Orchidaceae is one of the largest family within the flowering plants (Chase et al. 2015, Antonelli et al. 2023). This family includes approximately 27,000 species grouped in 750 genera (Lu et al. 2019, Antonelli et al. 2023). Orchidaceae is subdivided in five subfamilies: Apostasioidae, Vanilloideae, Cypripedioideae, Orchidoideae, and Epidendroideae (Chase et al. 2015). The phylogenetic relation among the subfamilies is shown in Figure 1. Orchids are mostly long-lived herbs (Hew & Yong 2004) and are distributed worldwide (except for polar and desert regions), particularly diverse in the tropics (Givnish et al. 2015). Most of the orchid species are epiphytes (Rasmussen & Rasmussen 2018), however, some of them are terrestrials, rupicolous or occur in aquatic environments (Shefferson et al. 2020).
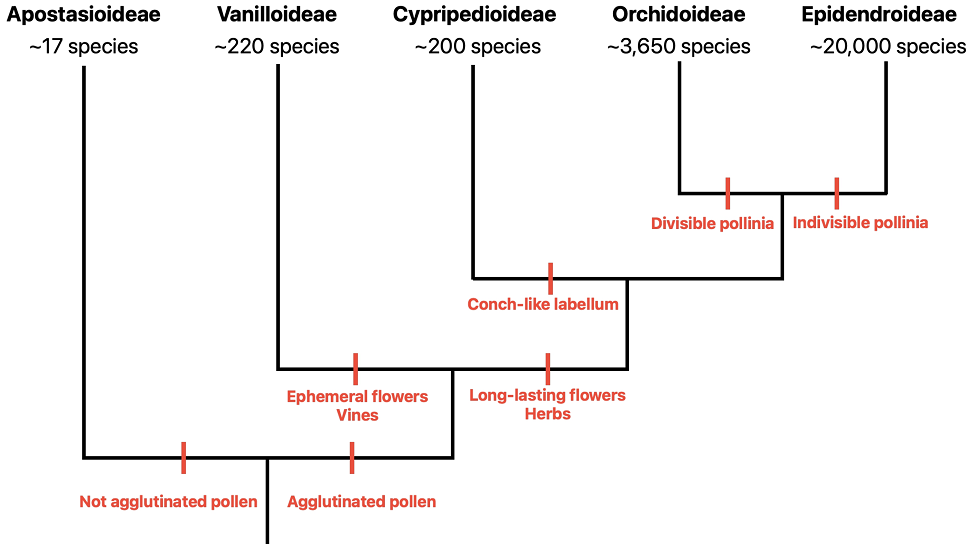
Figure 1 Subfamilies of Orchidaceae. Some differential traits among the subfamilies are highlighted in red. Figure constructed with data from literature (Chase 2005, Yin et al. 2016, Hu et al. 2022, Jolman et al. 2022, dos Santos & da Silva 2023, Kim et al. 2023).
Orchidaceae is considered as the most evolved plant family due their morpho-physiological particularities (Soltis et al. 2019, Hietz et al. 2022), such as the presence of pseudobulb and succulent leaves (Ng & Hew 2000), velamentous roots (Zotz & Winkler 2013, Pridgeon 2014), or the presence of crassulacean acid metabolism (Zhang et al. 2018). Another interesting feature about the biology of orchids is their seeds. Most orchids produce thousands of dust-like seeds with a non-developed embryo and no endosperm (Yeung 2017). For this reason, orchid seeds need to establish a symbiotic relationship with a mycorrhizal fungus, enabling germination (Otero et al. 2004, 2007, Porras-Alfaro & Bayman 2007). Symbiotic interactions between orchid seeds and mycorrhizae are a widely studied topic (Rasmussen 2002, Dearnaley 2007, McCormick et al. 2018, Sarsaiya et al. 2019, Favre-Godal et al. 2020, Li et al. 2021b, Selosse et al. 2022).
Orchids are remarkable by their flower diversity, much appreciated by the ornamental plant market. Among the ornamental orchids, the genera Cattleya, Cymbidium, Dendrobium, Phalaenopsis, Phaius, Paphiopedilum and Vanda are the most cultivated (Vij & Pathak 2012). Even though the flower is the most appreciated part of the orchids, some species such as Vanilla planifolia Andrews produce fleshy fruits, commercialized because of their aromatic and flavoring traits (Pérez-Silva et al. 2021, da Silva-Oliveira et al. 2022). Most orchid species exhibit hermaphrodite flowers, except for some species from the subtribes Catasetinae and Satyriinae, which produce unisexual flowers (Suetsugu 2020), whereas some orchids exhibit flowers whose sexual function is separated in time (Hurskainen et al. 2017).
The vast diversity of flowers makes orchids to be highly appreciated among collectors, resulting in overexploitation and trafficking of species. This is the main reason why approximately 1,970 species of orchids are threatened, according to the IUCN red list (Hinsley et al. 2018, IUCN 2023). For this reason, Orchidaceae is one of most heavily protected plant family, with comprehensive CITES (Convention on International Trade in Endangered Species of Wild Fauna and Flora) trade restrictions on the entire family; as a result, orchids represent almost 75 % of all CITES-listed species (Phelps 2015).
Several studies on orchids have focused on the diversity of floral characters (Pellegrino et al. 2017, Naczk et al. 2018, Dellinger 2020, Hu et al. 2020), flower rewards and pollinators (Cozzolino & Widmer 2005, Pansarin et al. 2008, Pansarin 2016, Fay 2018, Shrestha et al. 2020, Castro et al. 2021, Ray & Gillett-Kaufman 2022), and aromatic and other chemical compounds (Bohman et al. 2016, Wu et al. 2019, Ramya et al. 2020, Brzosko & Mirski 2021). In fact, several topics on orchid pollination have been studied since Darwin (1862). Here we aim to provide an update on the knowledge about the different types of reproduction systems observed in orchids (autogamy, allogamy, and xenia and metaxenia induction), along with the production of hybrids, with the purpose of promoting both the conservation and improvement of orchids through different pollination strategies.
Pollination. In general, pollination is defined as the transfer of pollen from the anthers to the stigma (Wurz et al. 2021). In most of the flowering plants, only a portion of the pollen produced by the anther is carried to the stigma in a single pollination event (Edlund et al. 2004). This is also true for the orchid subfamily Apostasioideae (Figure 1), and some Vanilloideae, such as in Cleistes (e.g., Pansarin 2003), in which the pollen is released as free monads. Pollination in Apostasioideae is more reminiscent of that observed in other families than in Orchidaceae (Kocyan & Endress 2001, Yin et al. 2016, Li et al. 2023). In the most of Orchidaceae subfamilies, the entire anther content must be removed by the pollinator (Endress 2016). In addition, one or more entire pollen package (i.e., pollinium) are deposited in the stigmatic surface in a single pollination event (e.g., Epidendroideae). As consequence, pollination in orchids has even been considered to be more specialized than other flowering plants (Jersáková et al. 2006). However, at the same time it is risky: if the pollinarium is removed by an inefficient pollinator, which can result in pollen lost, fruit set would be compromised (Cabrera-Reyes et al. 2021). For this reason, orchids commonly have specialized traits to attract effective pollinators, in order to ensure fruit set (Jersáková et al. 2006, Phillips et al. 2020).
