Estimates suggest that 5.1 million fungal species exist worldwide (Blackwell 2011), and that about 53,000-111,000 of them are macromycetes (characterized by the production of fruit bodies visible to the naked eye) (Mueller et al. 2007). Geographic variation in species richness and composition of macromycete communities has been related mainly to microclimatic conditions, which can be driven by plant distribution ranges, vegetation structure, and topographic factors (e.g., slope and aspect) (Mueller et al. 2007, Zhang et al. 2010, Singha et al. 2017, Gómez-Hernández et al. 2019).
Understanding how environmental factors relate to the diversity and distribution of macromycete species is a growing interest in ecological studies due to the roles these organisms play in most terrestrial ecosystems worldwide. Macromycetes interact with other groups as pathogens, where they can be detrimental to plant growth and fitness or regulate plant and animal populations (Termorshuizen 2014). As mutualists, macromycetes can help plants improve nutrient absorption, drought tolerance, and provide pathogen resistance (Barrico et al. 2012). Also, macrofungi are the most important decomposers of organic matter in terrestrial ecosystems and play major roles in the carbon and nitrogen cycles (Harley 1971, Dighton 2016).
Knowledge on the factors influencing macrofungal diversity and distribution may be helpful to ascertain which areas support higher diversity, evaluate how macromycete functional groups respond to environmental changes and their effect on ecosystem functioning, and detect processes structuring macrofungal communities in both natural and urbanized environments (Packham et al. 2002, Gómez-Hernández et al. 2012, Caiafa et al. 2017). Several studies have shown that variation in vegetation structure and tree species composition along vegetation types, affects macrofungal communities and the presence of species associated to woody plants, due to differences in the quality and quantity of the resources provided (Ferrer & Gilbert 2003, Richard et al. 2004, Brown et al. 2006, Zhang et al. 2010). In temperate communities, a greater canopy cover has been related to shade provision and increased soil moisture and water-holding capacity, all which influence macrofungal species richness (Ferris et al. 2000, Gabel & Gabel 2007). Also, some studies indicate that precipitation (Lange 1978, Salerni et al. 2002) or both humidity and temperature (Durall et al. 2006) are the main factors related to macromycete diversity (Brown et al. 2006). However, most macromycete studies address topics on taxonomy and systematics, and there is a lack of studies relating patterns of diversity and distribution to vegetation, microclimate, and topographic factors (Brown et al. 2006, Schmit & Mueller 2007, Braga-Neto et al. 2008, Gómez-Hernández et al. 2012, Gómez-Hernández et al. 2019).
In Mexico, estimates suggest the presence of more than 250,000 species of fungi (Guzmán 1998), and approximately 9,000-11,000 species of macromycetes (Aguirre-Acosta et al. 2014). Oaxaca is one of the most biodiverse regions in the world, and one of the most biologically diverse regions in Mexico (Flores-Villela & Gerez 1994, Villaseñor 2016), but mycological studies are scarce in this area (Garibay-Orijel et al. 2009) and there are only five studies assessing patterns of diversity and distribution within and across macrofungal communities. Caiafa et al. (2017) carried out a study along an elevation gradient in the Costa region and found that the functional diversity of macromycetes varies with elevation, is related more to microclimatic variables than to vegetation structure, and that heterogeneity of trait abundance and niche complementarity increased with elevation. Avendaño (2019) conducted a survey in the Valles Centrales region of Oaxaca, and the results showed that the functional diversity of macromycetes decreased from disturbed to conserved areas, and species composition differed largely between disturbed and conserved sites. The study by Gómez-Hernández et al. (2019) in the Sierra Norte region showed markedly different macrofungal communities in forest patches with different development stages of Pinus patula Schiede ex Schl. et Cham, where substrate availability and vegetation structure were the main factors related to the observed patterns of diversity and distribution. Ruiz-Almenara et al. (2019), carried out a study in the Mixteca region and their results indicated that intensive harvesting of wild edible mushrooms did not affect the diversity and distribution of macromycete species, showing that this can be an innocuous activity as long as the general environment and the macromycete habitat are not disturbed. The findings by Gómez-Hernández et al. (2021) indicated that some of the available resources in the niche space within the most urbanized sites are not being used, and that urbanization also causes a high degree of niche differentiation among macromycete species within communities in urbanized areas. The aim of this study was to evaluate patterns of macromycete diversity and composition along different vegetation types in forests of temperate affinity, and identify microclimatic, environmental, and vegetation structure factors related to the observed patterns.
