Forest fires are a natural part of the dynamics of most temperate and boreal forests because many of their species depend on fire to persist in these ecosystems (Frelich 2002, Rodríguez-Trejo 2014, Pausas & Keeley 2019). Therefore, species inhabiting these fire-prone ecosystems (or fire-dependent/influenced ecosystems, Hardesty et al. 2005) have developed mechanisms that allow them to complete their biological cycles and persist under natural fire regimes (e.g., frequency, intensity, and extent; Bond et al. 2005, Bond & Keeley 2005, Keeley et al. 2011, Pausas & Keeley 2014, 2019). However, natural or historical fire regimes are altered by different anthropogenic activities (e.g., agriculture, livestock, construction, fire exclusion or suppression policies, and cultural behavior) (Jiménez & Alanís-Rodríguez 2011, Bilbao et al. 2020, Ponce-Calderón et al. 2021) and climate change (e.g., alterations in temperature and precipitation patterns), thereby increasing the probability of high-severity fires and the degradation and loss of forests on unrecoverable scales (Westerling et al. 2006, Abatzoglou & Williams 2016, Liu & Wimberly 2016, Montoya et al. 2023).
Fire severity is the response of ecosystems to fire intensity (i.e., the amount of heat released in a given area and time) (DeBano et al. 1998, Keeley 2009, Rodríguez-Trejo 2014). High-intensity fires, resulting from increased fuel accumulation (organic matter in the soil, leaf litter, and fallen trunks) and extreme dry environmental conditions, favor the occurrence of high-severity fires (Bilbao et al. 2020, Ponce-Calderón et al. 2021). Consequently, these types of fires promote drier soil conditions, trigger soil erosion processes (Certini 2005, Rodríguez-Trejo 2014, Agbeshie et al. 2022), and even significantly affect the survival capability of fire-adapted species. This alteration often disrupts species composition (Johnstone & Chapin 2006, Crotteau et al. 2013, Gärtner et al. 2014, González-De Vega et al. 2018, Boucher et al. 2020, Etchells et al. 2020, Cadena-Zamudio et al. 2022) and the expected successional trajectory of communities (Connell & Slatyer 1977, Pulsford et al. 2016). In this sense, fire severity estimations are based on the degree of change in biotic and abiotic components, and the resilience of the vegetation to a given fire regime (Rodríguez-Trejo 2014). For instance, in the field, severity can be estimated as the proportion of dead plants and burning of vegetation, the loss of surface organic matter (e.g., leaf litter, duff, fine roots, fallen trunks), and the level of soil degradation (Parsons et al. 2010, Silva-Cardoza et al. 2021).
In fire-prone ecosystems, the natural regeneration patterns of plants are mainly defined by attributes related to their persistence capacity (pre-existing seed bank, regrowth capacity, time and type of dispersal), their resistance or their tolerance to the passage of a fire (thick bark, seeds with thick testa), and the post-fire microenvironmental conditions that influence their germination and establishment (Noble & Slatyer 1980, Keeley et al. 2011, Chapin et al. 2014, Rodríguez-Trejo 2014, Pausas 2015, Boucher et al. 2020). Typically, post-fire natural regeneration of oak-pine forests (OPF) begins with the establishment of fast-growing, short-lived, heliophilous pioneer species that can tolerate and prosper in these conditions (Rodríguez-Trejo & Fulé 2003, Almazán-Núñez et al. 2016). The early stages of OPF succession often show the dominance of Pinus spp. seedlings due to their ability to germinate, tolerate, and grow under direct solar radiation but, in Mexico, the succession tendency is generally toward the dominance of Quercus species over time (González-Espinosa et al. 1991, Almazán-Núñez et al. 2016). In a post-fire chronosequence within a mixed pine-oak forest in NE Mexico, González-Tagle et al. (2008) observed that oak species dominated young and intermediate stands due to their resprouting ability. Contrarily, pine species tended to co-dominate with oaks in mature stands. Similarly, Barton & Poulos (2018) showed a transition of mixed pine-oak forest to oak shrublands after high-severity fires in Arizona. This transition is due to the limited recruitment of pines and the resprout of oaks, persisting even up to 14 years post-fire. These tendencies are consistent with the findings in similar ecosystems (Alanís-Rodríguez et al. 2012, An et al. 2019). While many of these studies prioritize describing the woody stratum, understanding the condition of the herbaceous stratum is equally crucial for proposing strategies addressed to facilitate soil recovery and accelerate regeneration of fire-affected areas. In warmer temperate forests, post-fire regeneration is dominated by oaks rather than by pines (Alfaro-Reyna et al. 2019) because of differential recruitment patterns between these groups, growth limitations, and their respective capacities to resprout in response to post-fire environmental challenges such as drought and temperature (Carnicer et al. 2014, Torres et al. 2016, Barton & Poulos 2018).