In most of the orchids, flowers do not produce any kind of rewards for pollinators, therefore, they are pollinated by food deceit (Pansarin et al. 2008), sexual deception or attraction (Cozzolino & Widmer 2005, Mant et al. 2005, Shrestha et al. 2020, Luo et al. 2021). Pollinators commonly land on a flower or on an inflorescence in order to search for a flower reward, commonly nectar or fragrances, which may or may not be present (i.e., deceptive flowers) (Pemberton 2013, Lozano-Rodríguez et al. 2022). Besides the production of a flower resource, pollen is deposited on the body of pollinators. Pollen deposition on the stigma occurs when a pollinator carrying pollen visit another flower (Pemberton 2013, Hallett et al. 2017). Another important pic in pollination ecology studies is the source of pollen (Tremblay et al. 2005, Kropf & Renner 2008), since orchids can reproduce by autogamy, allogamy, or both (Willmer 2011, Bateman 2020). As consequence, in pollination ecology studies it is recommended to carry out pollination by hand, in order to check their reproductive systems, or even to verify whether agamospermy could or could not occur (Tremblay et al. 2005, Sao Leao et al. 2019, Wurz et al. 2021).
Autogamy. Autonomous self-pollination is defined as the transfer of pollen from the anther to the stigma of the same flower (Bateman 2020, Johnson & Edwards 2000, Willmer 2011). Several studies have shown that orchids can be pollinated with their own pollen and produce fruits and viable seeds (Ackerman et al. 2023). However, the fact that a plant could be self-pollinated does not necessarily make it obligatorily autogamous, as the majority of orchids commonly show different degrees of facultative autogamy (Talalaj et al. 2017). In contrast, allogamous self-compatible species that can be self-pollinated manually are called “frequently cross-pollinated species” (Sasikumar 2010). This knowledge is widely applied in V. planifolia, an orchid species self-pollinated by hand (Hernández-Hernández 2018). Autogamy guarantees the production of fruits, if that is what is desired, even knowing that the seeds contained may not be viable (Sao Leao et al. 2019, Yeh et al. 2021). Another disadvantage observed in self-pollinated orchids is the production of albino protocorms, something not commonly observed in orchids pollinated through outcrossing (de Paiva-Neto et al. 2022). Endogamy depression can be expressed as auto-pollination or in crosses between closely related individuals, as reported by Emeterio-Lara et al. (2018).
Although orchid flowers are widely adapted to outcrossing, species capable of autogamy are found in several Orchidaceae subgroups (Johnson & Edwards 2000). Spontaneous self-pollination is relatively common in Orchidaceae compared to other plant families. In fact, 31 % of orchid species set fruits through autonomous self-pollination (Evans & Jacquemyn 2020). The occurrence of self-pollination is easy to study in orchids due to its floral architecture (Talalaj et al. 2017). In addition, facultative self-pollination is sometimes observed in biotic-pollinated plants when the environment is subject to changes such as anthropogenic disturbance (Talalaj & Skiercynski 2015, Talalaj et al. 2017). This transition has also been recorded in some plants that have migrated to areas outside their previous range (Sramkó et al. 2019, Evans & Jacquemyn 2020). However, autogamy is more common among orchids with a weedy habit or those found in habitats with marginal pollinator activity (Suetsugu 2015).
Orchids developed barriers to avoid the production of seeds with self-pollen, and these can occur in both before and after pollen transfer. Autogamy in orchids is often prevented or limited by various mechanisms to promote outcrossing, which enhances genetic diversity and consequently more adaptability. Some common barriers to autogamy in orchids are 1) Morphological barriers: in many orchid species, the anther and stigma are spatially separated by the rostellum, preventing self-pollination (Bory et al. 2008, Sugiura 2013, Zhang et al. 2021). 2) Temporal barriers: some orchids exhibit dichogamy, where the male and female reproductive organs mature at different times; this temporal separation prevents self-pollination because the receptive stigma is not yet available when pollen is released or vice versa (Hurskainen et al. 2017). 3) Genetic barriers: some orchid species possess mechanisms of self-incompatibility, where the pollen from a flower is unable to fertilize the ovules of the same flower or other flowers on the same plant; this genetic barrier prevents self-fertilization and promotes outcrossing (Zhang et al. 2021). Genetic barriers such as self-incompatibility, operate after pollinia transfer by ensuring that the pollen from one flower cannot successfully fertilize the ovules of the same flower or other flowers on the same plant (Hurskainen et al. 2017, Zhang et al. 2021). 4) Other mechanical barriers: orchids typically have specialized pollination structures associated with the pollinia or pollinaria, which are often adapted for specific pollinators; these structures may not easily come into contact with the stigma of the same flower, reducing the likelihood of self-pollination (Gravendeel et al. 2004). Overall, these barriers to autogamy in orchids contribute to the promotion of outcrossing, which facilitates genetic recombination and maintains genetic diversity within populations (Scopece et al. 2014, Suetsugu 2015, Zhang et al. 2021).
Often, autogamy in orchids is facultative, and self-pollination takes place in the final phase of anthesis, mainly caused by a low frequency of pollinator visits (Suetsugu 2015, Talalaj et al. 2017). However, self-pollination becomes mandatory when the pollinator is ineffective or is no longer in the area (Jin et al. 2014, Pedersen et al. 2018). For example, V. palmarum (Salzm. Ex Lindl.) Lindl. opens its flowers and remains available to pollinators, however, it also exhibits a mechanism of facultative self-pollination; if biotic pollination does not occur, flowers self-pollinate (Pansarin & Ferreira 2021).
