Forest ecosystem functioning is primarily determined by the role played by tree species such that any natural or induced alteration of tree stratum can modify the ecological processes of the forest (Cadotte et al. 2011). Even under temporary altered conditions, the capacity of ecosystems to maintain the direction and intensity of their ecological processes has been named functional stability (FS) (Donohue et al. 2013). This is a complex concept since it includes emergent ecosystem properties such as asymptotic stability, resilience, resistance, robustness, persistence, and variability (Donohue et al. 2013). Measurement of FS is intrinsically difficult, but it is commonly represented in models that indicate that ecosystems with high species richness and/or diversity will present higher stability (Peterson et al. 1998). These diversity-stability models are based on observations from forest systems with complex structures in their mature condition, such as tropical, subtropical, or temperate broadleaved forests (Tilman et al. 1996, Keith et al. 2008). Nevertheless, the diversity-stability relationship is non-linear, since the number of added species per se does not explain the complete response of the ecological processes. There is evidence that the response of the ecological processes also depends on the metabolic traits of a few species and the interactions between these species and other organisms, which is noticeable in forests with low tree species richness (Peterson et al. 1998, Wu et al. 2013).
Litter decomposition is a key ecological process that provides a route for nutrient recycling that directly affects primary productivity (Suseela & Tharayil 2018). It is described as the physical and chemical transformation of senesced plant biomass, mainly leaves and non-woody tissues, to CO2 and mineral ions as the ultimate products through microbial mineralization (Berg 2014, Suseela & Tharayil 2018). Litter decomposition, and consequently C mineralization, is controlled by environmental conditions and the interaction between litter chemical traits and the microbial metabolism (Gartner & Cardon 2004, Grossman et al. 2020). The process is therefore highly sensitive to tree stratum modification or gap effects (Zhu et al. 2014).
At the local scale, the mass and the chemical traits of litter and the microbial metabolic activity of its communities are the main drivers of litter decomposition (Meier & Bowman 2008, Aubert et al. 2010, Wu et al. 2013, Ge et al. 2017). These factors are controlled by plant community species composition and relative abundance, which are in turn influenced by the plant species richness (Prescott 2002, Purahong et al. 2014). Consequently, change in the plant species richness through modification of the species composition has been reported to affect litter decomposition (Li et al. 2013, Wu et al. 2013). The effect of an increase in species richness on litter decomposition is expected to be positive, where tree species richness promotes the decomposition process, reflected in high rates, with a consequent positive effect on nutrient recycling (Gessner et al. 2010) and a low coefficient of variation (Keith et al. 2008). This suggests that systems with high plant species richness, such as mixed forests, present high litter decomposition rates, low variability in forest litter decomposition and thus high stability (Bonanomi et al. 2010, Wu et al. 2013). Similarly, since most of the coniferous temperate forests are characterized by low species richness with a high tendency towards monospecificity, the addition of plant species to the system could increase the litter decomposition rate (Xiao et al. 2019). However, the effect of such an addition on the stability of litter decomposition is unknown.
The disturbances such as wildfires and forest management practices may modify the tree stratum in coniferous forests, affecting litter decomposition in different ways, like quick changes in the litter chemical composition or a modification of the microbial metabolism (Holden et al. 2013, Ge et al. 2017). Nevertheless, the long-term effects are related to a change in the composition of plant and microbial communities (Ficken & Wright 2017). In coniferous forests, the disturbances that modify the tree stratum by increasing plant species richness significantly impact litter decomposition (Torras & Saura 2008, Boch et al. 2013, Griffiths et al. 2014, Xiao et al. 2019). This functional consequence is promoted by the change in the chemical composition (e.g., nutrient concentration) of the litter under the new condition (Osono & Takeda 2005, Vivanco & Austin 2008, Berg 2014, Wang et al. 2018) and by the consequent saprotrophic microbial response, expressed as adjustments of metabolic capacity to degrade organic compounds by exoenzymes (Aubert et al. 2010, Purahong et al. 2014) in order to promote C mineralization and use C efficiently. In particular, an increase in the number of broadleaf species in the tree stratum promotes a higher concentration of nutrients and labile molecules. This favors the production and activity of microbial exoenzymes and consequently acts to increase C mineralization, which reflects a low efficiency of the microorganisms in terms of storing C in their biomass (Aubert et al. 2010, Mooshammer et al. 2014). Microbial activity therefore provides a reliable indicator of the litter decomposition under conditions of modification of the tree stratum, mainly when these are standardized by C in microbial biomass through the specific enzymatic activity (SEA) and metabolic quotient (qCO2) (Chávez-Vergara et al. 2014, 2018).
The sacred fir (Abies religiosa (Kunth) Schltdl. & Cham.) is an endemic conifer species from Mexico that is distributed in high mountain sites of the Trans-Mexican Neovolcanic Belt in an elevation range of 2,400 to 3,500 m asl (Rzedowski 1978). Natural mature communities have very low richness in the tree stratum structure since they are commonly monospecific. These forests are characterized by a highly dense and closed A. religiosa canopy that reaches up to approximately 50 meters in height, with a small number of accompanying species in the understory (Sánchez-González et al. 2005, Cuevas-Guzmán et al. 2011, Endara-Agramont et al. 2012, Zepeda-Gómez et al. 2018, Hernández-Álvarez et al. 2021). Furthermore, A. religiosa forests are one of the montane ecosystems with the highest soil organic carbon stocks in Mexico (Santini et al. 2020).
However, this forest ecosystem is exposed to natural and anthropic disturbances across a wide range of their natural distribution, including wildfires and management activities. This has promoted fragmentation and opening of the canopy and delayed tree establishment, with consequences for the tree stratum structure (Endara-Agramont et al. 2012, Zhu et al. 2014, Montoya et al. 2020) that include an increase in the plant species richness, which, as a result of silvicultural strategies, can be represented by naturally regenerated secondary broadleaf species and/or non-native reforested species (Sánchez-Velásquez et al. 1991, Pineda-López et al. 2013, Santibañez-Andrade et al. 2015). In addition, it is estimated under projected climate change scenarios that the potential area occupied by these forests will present a 69 % reduction by 2030, promoting new fragments with modified conditions in the tree stratum (Sáenz-Romero et al. 2012). However, few studies have evaluated the effect of the plant community changes that have taken place in A. religiosa forests on the processes that control soil carbon stocks in these forests, such as litter decomposition and its stability (Barajas-Guzmán et al. 2020).
This study aimed to determine the effect of altering the tree stratum on microbial C use efficiency as a driver of C mineralization, and on the stability of litter decomposition in a sacred fir (A. religiosa) forest. We hypothesized that the increase in species richness of the tree stratum of the A. religiosa forest will promote litter C mineralization through a reduced C use efficiency by microorganisms, and will enhace the stability of litter decomposition. The Importance Value Index (IVI) was measured in order to determine the status of the tree strata alteration in the heterogeneous condition. Litter samples were collected in both conditions to determine chemical composition, enzyme activities, C in microbial biomass, C mineralization, specific enzymatic activity, and the metabolic quotient. The coefficient of variation was used as a proxy for litter decomposition stability (Tilman et al. 2006, Bonanomi et al. 2010, Wu et al. 2013).
Materials and methods
Study site and plot selection. We selected two adjacent areas of the same continuous A. religiosa forest on the Sierra de las Cruces mountain ranges in the Trans-Mexican Neovolcanic Belt (Figure 1). In both areas, the soil is a humic Andosol derived from quaternary volcanic ash deposits. The climate is described as sub-humid semi-cold with summer rains (June to October) (Cb' (w2) (w) ig), with an annual mean temperature of 10 °C and average annual rainfall of 1,370 mm (Rodríguez-Palacios 2009, CONANP-SEMARNAT 2006, Tovar-Velasco & Valenzuela-Garza 2006).