Successional changes in species composition and vegetation structure in post-fire OPF are influenced not only by fire severity and the rate of recovery dependent on time since fire (Flores-Rodríguez et al. 2021, Cadena-Zamudio et al. 2022), but also by climatic and topographic conditions, edaphic characteristics, land use history, proximity to seed source, stochastic events, and microenvironmental conditions before and after the fire (González-Tagle et al. 2008, Rodríguez-Trejo 2014, An et al. 2019, Rago et al. 2023). On the other hand, local variations in resource availability (e.g., seeds, nutrients, organic matter, moisture, microbiota) due to topographic conditions (e.g., slope, elevation, and exposure) can determine species richness and abundance, as well as the composition and structure of vegetation over time (Alanís-Rodríguez et al. 2012, Chapin et al. 2014, Kane et al. 2015, Bassett et al. 2017, An et al. 2019). These factors lead to high heterogeneity in forest regeneration and resilience responses, posing a challenge when formulating effective restoration proposals (González-Tagle et al. 2008, An et al. 2019). Understanding the biological and physical processes of post-disturbance succession is the theoretical basis for restoration actions (active or passive). In this context, the description of successional stages is important, as it enables the identification of key species, or groups of species, that can facilitate or accelerate the regeneration process (Balaguer et al. 2014, Souza-Alonso et al. 2022, Rago et al. 2023).
Temperate forests in Mexico comprise ecosystems critically affected by human activity and forest fires (Zúñiga-Vásquez & Pompa-García 2019, Montoya et al. 2023). Historically, these forests have faced land-use changes, illegal logging, and anthropogenic fires (Challenger et al. 2009). It is estimated that 37 % of the total forest cover in this ecosystem is secondary vegetation (CONAFOR 2019), where most Mexican OPF remain in various successional stages (Challenger et al. 2009). In this context, successional studies based on a chronosequence approach (i.e., sites with different post-disturbance times) offer the advantage of evaluating time-dependent ecological processes without long-term monitoring, despite the difficulty in meeting basic assumptions (e.g., finding sites that vary only in age and controlling microenvironmental variables) and theoretical limitations (e.g., lack of predictability due to stochastic events), which challenge its use and ecological interpretation (Johnson & Miyanishi 2008, Walker et al. 2010). “La Primavera” Flora and Fauna Protection Area (APFFLP in Spanish), located in western Mexico, allows the study of successional changes in species composition and vegetation structure along a chronosequence using sites with different recovery times after high-severity fires. Studying plant succession through chronosequences will allow to explore natural regeneration patterns in OPF and their relationship with microenvironmental conditions.
Our hypothesis proposes that the deposition of seeds, nutrients, and organic matter tends to accumulate mostly in the flat landforms. Hence, these sites are expected to exhibit greater species richness and abundance of individuals as compared to hillside sites. In contrast to some Pinus species, some Neotropical Quercus species are more tolerant to drier conditions after forest fires and climate change (Alfaro-Reyna et al. 2019). Therefore, Quercus will have a higher importance value index (IVI) throughout the post-fire chronosequence. This study aims to (1) describe post-fire differences in species composition, species richness, and structure of mixed OPF based on the recovery time since the last high-severity fire and two topographic conditions (flat vs. hillside sites), (2) determine microenvironmental variables associated with post-fire plant communities, and (3) identify key species for active restoration of the OPF after high-severity fires. Thus, this study would contribute to the knowledge of the natural regeneration trends in areas affected by severe fires in OPF as a reference framework for designing restoration proposals.