The transition between allogamy-autogamy pollination systems is particularly well documented in the terrestrial genus Epipactis (Talalaj et al. 2017, Sramkó et al. 2019, Evans & Jacquemyn 2020). Epipactis flowers show a morphology adapted to self-pollination and ensure seed production such as a reduced gynostemium or the occurrence of cleistogamy ensure seed production. Autogamy has been recorded in E. atrorubens (Hoffm. Ex Bernh.) Besser, E. dunensis Godfery, E. helleborine (L.) Crantz, E. leptochila Godfery, E. microphylla (Ehrh.) Sw., E. muelleri Godfery, E. palustris (L.) Crantz, and E. youngiana A.J.Richards ex A.F.Porter (Bonatti et al. 2006, Talalaj & Brzosko 2008, Talalaj et al. 2017, Evans & Jacquemyn 2020). Nonetheless, this adaptation has reduced the genetic diversity of the populations (Squirrell et al. 2002, Evans & Jacquemyn 2020). Even though the transition from allogamy to autogamy has been considered as an evolutionary dead end, due to the consecutive acquisition of individuals with lower genetic diversity, and therefore, with less probability of developing mechanisms that allow them to adapt to environmental changes, which might lead to narrow the ecological niches and eventually the loss of the species, this is common in orchids, since they have strategies to persist (i.e. clonality) (Phillips et al. 2020, Stípková et al. 2020, Evans & Jacquemyn 2022, Anghelescu et al. 2023).
Allogamy. It is subdivided in two types: geitonogamy (the pollen is taken to the stigma of another flower, from the same individual), and xenogamy (the pollen is taken to the stigma of another flower, from a different individual) (Bateman 2020, de Oliveira et al. 2022, de Paiva-Neto et al. 2022). A graphical comparison between autogamy, geitonogamy and xenogamy is shown in Figure 2.
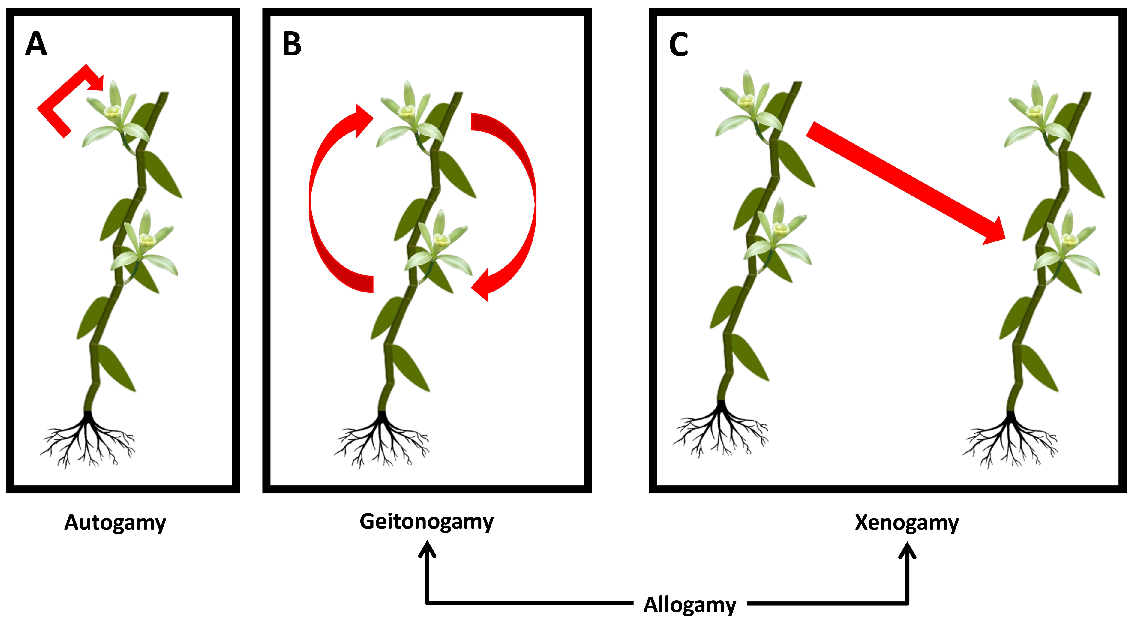
Figure 2 Comparison between autogamy (A) and allogamy (geitonogamy (B) and xenogamy (C)). Red arrows indicate the direction from pollen donator flower to pollen receipt flower. The diagram represents the species Vanilla planifolia, flowers are not in real scale.
Due to the structure of their flowers, allogamy is the most common breeding system among orchids (Lanzino et al. 2023). Even Darwin focused on explaining mechanisms that could promote cross-pollination, as he thought that such floral adaptations of the orchid were selected as consequence of pollinator pressures (Scopece et al. 2014). Orchid flowers commonly exhibit mechanical barriers (i.e., rostellum) that tends to reduce the formation of fruits with self-pollen (Zhang et al. 2021). This tendency to avoid self-pollination in orchids is due to the fact that self-incompatibility is a post-pollination barrier that avoids self-fertilization, however, self-incompatibility does not avoid pollen transfer (pollination) (Valdivia et al. 2010, Zhang et al. 2021). In partially self-incompatible orchids commonly occur a reduction in the number of seeds per fruit, reduction of the weight and size of the fruits, and a lower number of seeds with embryo (Tremblay et al. 2005, de Paiva-Neto et al. 2022). Partial self-incompatibility has been recorded in more than 750 species of Orchidaceae, mainly in Chondrorhyncha, Coelogyne, Dendrobium, Lycaste, Notylia and Oncidium (Johnson & Edwards 2000, Tremblay et al. 2005).
The first case of allogamy discussed here is geitonogamy. Almost all flowers from an inflorescence usually have the same probability of being visited by a pollinator, thus, geitonogamy results in the same effort as observed in xenogamy, because pollen is transported between two flowers (Lanzino et al. 2023). However, geitonogamy has been considered similar to autogamy, as the pollen transfer occur between flowers of the same individual (Kropf & Renner 2008). For this reason, some authors have considered that, in order to increase genetic diversity, geitonogamy is undesirable, along with autogamy (Sletvold et al. 2012, Gigant et al. 2016). Besides, it must be considered that there are many orchids that produce solitary flowers or racemes whose flowers open successively (one after another), which may reduce the probability of geitonogamy (Srimuang et al. 2010). Nevertheless, in some orchid species such as Chloraea crispa Lindl., Eulophia alta (L.) Fawc. & Rendle, or Phaius tankervilleae (King & Pantl.) Karthik., pollination by geitonogamy results in higher fruit set and seed germination than by autonomous self-pollination (Humaña et al. 2008, Johnson et al. 2009, Buragohain et al. 2016), in comparison to autogamy or even xenogamy, considered as the best way of pollinating (discussed below).
In contrast, pollination by xenogamy is the most widespread type of allogamy in orchids, which is facilitated by some floral structures such as the rostellum (Bory et al. 2008, Sugiura 2013, Freudenstein & Chase 2015, Ospina-Calderón et al. 2015). In truly allogamous orchids this structure is well-developed, while in autogamous species it is usually reduced (Suetsugu 2015, Endress 2016). Although today we know that xenogamy is not the only form of pollination, previous works, such as Darwin’s statement, mentioned that it must be the main way (Scopece et al. 2014).