Materials and methods
Study area. This study was carried out in Ayoquezco de Aldama in the political district of Zimátlan de Álvarez, Oaxaca, Mexico. Ayoquezo is located at 16° 36’-16° 44’ N and 96° 50’- 96° 57’ W, approximately at 1,598 m asl, and is characterized by a rainy season from May to October. The climate is Semi-warm A(C) (García 2004). Mean annual temperature is > 18 °C, and annual precipitation ranges from 600 to 800 mm (Unidad de Microrregiones 2005).
In the highlands of Ayoquezco de Aldama, where this study was carried out, the vegetation is represented primarily by oak-pine and coniferous forests, which include woody species like “cuatle” (Eysenhardtia polystachya (Ortega) Sarg.), “cuachepil” (Senna septemtrionali (Viv.) H.S. Irwin & Barneby), “tepehuaje” (Lysiloma acapulcensis (Kunth) Benth.), “cazahuate” (Ipomoea murucoides Roem. & Schult.), “enebro” (Juniperus flaccida Schlechtendal.), “sabino” (Taxodium mucronatum Ten.) and “cedro” (Cedrela odorata L.) (INAFED 2016).
Study sites. Four sites were selected to carry out the samplings, and the vegetation type in each one was defined based on the dominant genus of trees: oak forest (Site 1, dominated by Quercus), oak-pine forest (Site 2, presence of Pinus, dominated by Quercus), pine-oak forest (Site 3, presence of Quercus, dominated by Pinus), and pine forest (Site 4, dominated by Pinus). At each site, 10 permanent plots of 10 ×10 m were set up, with a distance of about 10 m between plots and 30 m from the forest edge, to avoid “edge effects” (Williams-Linera et al. 1998).
Macromycete sampling. From June to November 2019, macromycete fruiting bodies were collected twice a month in every plot of the four study sites. Sporomes of the same species growing within a 50 cm radius, caespitose growth, fairy rings, and fruit bodies of the same species growing on the same log or branch were all recorded as a single collection unit, and abundance was estimated as the number of collection units (adapted from Schmit et al. 1999). Hereafter, for practical purposes, collection units will be referred to as individuals. The collected specimens were identified based on their macro and micro morphological characters aided by identification keys and reference literature (i.e., Guzmán 1977, Largent 1986, Læssøe & Petersen 2019). Species that could not be identified were classified as a morphospecies.
Environmental variables. Air temperature and humidity, and soil temperature and humidity were recorded every sampling day at a permanent point in each plot. Slope and aspect (slope orientation) were measured once at each plot. The diameter, height, and number of woody plants with diameters > 10 cm at breast height (1.3 m above the ground) were also recorded. Vegetation structure was characterized as density of trees, mean and maximum height of trees, canopy openness and basal area (Supplementary Material, Table S1).
Diversity and species composition. The diversity of macromycetes was measured by species richness (number of species) and by the True Diversity index of first order (1D), which is a suitable metric to use for macromycete data because it weighs all species by their frequency, without favoring either common or rare species (Jost 2006). This index was calculated with the entropart package in R v. 3.2.3 (R Core Team 2017). The none-parametric species richness estimator Chao 2 was used to determine how complete were the species inventories recorded in every study site. The turnover of species composition between study sites was calculated with the abundance-based Chao-Jaccard similarity index using EstimateS (Colwell 2013). This index is neither sensitive to sample size nor to numerous rare species in a community, as in the case of macromycete communities (Chao et al. 2005).
Data analyses. Prior to statistical analyses, the assumption of normality was assessed performing Shapiro-Wilk tests. The Spearman correlation coefficient (ρ) was used to determine the relation between macromycete species richness and microclimatic, topographic and vegetation variables. A regression tree analysis was carried out to assess how the explanatory variables affect the variation of macromycete species richness in the study area. The relationship between the geographic distance between sites and changes in species composition was calculated using linear regression analyses. A Non-metric multidimensional scaling (NMDS) analysis was used to visualize the distance between each study site according to species composition. The relationship between the distribution of species and the explanatory variables in each community was determined with a Canonical Correspondence Analysis (CCA).
