is larger than that of broadleaf woodlands (Enright & Ogden 1995, Aiba et al. 2007). Different use of the light by conifers and broadleaf plants that have dissimilar aboveground architectures have been proposed as the reason for additive basal area, though belowground reserve partitioning may be also involved (Enright 1982, Ogden 1985, Lusk 2002, Midgley et al. 2002, Aiba et al. 2007).
We found that along our elevational gradient, an indication of the additive basal area is the increase in the basal area by the coniferous domain (Aiba et al. 2007). This can be interpreted as the findings of matching reserve usage by various life types (Cannel et al. 1992, Kelty et al. 1992, Aiba et al. 2007). In order not to compete with broadleaf trees for space (to expand their crowns), conifers emerge beyond the canopy of broadleaf trees. By reducing light competition between broadleaf and coniferous trees, these features allow for the most effective use of light at the stand level. Consequently, we indicate that the additive basal area of conifers is related to their growing prominence and improves productivity (Aiba et al. 2007).
We found higher values of basal area in young secondary forests at lower elevations. However, in secondary fallow forests after 15-20 years of regrowth, the basal area can quickly recover (Guariguata et al. 1997, Guariguata & Ostertag 2001, Montgomery & Chazdon 2001). This pattern appears to be the broad process of natural recuperation at low elevations (Álvarez-Yépiz et al. 2008). However, we found higher values of basal area in old-growth forests at mid and high elevations.
Mean tree height. In general, we found that old‐growth stands were of higher stature than secondary forests along the elevational gradient. There is a monotonic increase of mean tree height in old-growth forests with elevation, which is not a common pattern. In tropical mountains, it is expected that tree height typically decreases with increasing elevation (Leigh 1975, Bruijnzeel & Veneklaas 1998, Moser et al. 2011, Unger et al. 2012). Low altitude sites are exposed to more sunlight for longer periods than higher elevation sites, which is another reason why old-growth forests may have higher trees (Alves et al. 2010).
Otherwise, there is a hump-shaped pattern of mean tree height in secondary forests with elevation. This pattern represents that trees of secondary forests at mid-elevations recover faster due to favorable climatic factors. Normally diminishing accessibility of nutrients, particularly nitrogen, has been proposed to reduce tree height with growing elevation (Grubb 1977, Tanner et al. 1998, Alves et al. 2010), which can be the case in secondary forests at high elevations. Therefore, the highest tree heights at mid-elevations are due to the balance of climate, disturbance, and slope (Marshall et al. 2012).
Aboveground biomass. The AGB reported in this study varies widely along the elevational gradient. The lowest AGB occurred in Palmarejo, our site at 500 m. In general, at higher elevations, we found more AGB in old-growth forests than in secondary forests, which is like findings in other regions of Mexico (González-Zárate 2008, Ordóñez-Díaz et al. 2008). Also, Mendoza-Ponce & Galicia (2010) projected models of the future tree biomass indicating that the highest biomass will be found in secondary forests. This shows the importance of the species composition, age, and density (trees/ha) of these sites. For instance, presently, old-growth forests have the largest biomass, but in a 50-year-estimate, they will have the lowest possible biomass of all the woodlands due to the reason that they will not increase biomass and its density is low (trees ha−1).
The increase in aboveground biomass and carbon stocks of dead organic matter due to forest growth on abandoned agricultural land is one of the main processes to explain the C sink (Post et al. 1990, Johnson 1992, Mendoza-Ponce & Galicia 2010). The accumulation of AGB (tree) in secondary forests of the Cofre de Perote landscape may represent important reservoirs of C. These values suggest the significance of reassessing the position of these successional plots as C sinks.
Age, species composition, and population density of each vegetation stratum affect the ability of forest ecosystems to store C in biomass (Acosta-Mireles et al. 2002, Mendoza-Ponce & Galicia 2010). Mature sites contain the lowest C values because rates of C uptake and growth are lower than rates in young forests, so secondary forests will play the most important role in C storage (Mendoza-Ponce & Galicia 2010). This suggests that with appropriate thinning and harvesting rotations, secondary forests will reach AGB of old-growth forests in ∼20 years, allowing plans for sustainable production of wood and energy after the establishment of trees; this period is recommended considering the maximum growth rate of those species (Mendoza-Ponce & Galicia 2010). The forests that should be managed are located at the lower elevations, as well as in areas that under sustainable management plans could be reforested because they have been disturbed to create C sinks (Mendoza-Ponce & Galicia 2010). In nearly all studied elevations, the estimated AGB was higher than expected.