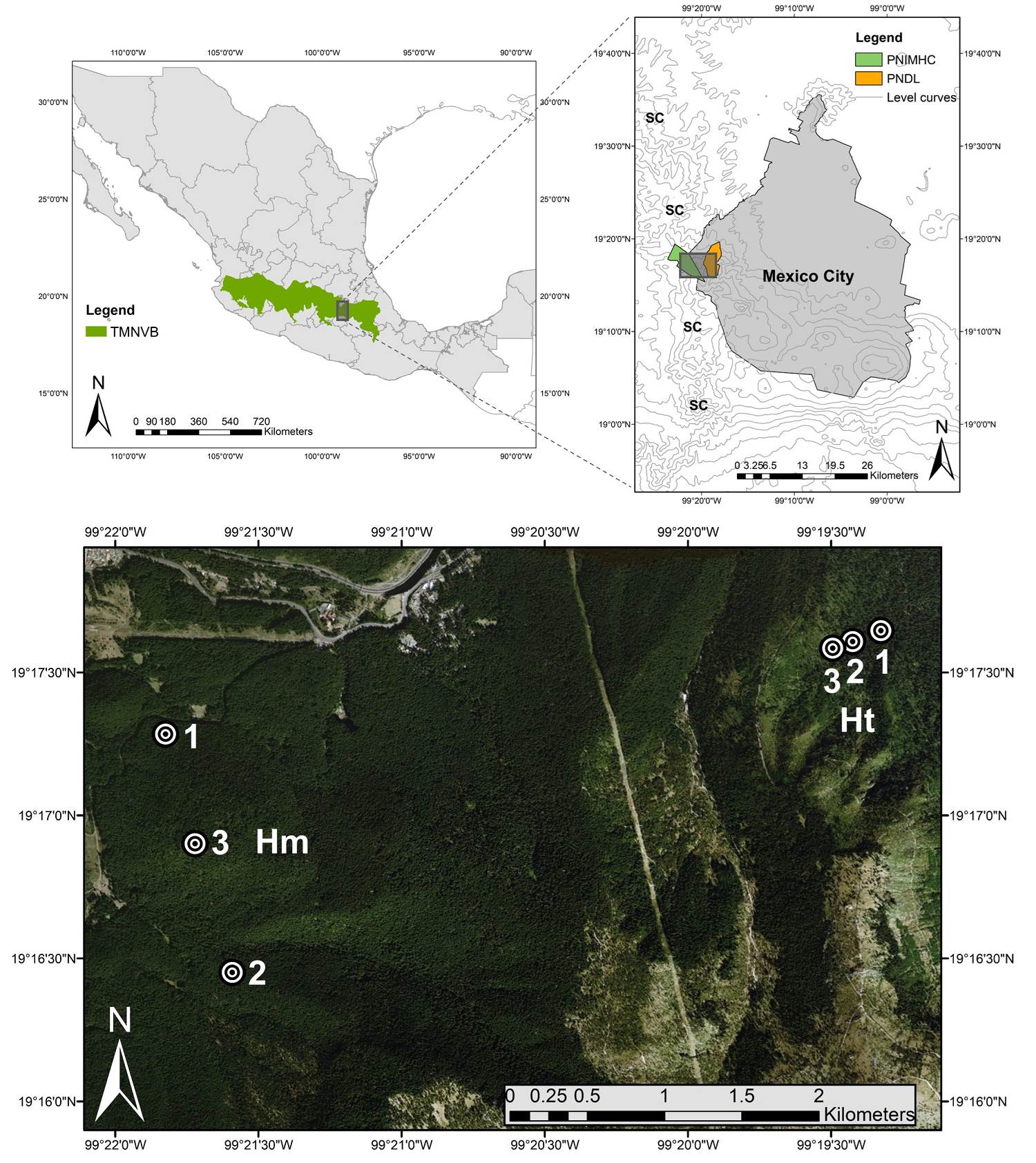
Figure 1 Study area and sampling plots (numbered circles). Hm: homogeneous condition; Ht: heterogeneous condition; PNDL: Desierto de los Leones National Park; PNIMHC: Miguel Hidalgo y Costilla National Park; SC: Sierra de las Cruces; TMNV: Trans-Mexican Neovolcanic Belt.
The two different areas selected were: a) a monodominant A. religiosa forest representing a seminatural condition (Chico-Avelino et al. 2015, Argüelles-Moyao et al. 2017), hereafter referred as the “homogeneous condition” (Hm) and b) an adjacent area that was subjected to a crown fire with varying intensity in 1998, affecting 400 ha of forest and causing the death of some adult A. religiosa individuals (CONANP-SEMARNAT 2006, Temiño-Villota et al. 2016). The consequent canopy opening favored the natural establishment of some broadleaf species typical of secondary succession (Garrya laurifolia Benth, Buddleja cordata Kunth, and Ribes ciliatum Humb. & Bonpl. ex Roem & Schult. ), along with the addition of non-native coniferous species (Pinus ayacahuite Ehrenb. ex Schltdl., Cupressus lusitanica Mill., and Pinus patula Schiede ex Schltdl. & Cham.), growing together with the surviving A. religiosa trees and derived from reforestation practices (CONANP-SEMARNAT 2006). Hereafter, this condition with the tree stratum modification in the A. religiosa forest is referred as the "heterogeneous condition" (Ht). In this condition, only one Pinus species was found, and although it is most likely to be Pinus patula, it was decided to refer to it as Pinus sp.
Tree stratum characterization. In July 2016, three 25 × 25 m (650 m2) random plots were established in each condition (Hm and Ht) based on previous studies by Argüelles-Moyao et al. (2017) and Pérez-Pazos et al. (2019) (Figure 1). These plots were all located within an altitudinal range of 3,040 to 3,215 m asl. All individuals of tree species with a diameter at breast height (DBH) ≥ 3 cm were identified to species or genus level, and DBH and total height were recorded in each case. The Importance Value Index (IVI) of the species within each site was estimated with the data obtained. This index measures the dominance of a species in a given forest area based on three attributes: relative dominance, relative density, and relative frequency. The IVI is the sum of these three measures, each expressed in ranges of 0 to 100 %. For this reason, the IVI ranges from 0 to 300 (Curtis & McIntosh 1951, Zarco-Espinosa et al. 2010, Gebrewahid & Abrehe 2019). The IVI was calculated as follows:
Relative dominance was obtained as follows:
where the basal area (BA) of the tree individuals was obtained with the following equation:
The relative density was calculated as follows:
The relative frequency was obtained as follows:
The relative and absolute values of tree dominance and density were recorded within each plot. The relative values of dominance, density, and frequency are expressed as a percentage (%), the absolute dominance as square meters per hectare (m2 ha-1), and the absolute density as the number of stems per hectare (stems ha-1).
Litter sampling. During the rainy season, in August 2016, a 50 m transect perpendicular to the main slope was established in each of the six study plots (three in the Hm condition and three in the Ht condition). Sampling points were established every 10 m along each 50 m transect. A 1 × 1 m square was delimited, from which sub-samples of the litter were obtained at the vertices and center of each sampling point. A 160 mm diameter polyvinyl chloride (PVC) ring was inserted down to the uppermost mineral soil layer, and the entire mass of foliar and woody tissues were obtained by hand from inside this ring (Chávez-Vergara et al. 2014) without considerating the litter stratification. The five subsamples were mixed to form a composite sample from each point, from which plant material > 2 mm in diameter was excluded. This study assumes that the litter is comprised of foliar and woody plant residues < 2 mm in diameter. In summary, five litter samples were collected per plot (30 samples in total). The composite samples were stored at 4 °C in darkness until analysis.
Laboratory analyses. Moisture content and mass in litter samples.- Following transportation to the laboratory, the complete fresh samples were weighed. An aliquot of 5 g of each sample was taken and dried in an oven at 70 °C for 72 h to obtain constant weight dry material and to determine the moisture content for correct calculations of microbial activity and litter mass content (Chávez-Vergara et al. 2018).