All data analyses mentioned in this section were performed in R v. 3.2.3.
Results
A total of 617 macromycete individuals were registered, belonging to 186 species, 60 genera, and 42 families. Of the species collected, 22 belong to ascomycetes and 165 to basidiomycetes (Supplementary Material, Table S2). The highest species richness was found in Site 3 (75 species) (pine-oak forest), followed by Site 1 (67) (oak forest), Site 2 (60) (oak-pine forest), and Site 4 (49) (pine forest) (Supplementary Material, Table S3). The True Diversity index showed that Site 1 had the highest diversity (49.83), followed by Site 3 (40.86), Site 2 (36.57), and Site 4 (31.17). The species richness estimator Chao 2 indicated that the completeness of the macromycete species inventory in Site 1 was 51.56 %, whereas in Site 2 was 50.21 %, Site 3 was 53.10 %, and Site 4 was 48.42 %.
Spearman correlations indicated a significant negative correlation between macromycete species richness and aspect (ρ = - 0.4307, P = 0.0055) as well as canopy openness (ρ = -0.4321, P = 0.0053). The regression tree analysis (residual mean deviance = 40.05) indicated that from a total of 40 plots in the study area, the variation of species richness in 42.5 % of them occurred when air humidity was under 48.85 %. From this 42.5 % of all plots, species richness in 52.99 % of them was influenced by canopy openness under 27.56 %. Additionally, from the 40 plots, the number of species in 57.5 % of them was affected by air humidity over 48.85 %, and species in 73.91 % of the mentioned 57.5 %, were affected by air temperatures higher than 20.98 °C. Species richness in 52.94 % of the 73.91 % mentioned before, varied when soil humidity was less than 35.41 % (Figure 1).
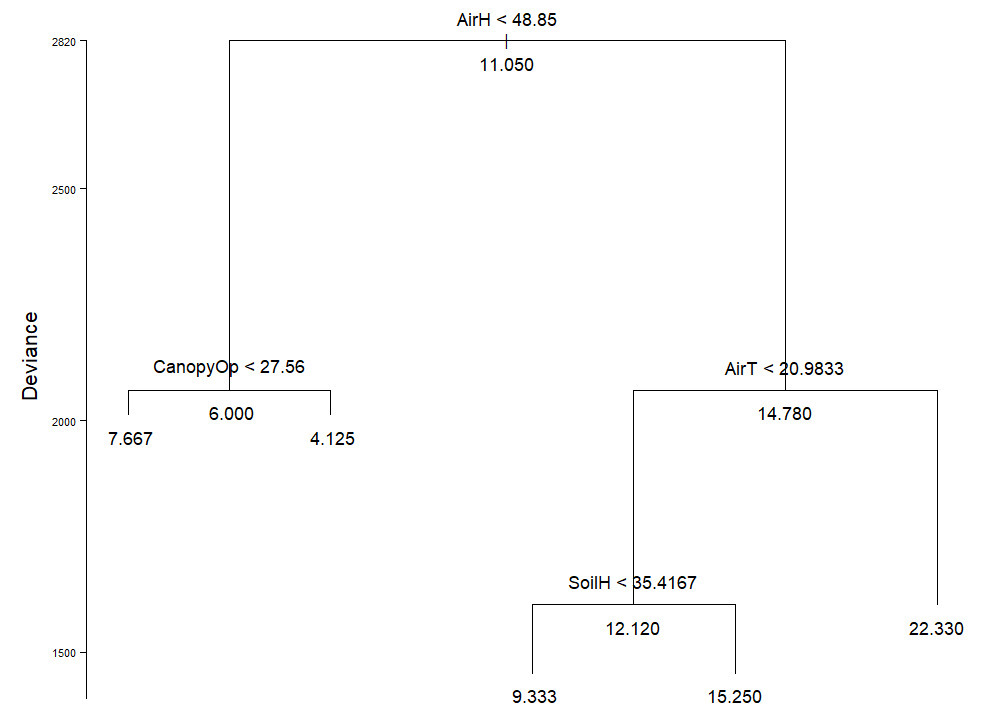
Figure 1 Regression tree for macromycete diversity. Partitions show the environmental variables and the threshold at which the partition was made. The average value of variable effect is indicated at the tips and nodes. Variables are: air humidity (AirH), canopy openness (CanopyOp), air temperature (AirT), and soil humidity (SoilH).