The old-growth forests at higher elevations had the highest AGB, hence, historical deforestation rates have produced C emissions. Nowadays, old-growth forests at higher elevations have the highest AGB (C stock) but with little potential for future C storage. In contrast to secondary forests at higher elevations, which have the smallest AGB (C stock) but show encouraging projections for C storage in the future. In the regeneration of coniferous forests, abandoned areas are important due to their potential to reduce C emissions and C storage. Finally, we found that forests at low elevations (500 and 1,000 m) along this elevational gradient are more resistant to human-caused disturbance.
In contrast to an expected constant decrease in AGB, our results indicate a unimodal pattern, which increases with elevation and reaches its peak at 2,500 m. Mid-elevation peaks in AGB have been found by other studies (Culmsee et al. 2010, Marshall et al. 2012, Ensslin et al. 2015). However, AGB estimated by Marshall et al. (2012) peaked at 1,500 m but reached values up to 540 t/ha. Ensslin et al. (2015) found a peak at 2,200 m, which is a similar elevation compared to the estimates of this study, even though their estimate of 365 t/ha is lower than the AGB at 2,500 m of this study. An increase of AGB with increasing elevation, which did not exceed elevations of 1,500 m, was also documented by Alves et al. (2010) and Rosenfield & Souza (2014). Overall, these results indicate that a unimodal trend in AGB with peaks around 2,200 to 2,500 m might be common for tropical elevational transects.
In both high-elevation sites, old-growth forests contain more than twice as much AGB as secondary forests. Ongoing reforestation is implemented by the local population and carried out in the form of monoculture plantations growing primarily Pinus patula, P. teocote, P. pseudostrobus var. apulcensis, and P. montezumae (Mendoza-Ponce & Galicia 2010). An increase in AGB with succession at higher elevations could be found.
Overall, we found that forest recovery of stand parameter is occurring faster at lower elevations. We found that mean dbh, basal area, and AGB recovers fast (10 to 15 years) in secondary forests at low elevations, which emphasizes their capacity for biodiversity preservation in human-altered tropical landscapes (Chazdon et al. 2016, Poorter et al. 2016, Rozendaal et al. 2019). Rapid recovery of dbh, basal area, and AGB could promote the provision of other ecosystem services, such as carbon sequestration and storage, by maintaining forest cover in the surrounding landscape to conserve seed sources and dispersers (Chazdon et al. 2016, Poorter et al. 2016, Rozendaal et al. 2019). Recovery may be much slower at high elevations.
Secondary woodlands must be left to grow to advanced age to maintain species groups in the landscape and to improve landscape connectivity, especially where old-growth forests are near (Chazdon et al. 2016, Poorter et al. 2016, Rozendaal et al. 2019). Our results indicate that to maintain tree biodiversity, natural regeneration is an effective solution. Species composition, by contrast, can take centuries to recover. Therefore, conservation strategies and restoration efforts must protect both secondary and mature forests in the landscape to promote this re-establishment of original biodiversity in secondary forests (Chazdon et al. 2016, Poorter et al. 2016, Rozendaal et al. 2019).
Our study reveals strong differences in AGB and forest structure among elevational belts and successional stages. Old-growth forests at 2,500 m had the highest aboveground biomass and the greatest difference between both successional stages. Regarding these results, remaining old-growth forest fragments in these elevations are in need to be conserved for their capacity to accumulate higher quantities of biomass and to provide ecosystem services, such as climatic regulation, water purification and collection, prevention of inundation and drought, and protection against erosion and landslides. Consequently, forest management and regulations of urban development are necessary to protect these old-growth forests. Williams-Linera et al. (2007) proposed the implementation of an archipelago reserve, a conservation concept that connects the remaining forest fragments with the help of riparian forests and biodiversity-friendly agricultural land. However, to create a sustainable management plan, it is important to cooperate with the local population. Further work is needed to transfer the results of this study to local communities and to provide data for the local nature conservation agency "Dirección del Área Natural Protegida (ANP) Cofre de Perote", to place priority areas under conservation.











 nueva página del texto (beta)
nueva página del texto (beta)