Materials and methods
Study site. “La Primavera” Flora and Fauna Protection Area (APFFLP; 30,500 ha) is located in Sierra Madre Occidental, western Mexico (20° 32' 36.33'' - 20° 43' 33.36'' N; 103° 27' 16.51'' - 103° 41' 12.65'' W, Figure 1) (SEMARNAT 2000). The APFFLP spans an elevation range of 1,400 to 2,200 m and features rugged landscape topography, regosol soil (92 %, characterized by low organic matter content), and high vulnerability to water erosion due to its recent geological history (Dye 2012). The prevailing climate is temperate subhumid, marked by distinct seasonality, summer rains and seven-month dry seasons. Precipitation ranges from 800 mm to 1,000 mm annually, with mean annual temperature of 18.7 °C (min and max values: 5 and 32 °C, CONABIO 2017), classifying the OPF as a dry ecosystem (IPCC 2003). The canopy cover of the OPF is dominated by Quercus castanea Née, Q. laeta Liebm., Q. resinosa Liebm., Q. viminea Trel., Q. magnoliifolia Née (Fagaceae), Pinus oocarpa Schiede ex Schltdl., P. douglasiana Martínez (Pinaceae), and Clethra rosei Britton (Clethraceae) (SEMARNAT 2000).
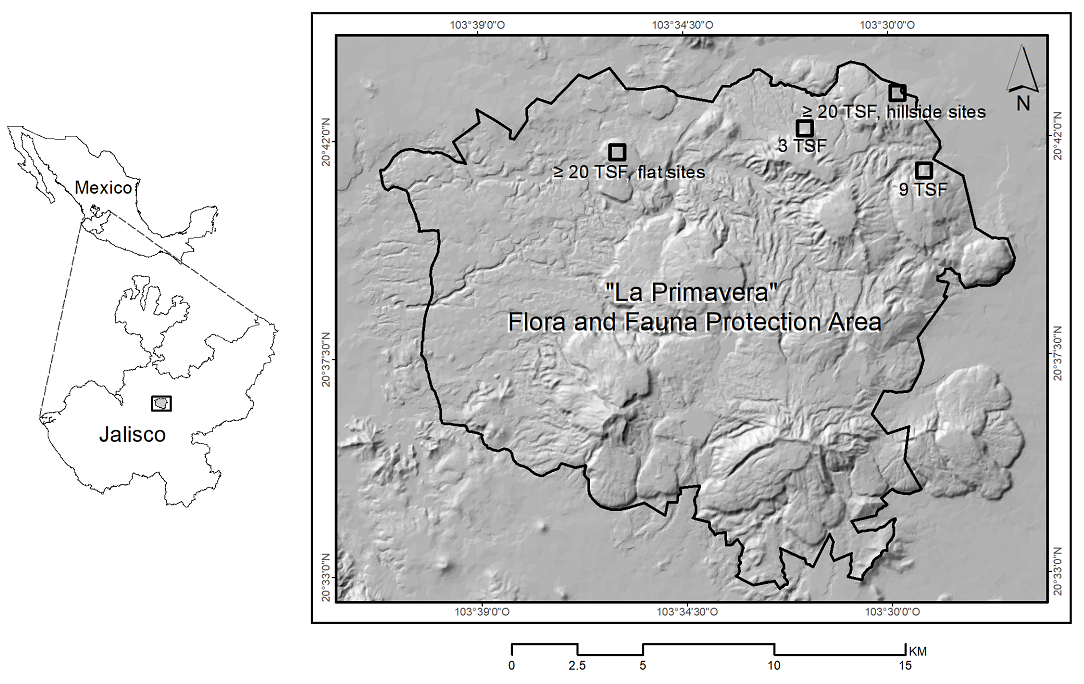
Figure 1 Location of sampling sites with 3, 9, and more than 20 years since the last high-severity fire (3 TSF, 9 TSF, and ≥ 20 TSF) in “La Primavera” Flora and Fauna Protection Area, western Mexico. Black outlined box symbols encompass sampling groups of 3 or 6 sites by post-fire time (N = 3), and topographic condition (N = 2). The 3 TSF and 9 TSF box with 3 flat sites and 3 hillside sites each; ≥ 20 TSF box with 3 sites each.