There is a tendency observed in allogamous plants (not only in orchids) to be pollinated by xenogamy instead of geitonogamy (Pang & Saunders 2015, Kundu & Karmakar 2022). It is hypothesized that the lack of nectar evolved to reduce geitonogamy, because pollinators (mainly bees), tend to avoid non-rewarding flowers (Johnson et al. 2004, Kropf & Renner 2008). A consequence of xenogamy is the genetic variation, allowing a better adaptation than to obligatory autogamous species (Willmer 2011). In addition, it provides some defense against natural mutations in the genetic material, since if one of the nuclear genomes is damaged, the effects of non-functional alleles may be masked by the correct functioning of the equivalent alleles on the chromosome inherited from the other parent (Willmer 2011, Talalaj & Skiercynski 2015). Autogamy reduces genetic diversity and favors the expression of harmful genes (Willmer 2011, Emeterio-Lara et al. 2018).
Pollination by xenogamy can also influence both the fruit set and the seed production (Tremblay et al. 2005). Even in self-compatible plants, this mode of pollination commonly increases fruit set (Borba et al. 1999, Caballero-Villalobos et al. 2017, Emeterio-Lara et al. 2018, Sao Leao et al. 2019), and improves seed viability (Johnson 2000, Vale et al. 2010, Caballero-Villalobos et al. 2017, Capó et al. 2022). Several studies have shown that allogamy generally tends to be more advantageous compared to autogamy in terms of higher fruit set, as observed in Epidendrum denticulatum Barb. Rodr. (Sao Leao et al. 2019), or Vanilla palmarum (Salzm. Ex Lindl.) Lindl. (Pansarin & Ferreira 2021), species that exhibited a fruit set between 43-76 % through autogamy, and 82-95 % through allogamy. This advantage stems from the genetic benefits associated with outcrossing, such as increased genetic diversity and reduced risk of inbreeding depression (Valdivia et al. 2010, Zhang et al. 2021). Cross-pollination increases the chances of successful fertilization by introducing genetic diversity and potentially overcoming self-incompatibility mechanisms (Zhang et al. 2019). Also, xenogamy generally results in seeds with higher viability compared to autogamy, as recorded in Cattleya coccinea Lindl. (Caballero-Villalobos et al. 2017), or Cyrtopodium punctatum (L.) Lindl. (Dutra et al. 2009), since these species exhibited a germination percentage ranging from 9 to 65 % through autogamy, and 42 to 97 % through xenogamy.
Many orchid species set fruits exclusively by cross-pollination (Fantinato et al. 2017, Mosquera-Mosquera et al. 2019, Zhang et al. 2021). Epidendroideae orchids exhibit a greater number of self-sterile species, and few Orchidoideae orchids can be self-fertilized (Fantinato et al. 2017, Sao Leao et al. 2019, Zhang et al. 2021). In contrast, orchids from Vanilloideae and Cypripedioideae species can set fruits through self-pollination, although their floral morphology promote cross-pollination (Suetsugu & Fukushima 2014, de Oliveira et al. 2022). Finally, Apostasioideae orchids might be pollinated by xenogamy (Kocyan-& Endress 2001, Yin et al. 2016). A comparison of pollination systems among different orchid subfamilies is shown in Table 1.
Table 1 Comparison among the different subfamilies of Orchidaceae regarding main breeding system exhibited.
| Subfamily | Main conclusions | Cites |
|---|---|---|
| Apostasioideae | There are not formal studies about pollination ecology within this subfamily. It is hypothesized that pollination might occur by xenogamy, and pollen grains (pollen is not agglutinated) might be offered as a reward to their pollinator, possibly meliponini bees. | Kocyan & Endress 2001, Yin et al. 2016 |
| Vanilloideae | Although historically hand-pollinated through autogamy, the genus Vanilla displays physical barriers (rostellum) on the flower, suggesting a predisposition to pollination by outcrossing rather than autogamy. The natural pollinators (when available) are euglossini bees. There is a single anther, with agglutinated pollen which is released as monads or tetrads. | Sasikumar 2010, Pansarin & Ferreira 2021, de Oliveira et al. 2022 |
| Cypripedioideae | Most of the species are self-compatible, but their flowers are designed as one-way traps, and because of their morphology, they promote cross pollination. They require insects (mainly wild bees) to transfer pollen from the anther to the stigma. There are two fertile anthers, whose pollen is agglutinated and paste-like. | Pemberton 2013, Suetsugu & Fukushima 2014 |
| Orchidoideae | It presents authentic pollinia, but divisible. There are some self-compatible species, they do not exhibit a rostellum, and if they do, it is very narrow or degrades during floral opening. However, xenogamy is considered as the predominant way of pollinating. Pollinators are mainly bees and lepidoptera. | Pansarin & Ferreira 2015, Fantinato et al. 2017 |
| Epidendroideae | It presents authentic pollinia, and it is indivisible. Xenogamy is considered as the predominant way of pollinating since self-compatibility is exhibited in most of its species. Epidendroideae orchids have often been considered to be nectar-rewarding or nectarless, however, some few species reward with nectar to their pollinators. Pollinators are flies and lepidoptera, or even hummingbirds. | Pansarin & Pansarin 2016, Mosquera-Mosquera et al. 2019, Sao Leao et al. 2019, Zhang et al. 2021 |
If pollination by allogamy (mainly by xenogamy) exhibits so many advantages over autogamy, why are there species that remain autogamous? In true autogamy can be an important sexual process of reproduction in flowering plants. Although it is not as effective as xenogamy, pollination by autogamy might exhibit a certain degree of genetic recombination (Willmer 2011). Additionally, autogamy does not require a substantial energy investment in the reward production (Eckert & Herlihy 2004). The participation of pollinators is no longer necessary, which makes pollination by autogamy advantageous in the case of pollinator decline (Pedersen & Ehlers 2000). Many orchids, especially in the tropics, show populations with few individuals, and successful cross-pollination events can be rare (Bernhardt & Edens-Meier 2010, Cabrera-Reyes et al. 2021). A species under such circumstances would develop strategies to ensure its offspring production, where autogamy would serve as a mechanism for this, albeit at the expense of potential adverse effects as mentioned above. However, cross pollination prevails throughout most members of this family and autogamy only happens as a last resort, which could mean orchids prioritize the advantages of cross-pollination over the potential security of fruit and seed production through autogamy, which does not ensure the production of viable seeds (Sao Leao et al. 2019, Yeh et al. 2021, de Paiva-Neto et al. 2022).
Metaxenia and xenia. All pollination strategies previously mentioned involve only one species (intra-specific pollination). Conversely, in metaxenia and xenia two species are involved (inter-specific pollination). Metaxenia is understood as the effect of foreign pollen (different species, but from the same genus) on fruit formation, including size increase and changes in traits as texture, shape, scent, flavor, and chemical composition (MacInnis & Forrest 2020). In addition, biochemical changes are also presented, as an increase in the concentration of different metabolites, highlighting carbohydrates and phenolic compounds (Suaib et al. 2020, Shahsavar & Shahhoseini 2022).