The Chao-Jaccard similarity index indicated that the similarity of species composition was higher for sites 1 and 2 (0.36) (oak, oak-pine), followed by sites 3 and 4 (0.26) (pine-oak, pine), sites 2 and 3 (0.22) (oak-pine, pine-oak), sites 2 and 4 (0.17) (oak-pine, pine), sites 1 and 4 (0.14) (oak, pine), and lastly site 1 and 3 (0.09) (oak, pine-oak). NDMS showed that sites 1 and 2 are the closest in terms of their species composition while sites 2 and 4 are the farthest apart (Figure 2). The CCA for microclimatic, topographic and vegetation variables included 187 macromycete species. The model showed that sites 1 and 2 are clearly separated from sites 3 and 4 along Axis 1 (eigenvalue = 0.7151) and site 3 is separated from site 4 along Axis 2 (eigenvalue = 0.6393). Axis 1 (14.85 %) and Axis 2 (12.83 %) together explained 27.68 % of the total variance (Figure 3).
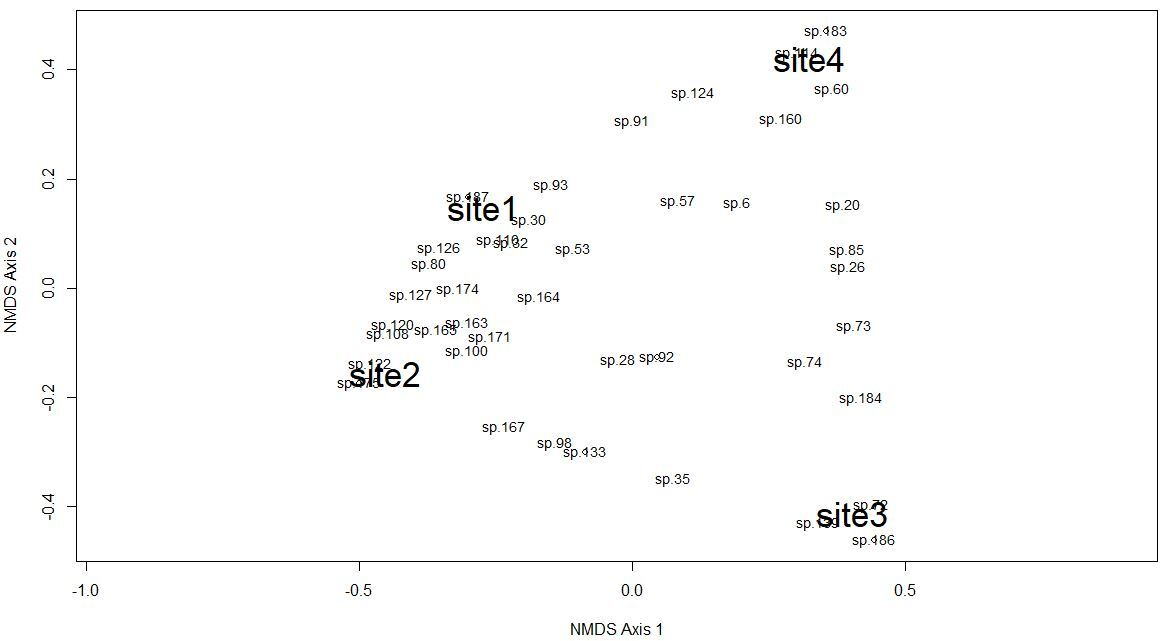
Figure 2 Non-metric Multidimensional Scaling analysis for macrofungal species complementarity among the study sites; Site 1 (oak forest), Site 2 (oak-pine forest), Site 3 (pine-oak forest), and Site 4 (pine forest). “sp1, sp2, sp3, sp4…” represents the species recorded in the study area, and the numbers of the species correspond to the numbers (#) in Supplementary Material, Table S2.