The historical fire regime of some mixed temperate forests in the Sierra Madre Occidental is characterized as superficial, with low intensity (burning at ground level and understory), and a return interval of 4-6 years (Fulé & Covington 1997, 1998, Rodríguez-Trejo 2008). This fire regime maintains a certain level of forest openness, regulates biomass accumulation, recycles nutrients, and promotes herbaceous species diversity (Fulé & Covington 1997). However, this regime has changed in recent decades due to fire suppression during certain periods, increasing local fuel loads (Jiménez & Alanís-Rodríguez 2011). Historically, APFFLP has faced various anthropogenic threats, including land-use changes for agriculture, extensive uncontrolled livestock grazing, forest resource exploitation, periodic use of fire for sugar harvest, recreational activities and, more recently, urban expansion (Huerta-Martínez & Ibarra-Montoya 2014). These factors increase the susceptibility of forest to fires, particularly during extreme conditions associated with climate change, given that most forest fires have an anthropogenic origin (Ibarra-Montoya & Huerta-Martínez 2016, Bassaber-Zuñiga et al. 2024). Consequently, forest fires have affected over 90 % of APFFLP, with a rising trend in frequency (approximately every seven years) and severity (Gallegos-Rodríguez et al. 2014, Huerta-Martínez & Ibarra-Montoya 2014). These fires impact on the carbon sequestration potential of forest and contribute to create heterogeneous landscape characterized by different successional stages (Balderas-Torres et al. 2013). However, there is no specific fire management plan implemented in the APFFLP.
Site selection. The Forest Fire Hazard Prediction System in Mexico (Vega-Nieva et al. 2020) allowed us to identify burned areas over the last 10 years. The severity of burned areas for each year was verified using the difference in Normalized Burn Ratio (dNBR = pre-fire NBR - post-fire NBR) (Key & Benson 2006). These indices were derived from the near-infrared (NIR) and short-wave infrared (SWIR) wavelengths of Landsat satellite images (Escuin et al. 2008). For the NBR spectral index, satellite images were selected for each year (earthexplorer.usgs.gov), followed by field verification. In 2021, two polygons were identified, where high-severity fires occurred (dNBR > 380) based on the methodology for temperate forests described by Silva-Cardoza et al. (2021). One had three years and the other had nine years since the last high-severity fire (referred to as time since the last fire, 3 TSF, and 9 TSF, respectively). High severity was confirmed at each site through a visual assessment of the burned or scorched crown extent (identified by brown or desiccated leaves and needles). More than 70 % of the tree canopy at each location showed signs of burning or complete absence (Silva-Cardoza et al. 2021). As a reference area, a polygon without records of forest fires over more than 20 years (hereafter ≥ 20 TSF) was selected to describe the composition and structure of the vegetation in absence of recent fires. The ≥ 20 TSF estimation was obtained through informal interviews with current APFFLP managers (M. Carrillo, pers. comm.). In each polygon (3 TSF, 9 TSF, and ≥ 20 TSF, Figure 2), six sites were selected (a total of 18 sites) and half of them corresponded to each topographic condition (TC: flat and hillside sites), with a separation of at least 100 m each other. All hillside sites were located in NE aspects. Although there is criticism about using chronosequences for analyzing plant community succession due to the theoretical limitations of this approach (e.g., predictability of successional replacement) (Johnson & Miyanishi 2008, Walker et al. 2010, Freestone et al. 2015), we consider that our study area meets the basic assumptions: sites differing only in age, within a sufficiently homogeneous landscape with similar climate and low number of tree species to describe (Walker et al. 2010, Freestone et al. 2015), and with clues about species-abundance-microclimate associations in relation to post-fire time.