Total nutrient concentration, stoichiometric ratios and nutrient contents.- The dried material was pulverized in a ball mill (Retsch model MM 400) for 30 seconds at 30 strokes s-1. From the dried and ground material, the total C concentration (Ct, mg C g-1) was quantified in each sample by dry combustion and coulometric detection (Huffman 1977 in Chávez-Vergara et al. 2018) in a coulometric total carbon analyzer (UIC model CM5012). The total N and P (mg g-1) (Nt and Pt) concentration was determined colorimetrically after acid digestion in an autoanalyzer (SEAL AA3). The N concentration was measured by the Kjeldahl method (Bremner 1996) and P concentration through the phosphomolybdic complex's colorimetric method after reduction with ascorbic acid (Murphy & Riley 1962). Stoichiometric relationships (C: N, C: P, and N: P) and nutrient contents (g m-2) were then calculated using the obtained total concentration data.
Carbon in microbial biomass.- Carbon in the microbial biomass (Cmic) present in the litter samples was determined by the extraction-fumigation method with chloroform (Vance et al. 1987, Joergensen 1996, Joergensen & Mueller 1996). A total of 2 g of fresh sample was fumigated in a chloroform atmosphere (fumigated sample) or incubated in the absence of chloroform (unfumigated sample). After fumigation, samples were extracted in K2SO4 0.5M. The extracts were analyzed to determine the total organic C (mg Cmic g-1) in a carbon analyzer (Shimadzu model TOC-L CSH/CSN) by catalytic oxidation. The Cmic values were obtained as the difference between the fumigated and unfumigated samples following the application of a correction factor kEC 0.45 (Vance et al. 1987).
Enzymatic activity.- The activity of dehydrogenase (DH) and the extracellular enzymes β-1,4-glucosidase (BG), butyrate lipase (LP), and polyphenol oxidase (PPO) was determined in each fresh litter sample. Determination of the DH activity was performed following a modification to the method described by Alef (1995) and modified by Chávez-Vergara et al. (2018), which is based on the reduction of 2, 3, 5-triphenyl tetrazolium chloride (TTC) for the development of triphenyl tetrazolium formazan red (TFF). The results are expressed in mg TFF g-1 d-1.
Determination of the hydrolase activities (BG and LP) in the litter samples was performed according to Chávez-Vergara et al. (2014), with specific substrates linked to p-nitrophenol (pNP). The concentration of the pNP detected was corrected by subtracting the absorbance values at 410 nm of the combined sample and substrate controls. Hydrolase activities are reported in µmol pNP g-1 h-1. The PPO activity was determined by quantifying 2,2'-azino-bis 3-ethylbenzothiazoline-6-sulfonic acid (ATBS) subject to oxidation, measured directly by colorimetry at 460 nm, using the same equipment described. This activity is reported as µmol-tyr g-1 h-1.
In vitro carbon mineralization.- The potential C mineralization was quantified by aerobic in vitro incubations according to the method described in Chávez-Vergara et al. (2014). Two grams of the fresh and crushed litter were placed in PVC pipes of 5 cm in diameter with the bottom covered by a polyester mesh (0.15 mm). The tubes were placed in airtight 1 L glass jars and incubated at 25 °C for 60 days in the dark. On seven dates during this period, the CO2 released in each bottle was collected in a vial with 10 mL of 0.5N NaOH and subsequently quantified by precipitation with 5 mL of 1 N BaCl and titration with 0.5N HCl and phenolphthalein as the indicator (Coleman et al. 1977). The potential mineralization of C (respiration) over 60 days is expressed in μg CO2-C g-1.
Microbial metabolic adjustments.- We weighted the enzymatic activity according to the size of the litter microbial community (Waldrop et al. 2000, Steinweg et al. 2013). The enzymatic activity was transformed to specific enzymatic activity through the following equation, modified by Chávez-Vergara et al. (2016):
Where SEA is the specific enzyme activity expressed as mg of TPF produced per mg of Cmic per day (mg TPF mg-1 Cmic d-1) for DH activity, and µmol of pNP produced or tyrosine oxidized per milligram of Cmic per hour (µmol mg-1 Cmic h-1) for the rest of the enzymes; A is the activity of some of the enzymes and Cmic represents the concentration of C in the microbial biomass. This variable evaluates whether the C acquired by the microbial communities is destined for the production of enzymes or is stored in the microbial biomass (Steinweg et al. 2013, Wang et al. 2013). This reflects the efficiency of the metabolism in terms of ability to store C in biomass through the production of enzymes (Romaní et al. 2006).
According to Anderson & Domsch (1993), the metabolic quotient (qCO2) was calculated as the potential C mineralization over 60 days (respiration) per unit of C in the microbial biomass (Cmic) (CO2-C μg Cmic µg-1). This variable indicates which proportion of C acquired by microbial communities is released as CO2 or accumulated in microbial biomass. It therefore reflects the efficiency with which the metabolism of these communities allows them to accumulate C in their biomass (Manzoni et al. 2012, Pinzari et al. 2017).
Statistical analyses. A Mann-Whitney U test was used to compare the values of DBH and tree height between the two sites studied (hereafter referred to as “conditions”). In addition, these variables were compared among the study plots using a Kruskal-Wallis test of independent samples. Differences in the chemical quality of the litter (Ct, Nt, Pt, C: N, C: P and N: P), Cmic, enzyme activity, SEA, respired CO2, and qCO2 were evaluated using two-factor nested ANOVA models, followed by a Tukey´s honestly significant difference (HSD) test. The ANOVA model was performed with the "condition" factor ("Hm" and "Ht") and the nested "plot" factor, which contained three levels (1, 2, and 3) representing the different tree stratum conditions within each condition. To comply with the ANOVA assumptions, the data were transformed to log10 where required. Together, the coefficient of variation of the chemical quality and microbial activity parameters of the litter at each site was estimated to evaluate the stability of the decomposition process (Bonanomi et al. 2010, Wu et al. 2013). The significance level, or α, was considered as ≤ 0.05 and all analyses were performed using Statistica v. 10.0.
Results
Tree stratum characterization in homogeneous and heterogeneous conditions. In the monospecific condition (Hm), only A. religiosa was present, showing the maximum possible IVI value (Figure 2A, Table S1) and a tree density of 240 ha-1 (Table S1). The most frequent DBH was around 42 to 68 cm (Figure 2B) and total height was between 18 to 24 m (Figure 2C). In the heterogeneous condition (Ht), A. religiosa was the most important species, followed in descending order by the species: Pinus sp. > Garrya laurifolia > Buddleja cordata > Ribes ciliatum (Figure 2A, Table S1). Variations in composition among the tree strata were found in the Ht plots. However, A. religiosa showed the highest relative dominance (65-92 %) in the three plots. The Ht condition had a density of 506.7 stems ha-1, where A. religiosa presented an IVI value that was reduced to 118.8. Nevertheless, the most frequent DBH was between 3 to 16 cm (Figure 2B), while height had a bimodal distribution with a first peak between 1 to 12 m and a lower second peak at 18 to 30 m (Figure 2C). Moreover, DBH (U = 646; P < 0.001) and height (U = 512; P < 0.001) values were higher compared to those of the Hm condition (Table S2).