Although there is no explanation regarding the molecular mechanisms related to metaxenia, current hypotheses point out to an increase in the concentration of growth regulators such as auxin, cytokinin, and gibberellin from the new pollen source, as fruits of larger size in other cultivars tend to exhibit higher levels of these regulators (Cheng et al. 2020, Deng et al. 2022). Another hypothesis suggests an enzymatic change in the fruits, particularly those enzymes related to processes such as fruit expansion and the production of sugars and phenolic compounds (Deng et al. 2022, Shahsavar & Shahhosseini 2022). Besides, chemical signaling related to volatile compounds could be another plausible explanation, since some chemical signals produced by the male part of the flower (released by the pollen) may interact with female reproductive structures and influence fruit development (Piotto et al. 2013, Deng et al. 2022). These hypotheses have been proposed based on observations in other crops of importance (i.e., date, pear, plum and tomato); however, these postulates have not been verified in orchids or in wild plants.
A related concept is xenia, sharing the same definition mentioned for metaxenia, but applied to seeds (Sabir 2014). It has been reported that xenia seeds usually exhibit greater viability, manifested as greater germination and development of seedlings, as well as an increase in size and weight (Sattler et al. 2016, van Esse et al. 2020). Xenia is considered the previous step for the formation of hybrids, since compatibility between the species must be observed, in addition to observing the immediate effects of the pollen (Sari et al. 2023). It has been considered that these changes in the seed could be attributable to greater control of seed development by paternal genes, and that maternal genes may lose part of the control, by allowing the formation of large seeds (de Jong & Scott 2007). However, this hypothesis was proposed for other angiosperms but the mechanisms of xenia induction in orchids are still unknown.
The study of metaxenia and xenia emerged in the last century, with the study carried out by Swingle (1928) focused on the date palm. Metaxenia as a concept is relatively recent, considering that the study of pollination ecology formally began in 1793 (Faegri & Pijl 1979). Studies about the effect of xenia and metaxenia induction have focused mainly on crops of economic importance, such as apple (Bodor et al. 2008, Militaru et al. 2015), pear (Cheng et al. 2020), strawberry (MacInnis & Forrest 2020), date (Swingle 1928, Shahsavar & Shahhoseini 2022), tomato (Piotto et al. 2013), cucumber (Olfati et al. 2010), grape (Sabir 2014), hazelnut (Balik & Beyhan 2020), and corn (Suaib et al. 2020), among others. In general, a positive effect could be observed regarding fruits and seeds production. Therefore, it is recommended to carry out this breeding system to obtain improvements in crops.
No formal studies about xenia and metaxenia have been carried out in Orchidaceae, except for two subfamilies: two species from Vanilloideae (V. planifolia and V. pompona Schiede) (Menchaca-García et al. 2011, Barreda-Castillo et al. 2023a), and three species from Epidendroideae (Bulbophyllum weddellii (Lindl.) Rchb.f, B. involutum Borba, Semir & F. Barros, and B. ipanamense Hoehne) (Borba et al. 1999). Regarding the Vanilla species, interspecific pollinations were carried out since both vanillas coincide phenologically (Barreda-Castillo et al. 2023a). A beneficial effect due to the induction of metaxenia could be observed only in V. planifolia when was pollinated with V. pompona, manifested as an increase in size and weight in the fruits in comparison to autogamy in each species, while in V. pompona it was the opposite effect, presenting smaller fruits. So, although it is beneficial in most crops, the induction of metaxenia does not necessarily provide better results compared to traditional pollination. Moreover, Menchaca-García et al. (2011) reported xenia effect on both V. planifolia and V. pompona species, obtaining germination percentages close to 80 %, which means an increase, considering that V. planifolia presents germination percentages near to 5 % (Yeh et al. 2021), and V. pompona germination has not been reported (Menchaca-García et al. 2011). This study was originally called “hybrid production”, however, xenia and hybrid production are related concepts, as we discussed in the next section.
Metaxenia and xenia induction are related to interspecific hybrids production, something useful to the most commercially important orchid, and one of the highest yielding crops in the tropics, Vanilla. It is recommended, first of all, to continue searching for pollen donor species for V. planifolia, aiming to induce changes in the chemical composition of its fruits, as well as an increase in their size (Chambers 2019). In addition, hybrid organisms would be obtained indirectly, combining qualities of the parental species (Sari et al. 2023). Furthermore, by selecting pollen donor species, these could accompany the cultivation of V. planifolia, which in turn could contribute to breeding programs for vanilla species (Watteyn et al. 2023) or even species with agro-economic value.
Regarding the Bulbophyllum species, crosses between B. involutum and B. ipanamense (species with greater genetic proximity) exhibited fruit formation rates and seed viability similar to those obtained in intraspecific crosses, whereas crosses between B. weddellii with both B. involutum and B. ipanamense exhibited a higher rate of fruit abortion (Borba et al. 1999). Therefore, better results are obtained in metaxenia and xenia induction when the species reflect genetic proximity. This study was reported as “crossing potential”, although they actually reported metaxenia and xenia effect. Research on the effect of xenia and metaxenia in orchids is necessary, given the lack of information that currently exists.
Hybrids. Although the effect of xenia induction has not been properly studied in orchids, the capacity of hybridization is well known in this plant family. A hybrid is defined as the organism result of cross fertilization between different species from the same genus (Figure 3A) or between species belonging to distinct genera (Figure 3B) (Kishor & Sharma 2009, Kempe & Gils 2011, López-Caamal & Tovar-Sánchez 2014, Chambers 2019). Despite metaxenia and xenia induction along with hybrids production involve cross pollination, metaxenia effect is just observed in fruit formation (Balik & Beyhan 2020), xenia effect is related to seed production (Sabir 2014), whereas hybrid production relates to the new generation of organisms produced (Preston & Pearman 2015, Goulet et al. 2017).