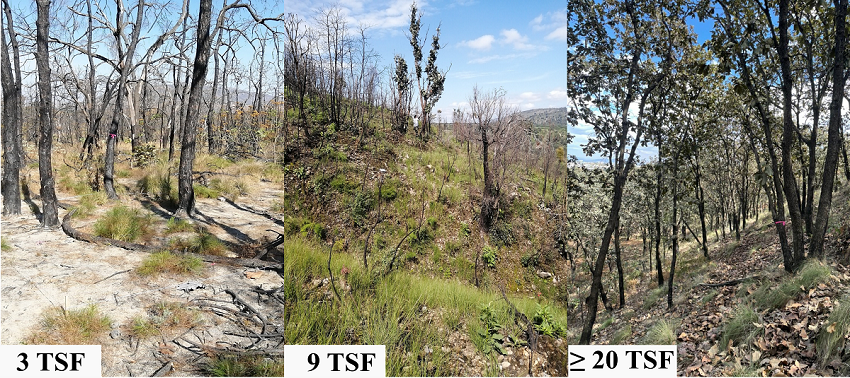
Figure 2 Photos of some sampling sites with 3, 9, and more than 20 years since the last high-severity fire (3 TSF, 9 TSF, and ≥ 20 TSF) in “La Primavera” Flora and Fauna Protection Area, western Mexico.
Sampling design. In recently burned sites at 3 TSF and 9 TSF, vegetation measurements were conducted between September and October 2021, while sites with ≥ 20 TSF were surveyed in January 2022. In the field, a 60 × 48 m grid was established at each burned site and TC using a stratified random design (with modifications; Vázquez-García & Givnish 1998). Within each grid, a subdivision was made at 12 m intervals, resulting in 20 subdivisions, with 10 squares randomly selected. In the center of each selected square, a circular plot (r = 5.64 m, 100 m2) was established, with a total sampling area of 1,000 m2 (0.1 ha) per site.
Field measurements. In each circular plot, the following data was collected for the woody stratum: number of tree individuals per species and total height (m) per individual/species, while diameter at breast height (DBH > 2.5 cm) per individual/species was also registered to estimate basal area (m2). Additionally, the number of resprouting individuals per tree species, originating from existing structures (stumps, root sprouts, etc.), was recorded. In the herbaceous stratum, four squares of 1 × 1 m were systematically established within each circular plot, where the number of individuals, average height (cm), and average cover (%) per species were registered. Species that could not be identified in the field were collected for herbarium preservation and later identification at the IBUG herbarium. To characterize the plant communities, absolute and relative abundances (ABUN), frequency (FREQ), dominance (DOM, basal area), and cover (COV, only for herbaceous species) were calculated (%) for each species. Additionally, to identify key species associated with TSF, the species’ importance value index (IVI) was calculated according to Curtis & McIntosh (1951) (Alanís-Rodríguez et al. 2020).
Microenvironmental variables were directly measured within the sites (3 sites × 3 TSF × 2 TC). Soil sampling was conducted in March 2022, where five independent samples were obtained from a depth of 30 cm at each site. These five samples were subsequently mixed in a single composite sample per site, resulting in 18 mixed samples. Then, the following soil determinations were conducted at the Soil Laboratory of Centro Universitario de Ciencias Biológicas y Agropecuarias of the Universidad de Guadalajara: pH (using a HI 2211 potentiometer, Hanna instruments), electrical conductivity (µmho/cm; with a YSI model 35 conductance meter), texture (using a Bouyoucos hydrometer), organic matter (%; Walkley & Black 1934), bulk density (g cm−3, Blake & Hartge 1986), and cations (mg/L), including K, Na, Ca, and Mg, following the methods recommended in NOM-021-SEMARNAT-2000 (SEMARNAT 2002). In November 2022, within each site, microenvironmental variables were directly measured at five random locations at midday (3 sites × 3 TSF × 2 TC, 90 measurements per variable). Photosynthetically active radiation available for plants (PAR, μmol m-2s-1) was measured with a photoradiometer (Apogee, Instruments USA). The quality of light (red/far red ratio) was determined with an SKR 100/116 sensor (Skye Instruments Ltd., Powys, UK). Soil moisture content (SMC, %) and soil temperature (ST, °C) were recorded using an SM 100 Waterscout sensor (Spectrum. Technologies, inc.). At each circular plot, we also recorded elevation (m asl), slope (%), percentage of woody cover, herbaceous cover, leaf litter cover, and rock and bare soil cover. All recorded variables are available in Appendix 1.