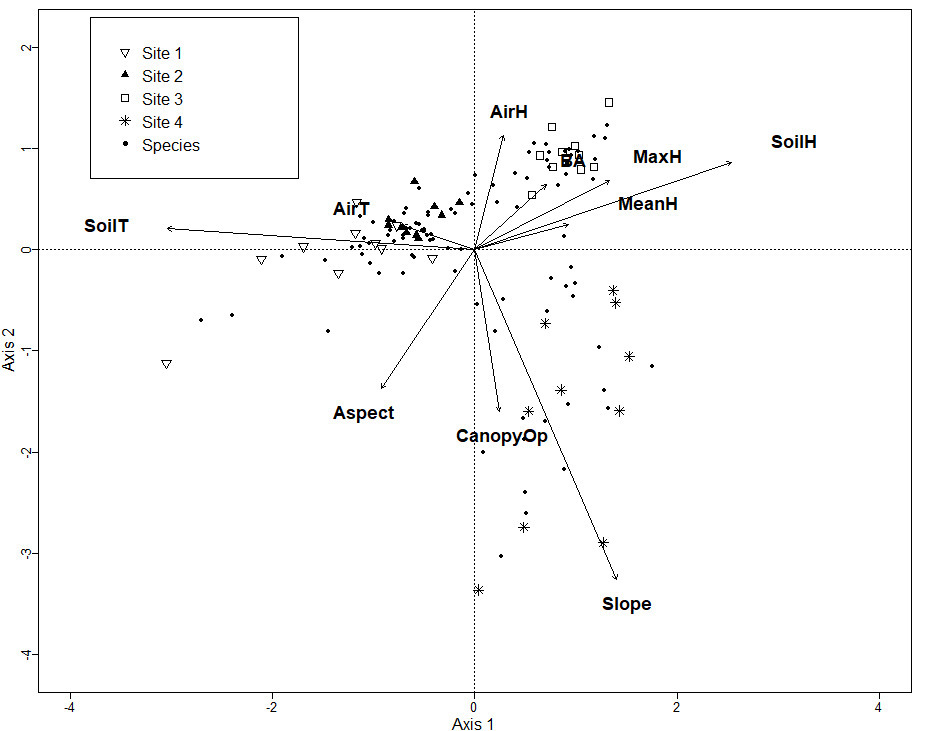
Figure 3 CCA for all the macromycete species recorded in the study; Site 1 (oak forest), Site 2 (oak-pine forest), Site 3 (pine-oak forest), and Site 4 (pine forest). Vectors represent environmental explanatory variables: AirH (air humidity), BA (basal area), MaxH (maximum height of trees), MeanH (average height of trees), SoilH (soil humidity), Slope, CanopyOP (canopy openness), Aspect, SoilT (soil temperature), AirT (air temperature).
Discussion
Our results show significant variation in macromycete diversity between vegetation types. In correspondence with our findings, several studies assessing local patterns of macromycete diversity in both temperate and tropical forests, suggest that the number of macrofungal species and fruitbody production are related to topographic and microclimatic factors, mainly air and soil humidity and temperature (O’Dell et al. 2000, Brown et al. 2006, Gómez-Hernández & Williams-Linera 2011, Caiafa et al. 2017).
In the present study, the regression tree analysis indicated that the number of macrofungal species in the study area is influenced mostly by changes in air humidity, air temperature and soil humidity. The analysis suggests that species richness in most of the sampled area is favored when air humidity is above 48.8 %, however, it has been reported that air humidity can be more advantageous for macromycete productivity when it is around 80 % (Shuhada et al. 2020). Comparable to studies showing that macromycete species richness decreases when temperature is over 27 °C (Jang & Hur 2014, Ghate & Sridhar 2016), our results indicated that macromycetes benefit with air temperatures below 20.9 °C. Similarly, it is broadly known that excessive soil water content can inhibit macromycete production and hence, species richness (O’Dell et al. 1996, Lodge et al. 2004), which corresponds with our finding suggesting that macromycetes benefit with soil humidity under 35.4 %. However, fruitbody production and occurrence of the different macromycete species along the rainy season depend on specific humidity and temperature ranges (Wilkins & Harris 1946, Thoen 1976).