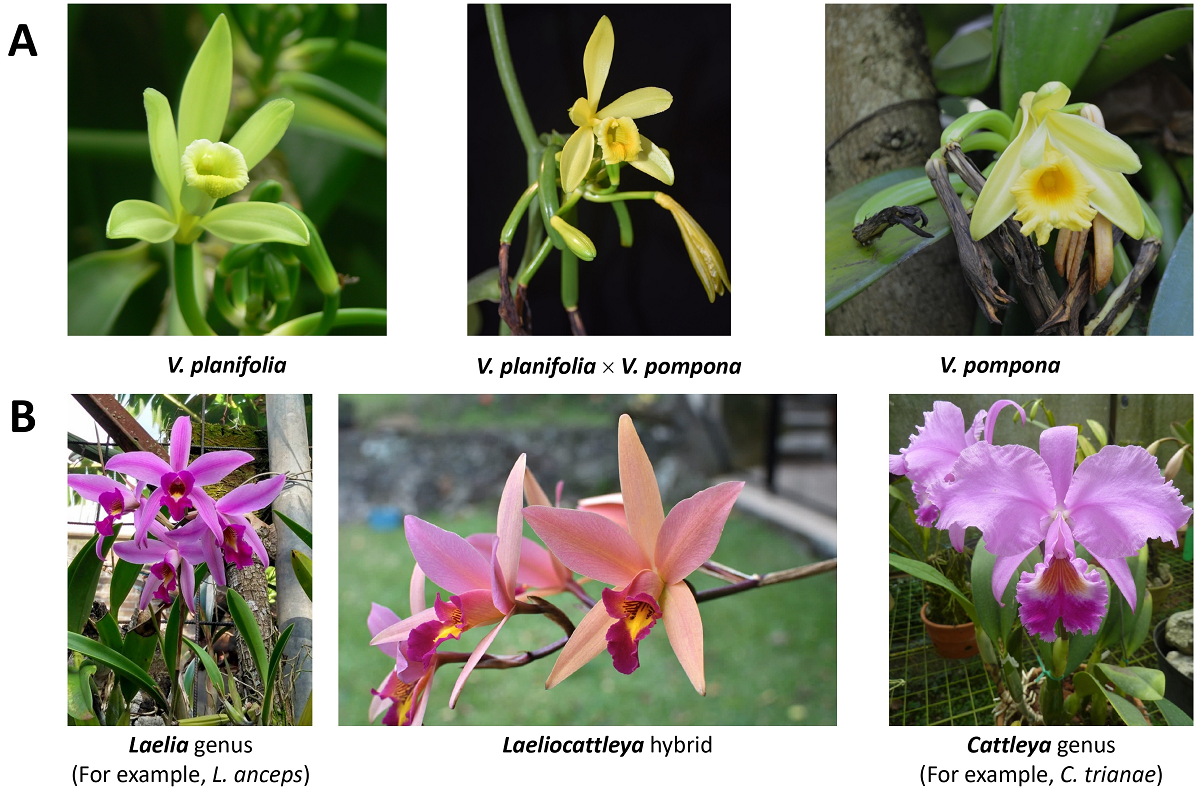
Figure 3 Representative images of hybrid production. In orchids, hybrids can be obtained from species from the same genus, as observed in Vanilla planifolia × V. pompona (A), or different genera, as observed in Laeliocattleya (B). L. anceps and C. trianae are illustrative only.
Xenia is directly related to hybrid production, as xenia induction is a process in plant reproduction that involves manipulating pollination to produce specific characteristics in the offspring (Olfati et al. 2010). In the context of hybrid production, xenia induction is used to ensure the fertilization of an ovule by a specific pollen grain, resulting in a hybrid with desirable traits from both parents (Deng et al. 2022). In artificial hybridization, this process is used to combine the desirable traits of two different parental entities on the hybrid (Goulet et al. 2017). For example, if a hybrid with greater resistance to certain diseases and improved performance is desired, breeders can use xenia induction to ensure that the ovules of one plant are fertilized by the pollen of another plant with the desired characteristics.
On the other hand, interspecific and intergeneric hybrids are related to the theory of metaxenia or xenia in the context of plant breeding and reproduction. Interspecific hybrids are produced by crossing between two different species within the same genus; in the context of metaxenia or xenia, when these hybrids are formed, characteristics from both parent species can be influenced by xenia, leading to traits in the offspring that are influenced not only by the genetic makeup of the parents but also by the environmental effects on the maternal tissues surrounding the embryo sac during pollination and fertilization (de Jong & Scott 2007, Malaviya et al. 2019, Deng et al. 2022). In contrast, intergeneric hybrids are produced by crossing two different genera; similarly, in the context of metaxenia or xenia, when intergeneric hybrids are formed, the environmental effects on the maternal tissues during pollination and fertilization can influence the traits of the resulting offspring, along with the genetic contributions from both parent genera (Havkin-Frenkel & Belanger 2018, Li et al. 2021a, Vilcherrez-Atoche et al. 2022).
In the past, it was considered that the production of plant hybrids was useless, since it would not be possible to obtain viable organisms, and if any were obtained, they would be weak and inferior to their parental species (Stebbins 1958). Nowadays, it is known that the hybridization is a viable process observed in several plant groups (Paun et al. 2011), as it occurs even in natural conditions (Fay et al. 2007, Johnson 2018, Arida et al. 2021, Cantuária et al. 2021).
Natural hybridization in orchids have significant contributions in evolutionary, ecological, and taxonomic processes. Regarding evolutionary processes, natural hybridization is a common phenomenon and has long been suspected to be a potent evolutionary force (Li et al. 2021a, Fiorini et al. 2023). It has been suggested that a significant number of flowering plants may be of hybrid origin (Cozzolino et al. 2006). Orchid hybrids contribute to the ongoing evolutionary processes by introducing new genetic combinations and variations (Cozzolino et al. 2006, Johnson 2018, Evans et al. 2023). Also, orchid hybrids can play a role in speciation processes by serving as intermediates between parental species (Johnson 2018). Hybridization events can lead to the formation of new species through hybrid speciation, where hybrids become reproductively isolated from parental species and establish distinct evolutionary lineages (Fay et al. 2007, Pavarese et al. 2013, Marques et al. 2014). Hybridization is not merely a kind of ‘‘evolutionary noise’’ with little evolutionary significance but may instead sometimes play a positive role in evolution, either through hybrid speciation, or through the origin and transfer of novel adaptations (Cozzolino et al. 2006).
Natural hybridization is really common in Orchidaceae (Johnson 2018, Evans et al. 2023), as it has been observed in B. × cipoense (B. weddellii × B. involutum) (Borba & Semir 1998), Catasetum × sheyllae (C. boyi Mansf. × C. garnettianum Rolfe) (Cantuária et al. 2021), Epidendrum purpureum (E. denticulatum × E. orchidiflorum Salzm. ex Lindl.) (Arida et al. 2021), Laelia × meavei (L. dawsonii (J.Anderson) De B. Crawshay × L. rubescens Lindl.) (Cetzal-Ix et al. 2020), L. × oaxacana (L. halbingeriana Salazar & Soto Arenas × L. anceps Lindl.) (Salazar et al. 2014), Prostechea × chixoyensis (P. cochleata (L.) W.E.Higgins × P. radiata (Lindl.) W.E.Higgins) (Mó et al. 2014), Orchis × dietrichiana (O. tridentata Scop. × O. ustulata L.) (Cozzolino et al. 1998), or Vanilla × tahitensis (V. planifolia × V. odorata C.Presl), which was believed to be a species (Lubinsky et al. 2008), among others.