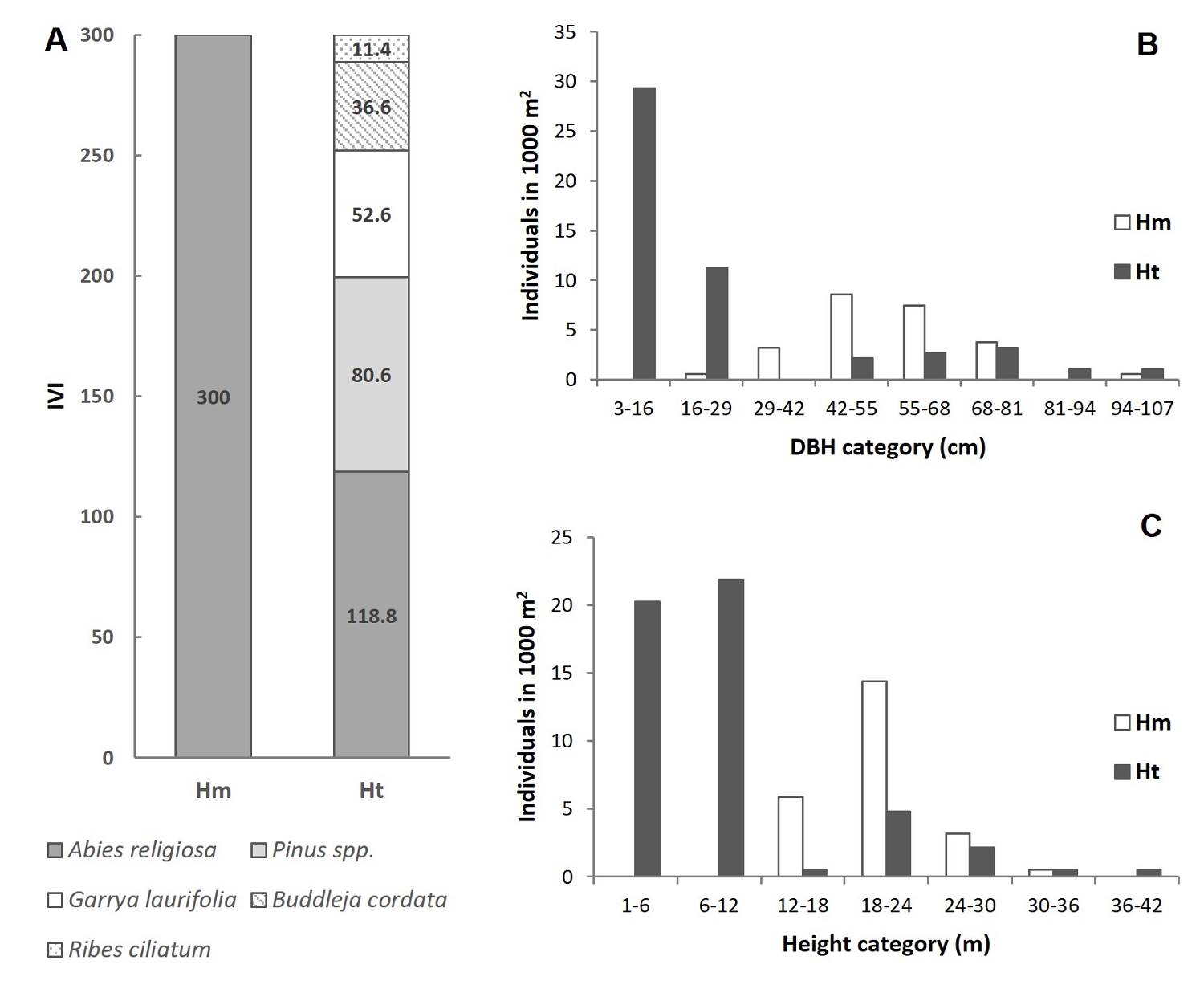
Figure 2 Importance Value Index (IVI) and tree size in the two study conditions. A) IVI in the study plots, B) Frequency of individuals in each category of DBH in each condition and C) Frequency of individuals in each height category in each condition. Hm: homogeneous condition; Ht: heterogeneous condition.
In particular, plot 1 showed three species, A. religiosa, Pinus sp. and G. laurifolia, among which A. religiosa presented 65.6 % of the relative dominance (Table 1). Nevertheless, Pinus sp. showed the highest density, with 240 stems ha-1, representing 70.3 % of all stems per ha-1 (Table 1). Five species were present in plot 2: A. religiosa, Pinus sp., G. laurifolia, B. cordata and R. ciliatum, and A. religiosa again showed the highest relative dominance with 83.6 % (Table 1). In plot 3, A. religiosa, G. laurifolia and B. cordata were present (Table 1). Abies religiosa presented the highest relative dominance (91 %) in this plot, followed by G. laurifolia (4.76 %) and B. cordata (4.09 %) (Table 1).
Table 1 Dominance and density of tree species in each plot in the two study conditions. The absolute (Abs) and relative (Rel) data of each parameter are presented. Hm: homogeneous condition; Ht: heterogeneous condition.
| Plot | Species | Dominance | Density | ||
|---|---|---|---|---|---|
| Absolute (m2 ha-1) |
Relative (%) |
Absolute (stems ha-1) |
Relative (%) |
||
| Hm | |||||
| 1 | Abies religiosa | 23.21 | 100 | 90.67 | 100 |
| Total | 23.21 | 100 | 90.67 | 100 | |
| 2 | Abies religiosa | 24.04 | 100 | 101.33 | 100 |
| Total | 24.04 | 100 | 101.33 | 100 | |
| 3 | Abies religiosa | 12.55 | 100 | 48 | 100 |
| Total | 12.55 | 100 | 48 | 100 | |
| HtL | |||||
| 1 | Abies religiosa | 14.99 | 65.63 | 32.00 | 9.38 |
| Pinus sp. | 4.77 | 20.88 | 240.00 | 70.31 | |
| Garrya laurifolia | 3.08 | 13.49 | 69.33 | 20.31 | |
| Total | 22.84 | 100 | 341.33 | 100 | |
| 2 | Abies religiosa | 14.11 | 83.66 | 32.00 | 31.58 |
| Pinus sp. | 0.21 | 1.23 | 21.33 | 21.05 | |
| Garrya laurifolia | 0.05 | 0.27 | 5.33 | 5.26 | |
| Buddleja cordata | 2.40 | 14.26 | 32.00 | 31.58 | |
| Ribes ciliatum | 0.10 | 0.58 | 10.67 | 10.53 | |
| Total | 16.86 | 100 | 101.33 | 100 | |
| 3 | Abies religiosa | 5.78 | 91.15 | 16 | 25 |
| Garrya laurifolia | 0.30 | 4.76 | 16 | 25 | |
| Buddleja cordata | 0.26 | 4.09 | 32 | 50 | |
| Total | 6.35 | 100 | 64 | 100 | |
Hm: homogeneous condition; Ht: heterogeneous condition
Litter C, N and P total concentration. The litter P concentration presented differences between conditions. It was lower in the Ht condition than in the Hm condition (0.72 mg g-1 and 0.82 mg g-1, respectively), which resulted in a significantly higher C:P ratio in the former (Figure 3E, Tables S3, S4). The C and N concentrations and the stoichiometric ratios C:N and N:P did not show significant differences between the conditions and/or plots (Figure 3, Tables S3, S4), but all variables presented higher coefficients of variation in the Ht condition than in the Hm condition (Table 2).