Changes in temperature and humidity affecting macromycete communities along environmental gradients are a function of the varying vegetation structure and factors like slope or aspect (Cavender-Bares et al. 2009, Zhang et al. 2010, Gómez-Hernández et al. 2019). Consistent with our results, studies have found that an open canopy exposes the surrounding soil to sunlight, which can cause high soil temperatures and loss of humidity, both unfavorable for macrofungal growth (Ferris et al. 2000, Egli et al. 2010, Ford et al. 2018). Our results also showed that the aspect of the terrain is negatively related to macrofungal diversity, because landscape topography influences water drainage and evaporation rate, thereby affecting fruit body production and species richness (Rubino & McCarthy 2003, Gómez-Hernández et al. 2012).
The turnover of macromycete species composition was high between the four studied sites, with the oak-dominated forests being the most similar. Although geographic distance has been related to species turnover between macromycete communities, there was no correlation between species turnover and geographic distance in our studied area, but the distribution of macromycete species can be explained by variables related to changes in the structure and composition of tree communities (Brown et al. 2006, Gómez-Hernández et al. 2019). Each of our study sites had their own characteristics, but results indicated that soil temperature, canopy openness, and maximum tree height were the main factors driving macromycete distribution. Studies in forests of temperate affinity have found that macromycete distribution can be influenced by the composition of woody plant species, vegetation structure (e.g., basal area, canopy openness, height of trees) and factors buffering temperature rise and humidity loss (Gabel & Gabel 2007, Gómez-Hernández et al. 2019). Variation in the composition of tree species and vegetation structure involve changes in microclimatic conditions and in the quality and quantity of available resources, which can highly influence macromycete communities (Lodge 1997, Ferris et al. 2000, Gómez-Hernández et al. 2019).
Patterns of diversity and composition of macromycete functional guilds along the different vegetation types were not analyzed in this study. However, information on this issue should be of interest and relevance for future studies since changes in vegetation type can alter macrofungal communities due to their trophic strategies make them require biological interactions such as mycorrhizal plant hosts or plant debris as substrates (Gabel & Gabel 2007, Newbound et al. 2010). The relationship between vegetation type and macrofungal communities is reflected in host trees affecting macromycete specialization and providing unique habitat availability and different resource quality (Villeneuve et al. 1989, Richard et al. 2004, Brown et al. 2006, Zhang et al. 2010). In pine and oak forests, both ectomycorrhizal and saprotrophic richness and composition of species have been observed to change meaningfully as the number of forest layers vary, suggesting that macrofungal communities are mainly shaped by host plants, forest structure, physicochemical attributes of the soil, and quality of the substrata (Dighton & Mason 1985, Villeneuve et al. 1989, Fernández-Toirán et al. 2006).
This study contributes with ecological knowledge on macromycete communities associated to temperate forests in Oaxaca. Results can be used to assess ecosystem quality and determine changes in ecological dynamics (Rojas et al. 2017). Also, the information generated can be useful for conservation decisions and management recommendations given that forests with high macromycete diversity were identified, and factors limiting species distribution and growth were detected (Gabel & Gabel 2007, Dejene et al. 2017).
Our findings showed that macromycete diversity and composition can change conspicuously along relatively small areas due to differences in the local environment regarding the heterogeneity of habitats and resources provided by woody plant species. It should be of interest for future studies to assess how this variation affects the different functional groups along vegetation types. Furthermore, assessing variables related to soil characteristics could be useful to better understand the observed patterns of diversity and distribution. To complement ecological studies on macrofungi and better understand how macromycete diversity and distribution are influenced by biotic and abiotic factors, it is necessary to carry out surveys in different ecosystems and include metrics based on functional and phylogenetic information to make inferences about the evolutionary and ecological processes structuring macrofungal communities.
Supplementary material
Supplemental data for this article can be accessed here: https://doi.org/10.17129/botsci.3012











 nueva página del texto (beta)
nueva página del texto (beta)