Regarding ecological processes, natural orchid hybrids can potentially reproduce with their parent species, leading to introgressive hybridization (Marques et al. 2014). This process involves the transfer of genetic material between hybrids and parent species, influencing the genetic diversity and adaptation potential of populations (Marques et al. 2014). Introgressive hybridization can facilitate the exchange of adaptive traits, contributing to the resilience and evolutionary flexibility of orchid populations in changing environments (Pinheiro et al. 2010). However, natural hybridization is typically considered deleterious for the conservation of biodiversity (Vereecken et al. 2010, Stull et al. 2023). Interspecific gene flow is often seen as a hazard in plant conservation genetics, especially when rare species come in contact and hybridize with more common and widespread related taxa as a consequence of habitat disturbance (Ferdy & Austerlitz 2002). Hybridization may lead to the loss of rare taxa as a consequence of outbreeding depression and genetic assimilation (Chung et al. 2005). Consequently, specific conservation strategies should be designed to protect individuals and hybrid populations, in order to maintain both the natural sources and the new organisms for future evolution (Fay 2018, Evans et al. 2023).
Regarding taxonomic contributions, natural orchid hybrids contribute to the overall diversity of the orchid family by generating new combinations of traits and morphologies (Goulet et al. 2017, Li et al. 2021a). These hybrids often exhibit unique characteristics that may not be present in either parent species, leading to the recognition of additional taxa (Bertrand et al. 2021). However, orchid hybrids can also present challenges for taxonomic classification due to their intermediate characteristics and complex genetic backgrounds (Cozzolino et al. 2006, Radak et al. 2019). Therefore, taxonomists need to carefully evaluate morphological, genetic, and ecological data to accurately classify orchid hybrids and understand their evolutionary relationships with parent species.
In addition to natural hybridization, there are several hand-made hybrids between species of different genera, for example, Aranda (Arachnis hookeriana (Rchb. f.) Rchb. f. × Vanda lamellata Lindl.), Brassocattleya (Brassovola × Cattleya), Laeliocattleya (Laelia × Cattleya), Odontocidium (Odontoglossum × Oncidium), and several interspecific hybrids within Cattleya, Cymbidium, Dendrobium or Phalaenopsis (Asociación Mexicana de Orquideología 2005). In orchids, the hybrid production is not limited to primary hybrids, since there are secondary hybrids (crossing of a hybrid with a species or crossing three species) reported (i.e., Vanilla × manitra ampotony (V. planifolia × V. × tahitensis)) (Grisoni & Nany 2021). In addition, hybrids can be cross-pollinated with other hybrids, and viable seedlings can still be obtained, combining more traits in each generation (Devadas et al. 2016).
Among the 25 largest plant families, Orchidaceae is the one with the largest number of known hybrids (Fiorini et al. 2023). The lack of endosperm and the phylogenetic closeness that orchids share with each other have been considered as the main reasons why so many hybrids are obtained in this family (Johnson 2018, Li et al. 2021a). Although phenological coincidence is helpful in the formation of hybrids because pollinators might cross-pollinate between species (Turchetto et al. 2022), as well geographical coincidence, also called “hybrid zones” (Marques et al. 2014, Johnson 2018, Evans et al. 2023), and morphological similarity of flowers (Calevo et al. 2021). However, sometimes pollen is preserved in cryogenic conditions to be used later, achieving both formation of fruits as well as viable seeds (Divakaran et al. 2016), but this happens under cultivation conditions or in horticulture.
The first list of hybrid orchids reported around 10,000 crosses (Adams & Anderson 1958). The first natural orchid hybrid ever recorded was Phalaenopsis intermedia (P. aphrodite Rchb. f. × P. equestris var. rosea Valmayor & D. Tiu) in 1853 (Li et al. 2021a), whereas the first hand-made orchid hybrid was “Calanthe” (Calanthe masuca (D. Don) Lindl. × C. furcata Bateman ex Lindl.), reported in 1856 (Li et al. 2021a). Nowadays, more than 100,000 orchid hybrids (natural and hand-made) are reported worldwide (The Royal Horticultural Society 2023), list that keeps growing every day.
In most of the cases, hybridization process results in the production of organisms with better responses to biotic and abiotic stress, in comparison to their parental species (Divakaran et al. 2006, Kumar & Singh 2016, Goulet et al. 2017, Li et al. 2021a). Some benefits of the production of hybrid organisms are reflected in qualities appreciated by the market, such as better blooms, larger flowers, long flowering period, or the combination of pigments (Tatsuzawa et al. 2004, Edens-Meier et al. 2013, Pramanik et al. 2022). Perhaps this is the main reason why there are so many artificial orchid hybrids worldwide.
Improvement of shape, size and aroma of the fruit might not be important for most orchids; however, it is very important in the case of vanilla. For this reason, research on hybrids in orchids is of greatest relevance in vanilla, due to its economic importance. For example, V. planifolia × V. pompona hybrids have exhibited a better response to water stress (Barreda-Castillo et al. 2023b), and to the exposition to Fusarium oxysporum f. sp. vanillae, its main pathogen (Barreda-Castillo et al. 2022). V. × tahitensis (V. planifolia × V. odorata) exhibits a vanillin concentration similar to V. planifolia (Brunschwig et al. 2016). V. × manitra ampotony (V. planifolia × V. × tahitensis) shows a concentration of vanillin (aromatic chemical marker of vanilla) up to 20 times higher than the observed in V. planifolia, in addition to an increase in certain phenolic compounds (Grisoni & Nany 2021). V. × tsy taitra ((V. planifolia × V. pompona) × V. planifolia) exhibits a better aromatic quality than V. planifolia, along with the resistance to F. oxysporum f. sp. vanillae (Varela-Quirós 2010, Havkin-Frenkel & Belanger 2018). More vanilla hybrids have been produced between aromatic species, such as V. planifolia × V. phaeantha Rchb. f., V. phaeantha × V. pompona, or V. pompona × V. odorata (Hu et al. 2019, Chambers et al. 2021), with the aim of obtaining higher quality fruits.
Even though historically so many hybrids have been produced within Orchidaceae, not all of them have been viable, or have shown optimal characters (Chambers 2019, Grisoni & Nany 2021). Hybrid production programs as a genetic improvement strategy have historically produced hundreds of lines, however, only certain lines of interest have been selected (Grisoni & Nany 2021). It is recommended to select the orchid hybrids since seed germination (Menchaca-García 2018), or during the development of seedlings (Divakaran et al. 2016), in order to conserve the lines with the desired characters, and once selected, multiply them by in vitro culture (Divakaran et al. 2016, Menchaca-García 2018). Although genetic improvement of orchids through hybridization is a long-term process, it is still the best method to obtain plants with improved qualities without having to resort to the use of transgenics, since hybrid organisms are not necessarily result of genetic modification (Chandler & Dunwell 2008, Goulet et al. 2017, Chambers 2019).