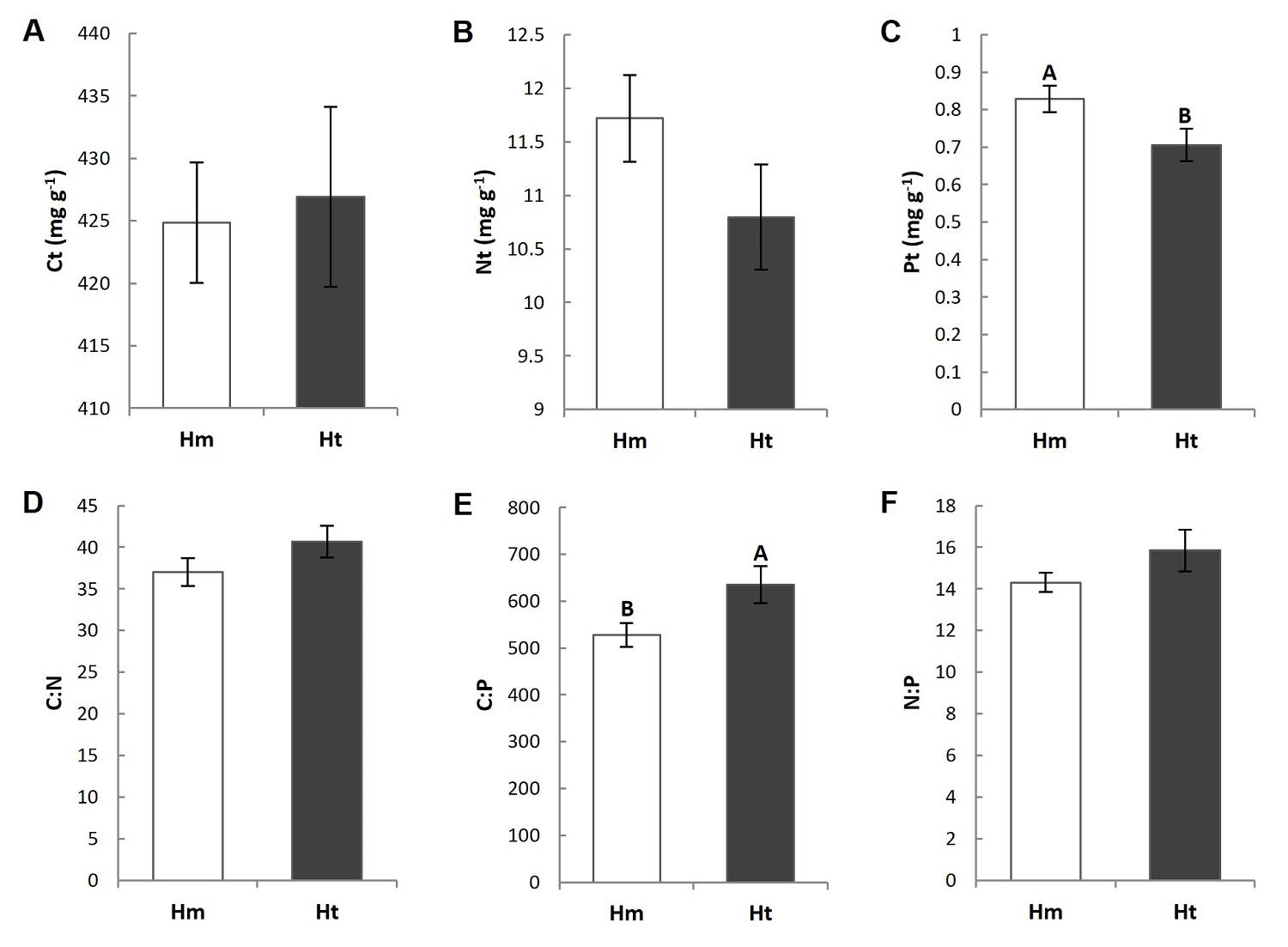
Figure 3 Chemical composition of the litter at the study conditions. Different capital letters indicate that the means are significantly different (P ≤ 0.05). Vertical lines indicate the standard error. Hm: homogeneous condition; Ht: heterogeneous condition; Ct: total carbon; Nt: total nitrogen; Pt: total phosphorus.
Table 2 Coefficient of variation of the chemical characteristics and microbial activity of the litter in the two study conditions. Hm: homogeneous condition; Ht: heterogeneous condition; Ct: total carbon; Nt: total nitrogen; Pt: total phosphorus; BG: β-1,4-glucosidase; LP: butyrate lipase; PPO: polyphenol oxidase; DH: dehydrogenase; SEA: specific enzyme activity; qCO2: metabolic quotient
| Coefficient of variation (%) | ||
|---|---|---|
| Condition | ||
| Variable | Ht | Ht |
| Total nutrients | ||
| Ct (mg g-1) | 4.4 | 6.5 |
| Nt (mg g-1) | 13.4 | 17.7 |
| Pt (mg g-1) | 16.6 | 23.9 |
| C:N | 17.5 | 18.3 |
| C:P | 18.9 | 24.0 |
| N:P | 12.4 | 24.5 |
| Enzyme activity | ||
| BG (µmol pNP g-1 h-1) | 122.2 | 52.5 |
| LP (µmol pNP g-1 h-1) | 24.1 | 36.4 |
| PPO (µmol Tyr g-1 h-1) | 49.3 | 44.6 |
| DH (mg TPF g-1 d-1) | 47.5 | 77.4 |
| Specific enzyme activity | ||
| SEA BG (µmol pNP mg-1 Cmic h-1) | 114.5 | 55.9 |
| SEA LP (µmol pNP mg-1 Cmic h-1) | 36.7 | 47.6 |
| SEA PPO (µmol Tyr mg-1 Cmic h-1) | 47.1 | 70.4 |
| SEA DH (mg TPF mg-1 Cmic d-1) | 42.0 | 78.4 |
| C mineralization | ||
| Respiration (μg CO2-C g-1 60 d-1) | 37.2 | 22.3 |
| qCO2 (μg CO2-C µg-1 Cmic 60 d-1) | 26.8 | 25.0 |
Enzymatic activities, carbon in microbial biomass and potential carbon mineralization. The main differences in microbial activity occurred between conditions. In particular, the litter from higher Ht condition presented a significantly higher activity of β-glucosidase (BG) and polyphenol oxidase (PPO), and lower CO2 respired in vitro over 60 days, than was the case in the Hm condition (Figures 4A, C, F, Tables S3, S4). Furthermore, the BG and PPO activity showed a lower coefficient of variation in the Ht condition, but a higher coefficient of variation in the CO2 respired in the same condition (Table 2). Other parameters, such as lipase (LP) and dehydrogenase (DH) activities, did not differ significantly between conditions (Figure 4B, D, Tables S3, S4), although their coefficients of variation were higher in the Ht conditions (Table 2). Carbon in the microbial biomass did not present significant differences between the study conditions but showed a tendency to be higher in the Hm condition (Figure 4E, Tables S3, S4).