Finally, it is recommended that in future hybridization programs the species with the most desirable characters must be used as ovule donor instead of the pollen donor, due to greater expression of maternal traits in the new organisms (Havkin-Frenkel & Belanger 2018, Barreda-Castillo et al. 2023b). There are two main hypotheses about the expression of maternal characters in a greater degree in hybrids: 1) It might be due to the inheritance of plastids and mitochondria genomes, since both organelles are usually inherited from the maternal parent (Daniell et al. 2021, Park et al. 2021) or 2) it might be due to epigenetic regulation, since this mechanism is more sensitive in plants (Baulcombe & Dean 2014, Kumar & Singh 2016). However, there is not a real consensus about this topic. All types of pollination mentioned so far (as well as the induction of metaxenia and xenia, and production of hybrids) are summarized in Figure 4.
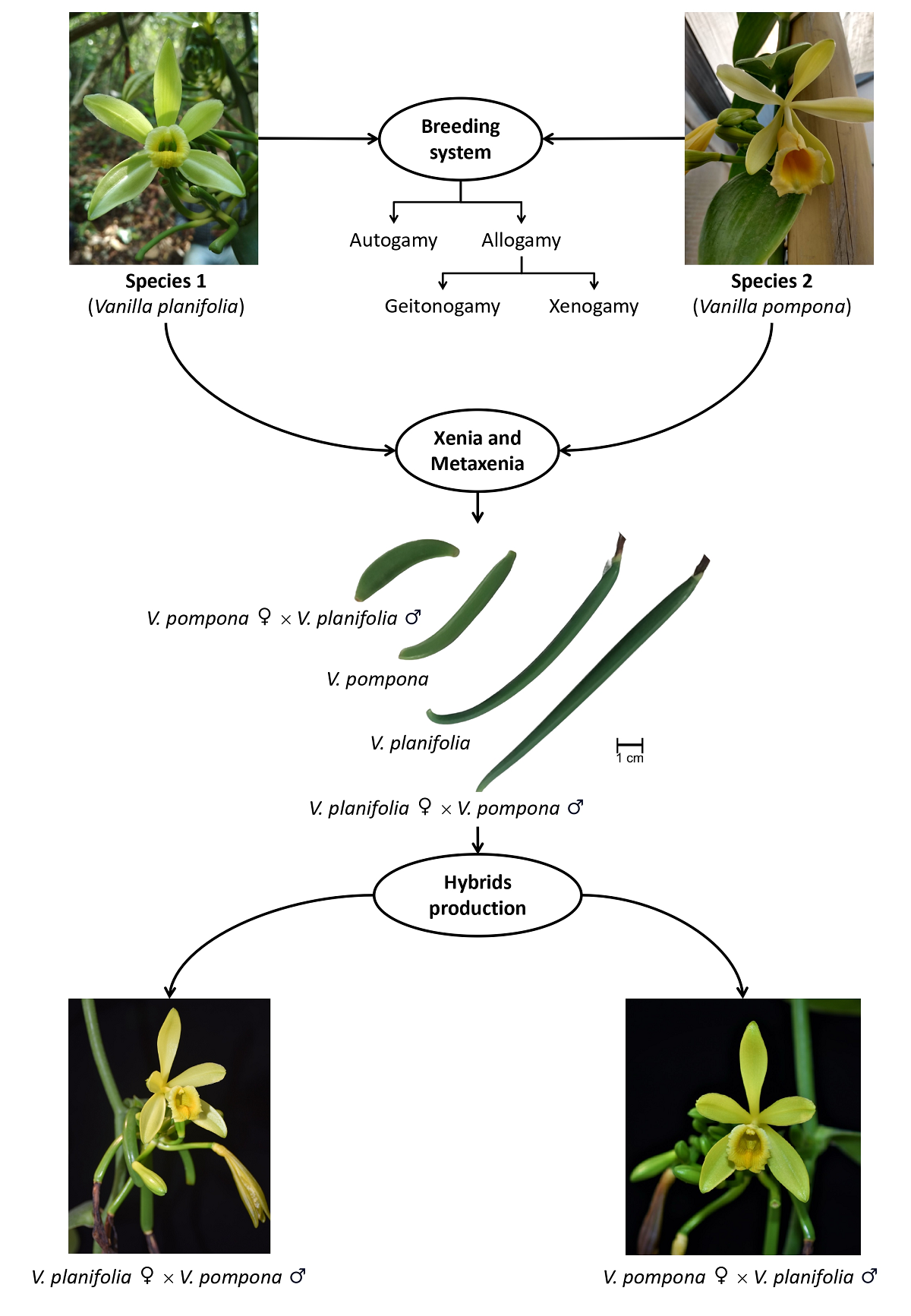
Figure 4 Breeding systems, induction of xenia and metaxenia, and production of hybrids in orchids. Breeding systems autogamy or allogamy (geitonogamy and xenogamy) involves only one species, whereas xenia and metaxenia require two. Although xenia and metaxenia effect only is associated with seeds and fruits production, respectively, hybrids production is related to this process, as the new organisms are also result of cross pollination.
Members of Orchidaceae offer several types of rewards to pollinators or are pollinated by any kind of deception, but fruit set by cross-pollination is strongly favored in this family. However, obligatory and facultative autonomous self-pollination has evolved independently several times in this huge plant family. Allogamy is favored by pre-zygotic, i.e., floral mechanisms, and post-zygotic (genetic) barriers. Moreover, pollination by xenogamy is more frequent than by geitonogamy, because xenogamy offers benefits such as greater genetic diversity.
Although Orchidaceae is well known for its capacity for interspecific pollination and viable production of fruits and seeds, there are scarcity of studies about biochemical and morphophysiological changes in fruits (metaxenia) or seeds (xenia) in these species. Paradoxically, hybrid production (result of interspecific pollination) is well documented in this family, since Orchidaceae shows the greatest production of these organisms. It is recommended to keep producing hybrids for ornamental and economical purposes, as increasing crop productivity, as in the case of Vanilla. Besides, these organisms usually exhibit better traits than their parental species, expressed as greater tolerance to adverse conditions such as biotic or abiotic stress.
In summary, although the majority of orchids can be self-pollinated, it is advisable to pollinate them through xenogamy when done by hand, since in this way it is possible to promote intraspecific genetic diversity and promote seed germination, something necessary in species in risk of extinction. Furthermore, induction of xenia (and hybrid production) could promote genetic improvement, and production or organisms with new and desirable traits.











 nueva página del texto (beta)
nueva página del texto (beta)