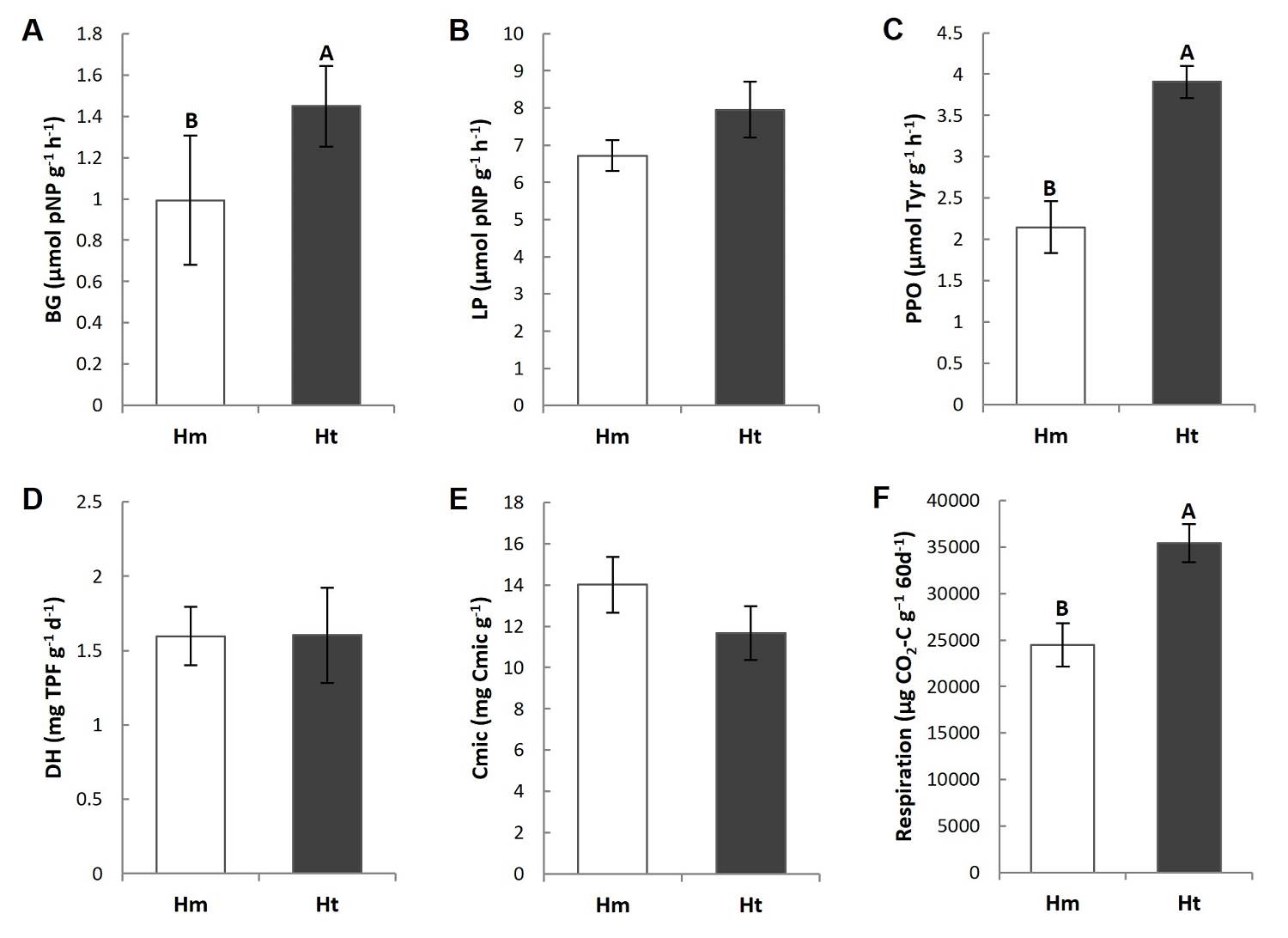
Figure 4 Mean value (standard error) of enzymatic activity of A) β-1,4-glucosidase (BG), B) butyrate lipase (LP), C) polyphenol oxidase (PPO), D) dehydrogenase (DH), E) Microbial carbon (Cmic) and F) C mineralization in the litter (respiration) of the study conditions. Different capital letters indicate that the means differ significantly (P ≤ 0.05).
Specific enzyme activity (SEA) and metabolic quotient (qCO 2 ). The main differences among the two conditions were the higher Specific Enzymatic Activity of BG (SEA BG), LP (SEA LP) and PPO (SEA PPO), and the almost two-fold higher metabolic quotient (qCO2) in the Ht condition than in the Hm condition (Figures 5A-C, E, Tables S3, S4). Of the enzymes evaluated, only the SEA LP presented differences among the plots in the Ht condition, where plot 1 of this condition was higher than plot 3 (P < 0.05) (Tables S3, S4). However, the SEA DH did not show significant differences between the conditions or among the plots (Figure 5D, Tables S3, S4). In addition, the coefficient of variation of the SEA of the LP, PPO and DH was higher in the Ht condition and lower in the case of the SEA BG and qCO2, than was the case in the Hm condition (Table 2).
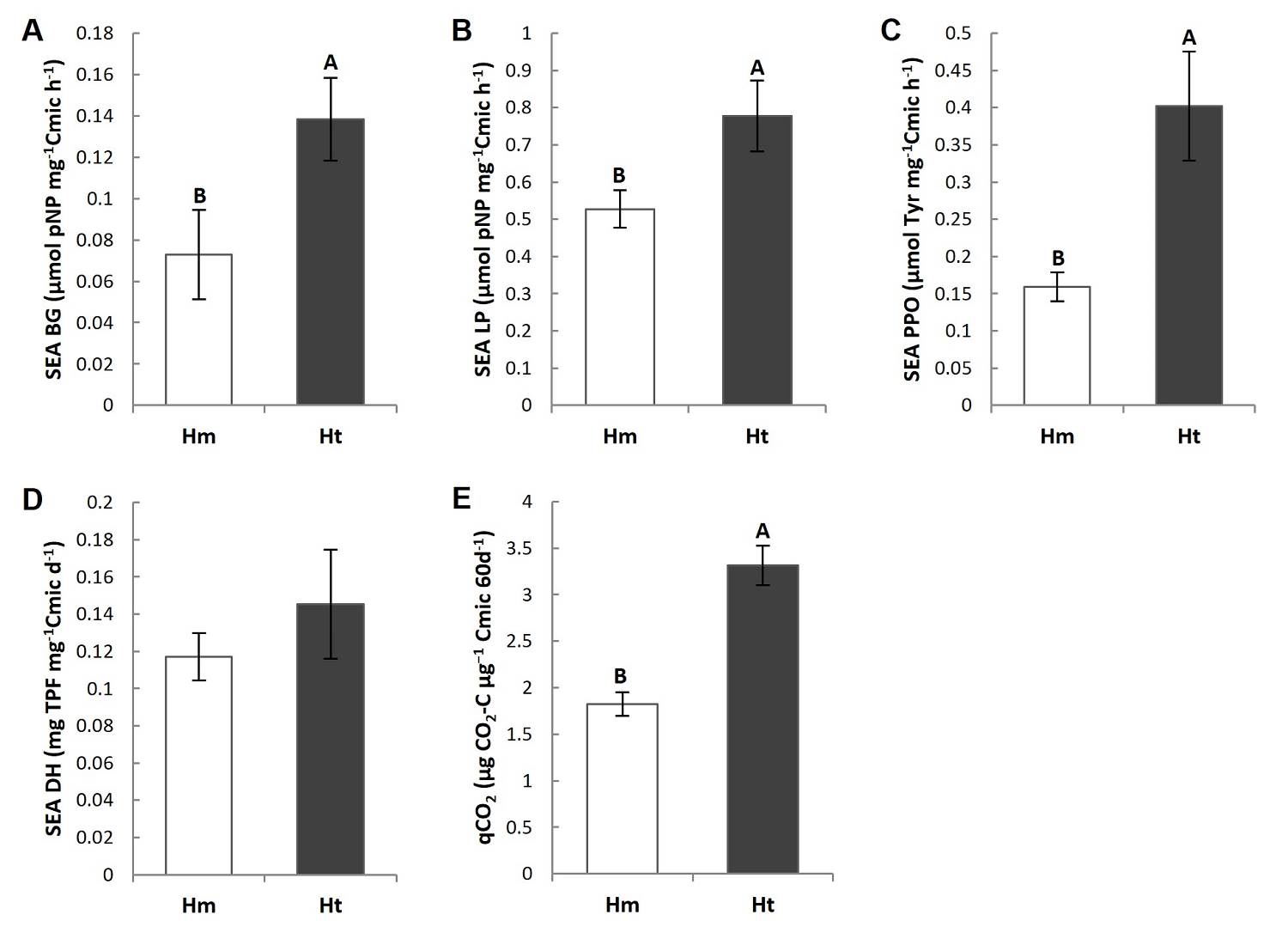
Figure 5 Mean value (standard error) of the specific enzymatic activity (SEA) of A) β-1,4-glucosidase (BG), B) butyrate lipase (LP), C) polyphenol oxidase (PPO) and D) the metabolic quotient (qCO2) in the litter of the study conditions. Different capital letters indicate that the means differ significantly (P ≤ 0.05).
Discussion
Modification of the tree stratum in a sacred fir forest. Our results showed differences in tree stratum attributes between the two conditions, derived from the different forest history of each, even though they both started from the same point as mature sacred fir (A. religiosa) forest. The tree stratum of the Hm condition maintains uniformity in its traits since it is represented only by A. religiosa and the higher values of DBH and tree height were similar among plots in the Hm condition. Moreover, the magnitude of these traits in A. religiosa is consistent with several studies in other preserved forests, supporting the idea that the Hm condition is a mature forest derived from a tree stratum of high longevity (Sánchez-Velásquez et al. 1991, Wang 2006, Avedaño et al. 2009, Razo-Zárate et al. 2013). This suggests that the tree stratum of the Hm condition does not present a notable modification from its initial condition since it preserves the characteristic low heterogeneity of A. religiosa forests (Cuevas-Guzmán et al. 2011).
In contrast, the traits observed in the tree stratum of the Ht condition showed an increase in its heterogeneity relative to its initial condition. Notably, there was an increase in species richness which promoted a change in the tree species composition, causing increased heterogeneity in the tree stratum. As a consequence of modifying the tree stratum in the Ht condition, the IVI values of A. religiosa decreased. Nevertheless, this species continued to present the highest IVI in the Ht condition, a finding that is related to maintaining its role as the dominant species in the site. Complementarily, the heterogeneity of the tree stratum can be seen in the change in the size of trees present, represented by different DBH and height values. These values were mostly low, and are associated with trees of younger age and low biomass (van Breugel et al. 2006, Cuevas-Guzmán et al. 2011).
The novel scenario in the Ht condition is attributed to the impact of, for example, wildfires, the consequent gap dynamic, and forest management (Arriola-Padilla et al. 2014). The Ht condition showed two trajectories of forest development; the first as a secondary successional stage denoted by the presence of the broadleaf angiosperms B. cordata and G. laurifolia, species that occur in early and intermediate stages of ecological succession, respectively (Quintana-Ascencio et al. 2004, Jiménez-Vazquez 2012, Mendoza-Hernández et al. 2013). The second trajectory consists of a reforestation stage with Pinus sp., a non-native species introduced as a common management practice in the temperate Mexican forests (CONANP-SEMARNAT 2006). This condition was mainly seen in plot 1 since Pinus sp. had the highest relative density among any of the plots of the two conditions. This reflects the fact that the tree plant community characteristics of a fraction of the Ht condition have been the result of targeted processes (Galeana-Pizaña et al. 2013). Thus, disturbances and management actions would be the factors that have determined the increase in heterogeneity of the tree stratum in the Ht condition, compared to the characteristic mature condition of a sacred fir forest observed in the Hm condition.
Is the modification of tree stratum reflected in litter chemical composition? Our results show that such tree stratum modification promotes a chemical change that is reflected in the C:P ratio between the conditions as a result of the change in tree species composition (Prescott 2002). Although there was no difference in total nutrients and their ratios, modification of the tree stratum in the Ht condition promoted a higher coefficient of variation of the litter chemical composition (Ct, Nt, C:N, and N:P), reflecting lower stability in the chemical composition. Consequently, this high variation would have precluded the occurrence of statistical differences between the chemical traits of the two sites. Alternatively, the higher IVI of A. religiosa in the Ht condition suggests that this species still influences the litter chemical composition in a manner similar to that which occurs in the Hm condition, thus producing the similarity observed between both conditions. This highlights the fact that A. religiosa has an important role in the definition of chemical composition despite its loss of dominance. It can also explain the consistency between the data observed in the Hm condition and other sacred fir forests (Alvarado-Rosales 1989, Galicia et al. 2016, Peña-Mendoza et al. 2017, Jasso-Flores et al. 2020).
Vergutz et al. (2012) showed that green and senescent foliar tissues in conifers present a lower P concentration and higher C:P ratio than deciduous angiosperms. In contrast, we observed that foliar tissues generated by A. religiosa in the Hm condition had higher or similar P concentrations compared to angiosperms such as those present in the Ht condition (Alvarado-Rosales 1989, Aguilar-Rodríguez & Barajas-Morales 2005, Domínguez-Bernal 2011, Peña-Mendoza et al. 2017, Jasso-Flores et al. 2020). Meanwhile, the foliar tissues of Pinus sp. needles in plantations located in a forest of A. religiosa showed lower P concentration than that observed in sacred fir forests (Salaya-Domínguez et al. 2012). This pattern in foliar tissues is similar in the litter, as observed by Jasso-Flores et al. (2020), where litter P concentration was lower in a Pinus forest than in both an A. religiosa forest and an Alnus jorullensis forest. By changing the identity of the tree species, the tree stratum heterogeneity in the Ht condition would therefore have promoted a reduced litter P concentration compared to that of the Hm condition.
Microbial activity adjustments related to the decomposition process as a consequence of tree stratum modification. It has been observed that the activity of different exoenzymes produced by microbial communities and the litter decomposition they carry out are processes that are influenced by nutrient concentration (Keeler et al. 2009, Talbot & Treseader 2012). In congruence, the results suggest that the modification in litter chemical traits as a result of increased heterogeneity in the tree stratum stimulates cellulose and lignin depolymerization in the litter through BG and PPO activities and promotes its decomposition, as commonly observed in litter mixtures from other forests (Prescott 2002, Purahong et al. 2014, Chávez-Vergara et al. 2018).
The higher SEA related to depolymerization of C-rich molecules (BG, LP, and PPO) in the Ht compared to the Hm condition indicates that the microbial community of the former site accumulates a low concentration of C in its biomass despite maintaining high depolymerization of cellulose, aliphatic compounds, and lignin through BG, LIP and PPO activities, respectively. This occurs mainly in plot 1 of the Ht condition, where the high SEA LP was observed in the presence of Pinus sp. Similarly, Sudachkova et al. (2004) found high LP activity, determined by high concentrations of aliphatic compounds in P. sylvestris needles. Moreover, the high qCO2 observed in the Ht condition indicates that its microbial community has a low efficiency in the use of C obtained from the litter since it accumulates a low proportion of C in its biomass, while the greatest fraction is respired as CO2 (Manzoni et al. 2012, Pinzari et al. 2017). A similar pattern has been observed in the soils and litter of mixed forests of Picea abies and Fagus sylvatica with high C:P ratios (Spohn & Chodak 2015). This may be attributed to the microorganisms favoring the conservation of limiting nutrients and, to a lesser extent, carbon protection (Mooshammer et al. 2014) as a consequence of dealing with a new and chemically different litter under heterogeneous conditions in the tree stratum.
The SEA and qCO2 therefore suggest that, given the reduction in litter P concentration caused by the increased tree stratum heterogeneity, the C consumed by the microbial communities allows them to maintain a high production of enzymes. This promotes a high C acquisition for energy from the organic molecules of the litter, but it occurs to the detriment of C conservation in its biomass and growth, generating higher CO2 emissions (Figure 6). This has been suggested as an indicator of the stress of microbial communities, and has been observed as a response to low P availability that allows these communities to survive under these limited conditions (Anderson & Domsch 2010, Spohn & Chodak 2015). These patterns could also be attributed to a change in litter microbial community structure in the Ht condition, which was not measured in this study. Nevertheless, further studies are needed to evaluate the influence of microbial community structure over the observed results.
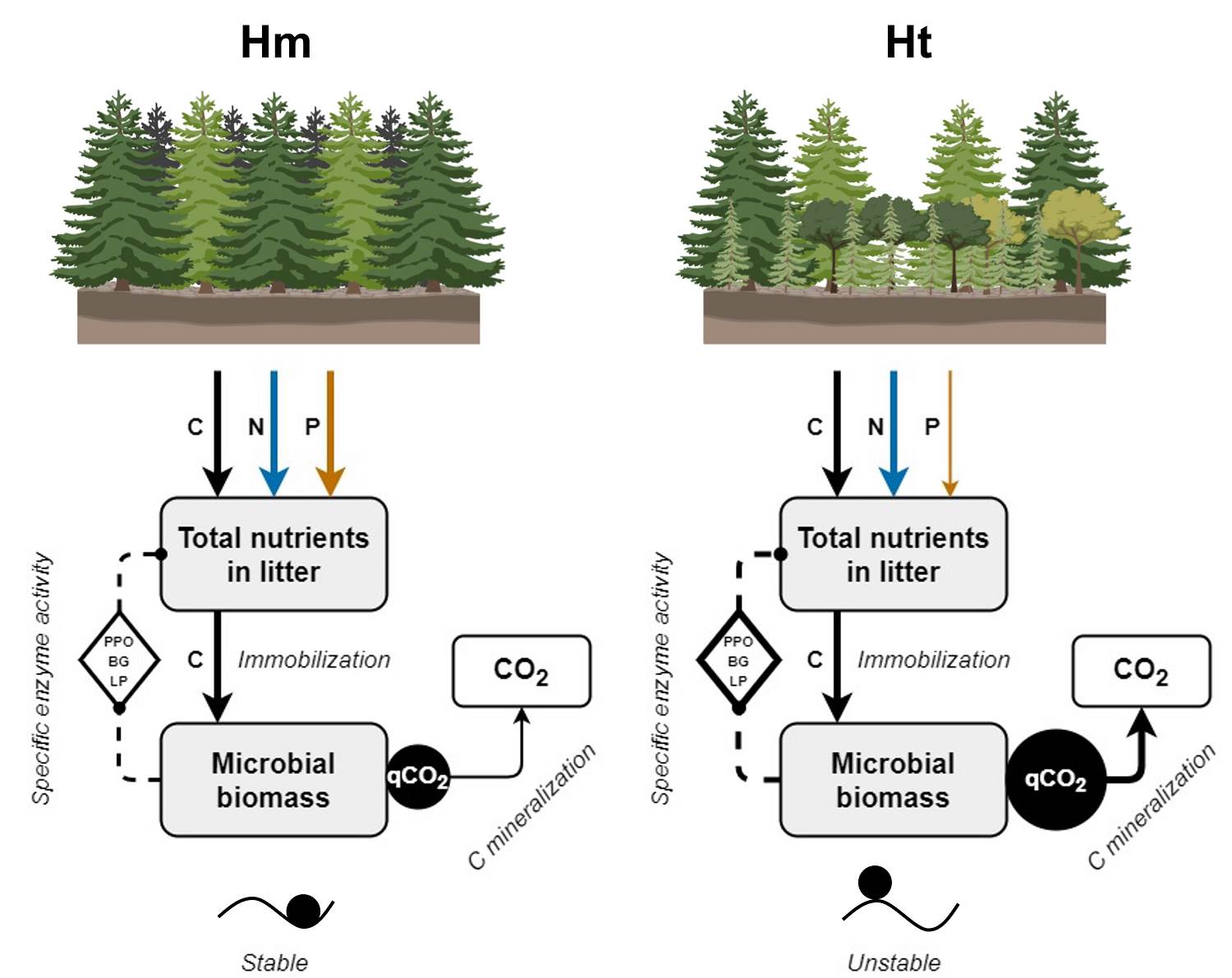
Figure 6 General effects of the modification of the tree stratum on litter decomposition. Solid lines indicate the C (black), N (blue) or P (orange) concentration that enters the litter or the microbial biomass, or that is mineralized, in each tree stratum condition. Dashed lines indicate the microbial activity related to obtaining C from the litter. The magnitude of the measeured variables is proportional to the thickness of the lines and the size of the black circles. Circles on the ridges or valleys represent the stability of the litter decomposition as indicated by a low (stable) or high (unstable) coefficient of variation in the litter nutrient concentration and specific enzyme activity.
In addition, it was difficult to disentangle the direct long-term effect that the wildfire that occurred in the Ht condition could have had over the results, even when wildfires can impact litter decomposition by directly affecting decomposer microbial community composition and activity (Mikita-Barbato et al. 2015). Nevertheless, in coniferous forests where the decomposition process occurs at slow rates due to environmental constraints (e.g., temperature and water), the parameters involved in litter and soil decomposition (e.g., microbial biomass, exoenzyme activity, and microbial respiration) have been found to return to the pre-fire levels in approximately 20-24 years (Holden et al. 2013, Pérez-Valera et al. 2020). Since the rate of litter decomposition has been reported to be fast in A. religiosa forests (Barajas-Guzmán et al. 2020), it is suggested that the direct effect of the wildfire over the litter decomposition has decreased over the 18 years since the fire occurred in the Ht condition. Instead, the results point to an indirect effect of the wildfire that, together with management activities, has a long-term impact on litter decomposition by increasing the heterogeneity of the tree stratum.
Is the stability of the decomposition process modified by the alteration of tree stratum in a sacred fir forest? In addition to recognizing the change in the magnitude and direction of the factors involved in litter decomposition, this study evaluated the stability of these factors under the study conditions through their coefficient of variation. This parameter has been used in studies related to the decomposition process (Bonanomi et al. 2010, Wu et al. 2013). In congruence with the diversity-stability models of ecological processes, a lower coefficient of variation in the C mineralization of the Ht condition was observed. Furthermore, there was an increase in the number of species in the tree stratum relative to the Hm condition. Initially, this suggests higher stability in the decomposition process of the litter of the Ht condition, as shown in other studies (Keith et al. 2008, Bonanomi et al. 2010, Wu et al. 2013, Setiawan et al. 2016). However, C mineralization only represents the final step of decomposition.
According to that observed in the study conditions, litter chemical traits and microbial metabolism present an interaction (Vivanco & Austin 2008, Aubert et al. 2010), in which the litter chemical traits and activity of the LP and DH enzymes show higher coefficients of variation at the Ht condition than at the Hm condition. This was particularly evident for variables that reflect the microbial metabolic efficiency to store C in biomass through the production of enzymes, where high coefficients of variation were observed in the SEA of the PPO, LP, and DH in the same condition. This suggests low stability in chemical attributes and C use efficiency in the litter during decomposition in the Ht condition. Complementarily, even when the coefficient of variation of qCO2 did not vary between conditions, a high qCO2 such as that observed at the Ht condition indicates low stability in the decomposition process since there is low efficiency in the use of resources by the microbial community. This pattern has mainly been observed in highly diverse forests in early successional stages (Pinzari et al. 1999), which are similar to Ht. Our results therefore suggest a contradictory pattern compared to common diversity-stability models, since they indicate that even a slight increase in the plant species richness of the tree stratum, by modifying plant species composition, generates a decrease in the stability of a part of the litter decomposition process (Figure 6); specifically, the part that involves the interaction between chemical traits and microbial metabolism related to C use. In contrast, the Hm condition, dominated by A. religiosa, would present greater stability in some of the factors involved in litter decomposition. This has been observed in ecological processes where plant communities are in advanced successional stages (Pinzari et al. 1999, Wales et al. 2020). This stability is congruent because the Hm condition is part of a mature A. religiosa forest.
The results suggest that, in the evaluated forest, the increase in the heterogeneity of the tree plant stratum structure as a result of disturbance and subsequent forest management events, such as reforestation, can generate conditions of instability in the litter decomposition process. This process could therefore be susceptible to less predictable modifications, new disturbances, and environmental variations (McGrady-Steed et al. 1997, Luo et al. 2015, Dietze 2017). It is also suggested that the stability of litter decomposition, rather than adjusting to the diversity-stability models, is related to the successional stage of the tree species stratum and its species composition, suggesting the potential use of endemic monospecific conifer forest ecosystems as novel models for research into biogeochemical cycles at temperate forests in tropical regions.
In conclusion, the increase in heterogeneity in the tree stratum due to the effect of fire and reforestation with non-native species, mainly represented by a change in the species composition and a higher tree species richness, increased the C:P ratio in the litter. This promoted the microbial activity related to C acquisition, but reduced the C use efficiency by the microorganisms, favoring a higher CO2 emission into the atmosphere. Furthermore, there was a low stability in the interaction between the litter chemical traits and the microbial metabolism during the decomposition process. The results highlight that conditions derived by wildfires and management practices have a lasting effect on both the rate and stability of the litter decomposition by increasing the tree stratum heterogeneity in a monospecific sacred fir forest.
Supplementary material
Supplemental data for this article can be accessed here: https://doi.org/10.17129/botsci.3029











 nueva página del texto (beta)
nueva página del texto (beta)