Data analysis. To evaluate the effectiveness of our sampling efforts, we calculated the average of four non-parametric estimators to determine the proportion of species captured un samplings in relation to the total expected species richness. This approach facilitated the determination of the general trend of the expected species that we aimed to record during samplings. The estimators used were Chao 1 and ACE, which are based on species abundance, and Chao 2 and Jackknife 2, which are based on presence-absence data (Gotelli & Chao 2013). All these estimates were conducted using EstimateS version 9.1.0 (Colwell 2013).
To graphically visualize the similarity in species composition between sites as a function of TSF and TC, a main matrix was integrated with all species having ≥ 5 % in abundance in one or more circular plots (Gauch 1982). The species matrix was standardized and subjected to a double square root transformation (Clarke et al. 2014). This matrix was then used for ordination via non-metric multidimensional scaling (NMDS), a technique suitable for non-normally distributed data primarily used to visually represent groups or differences within large complex data (McCune & Grace 2002). Subsequently, a one-way analysis of similarity (ANOSIM, based on Bray-Curtis’s distance) (999 permutations) was performed to evaluate differences in species composition and abundance as a function of TSF × TC.
We employed a two-way Generalized Linear Mixed Model (GLMM) using the “lme” function on the “lme4” package (Bates et al. 2015) to explore the interactive effects of time since fire (3 TSF, 9 TSF, and ≥ 20 TSF) and topographic conditions (TC; flat and hillside sites) on woody vegetation structure (richness, number of individuals, basal area, and total height). In these models, TSF and TC served as fixed factors, while circular plots (N = 10) were nested within sites and sampling dates and treated as random factors. Variation in basal area and height of woody species was specifically assessed for individuals ≥ 4 m in height. Due to the lack of normal distribution of the residuals for a GLMM, the DBH variation between TSF-TC categories (3 TSF - flat, 3 TSF - hillside, 9 TSF - flat, 9 TSF - hillside, ≥ 20 TSF - flat, ≥ 20 TSF - hillside N = 6) was evaluated with a Kruskal-Wallis test. Additionally, to assess whether the number of resprouting individuals varied between the three most important tree species (with the highest IVI value, N = 3) and TSF-TC categories (3 TSF - flat, 3 TSF - hillside, 9 TSF - flat, 9 TSF - hillside, N = 4), a two-way GLMM was conducted with sites as a random factor. Also, a two-way GLMM was used to evaluate the effect of TSF and TC on the richness, number of individuals, average cover, and average height of the herbaceous stratum (i.e., four subplots nested within each circular plot = 40 subplots per site).
To evaluate variation in soil attributes (pH, EC, OM, BD, K, Na, Ca, Mg) and microenvironmental variables (PAR, SMC, ST) in relation to TSF and TC, a two-way GLMM was performed, treating the site as a random factor. When necessary, response variables were transformed (e.g., log and square root) to satisfy assumptions of models. Each model was validated by graphically checking the fit of residuals using the “plot” function, and the significance of the “t-values” of the model was evaluated with the “ANOVA” function. Post-hoc analyses were performed using the “emmeans” function (package “emmeans”; Russell et al. 2021). These analyses were carried out using R Studio v. 2022.02.2 (R Studio Team 2022).
To identify microenvironmental variables associated with species composition and abundance in post-fire plant communities, a matrix of variables was generated, excluding those with collinearity (Soil pH, soil texture, OM, K, Na, Mg, red/far red ratio, SMC, ST, elevation, slope, leaf litter cover, rock, and bare soil cover). The final matrix included time since the last fire (TSF), topographic condition (TC), electrical conductivity (EC), soil bulk density (BD), calcium (Ca), photosynthetically active radiation (PAR), average herbaceous species cover (Cher), and average canopy cover (CC). Qualitative TSF and TC variables were converted into auxiliary (or dummy) variables and treated as quantitative. Detrended Correspondence Analysis (DCA) was conducted using the species matrix to estimate gradient amplitudes, extracting a total inertia < 2. A direct linear ordination technique was used because scatter plots between species and microenvironmental variables did not exhibit unimodal relationships (McCune & Grace 2002). Canonical redundancy analysis (RDA) was also performed because, unlike other techniques, they represent the interactions between the species matrix and the microenvironmental variables matrix (predictor) (Legendre & Andersson 1999), in addition to identify species associated with each post-fire site. NMDS and ANOSIM analyses were performed using the Primer v. 7 (Clarke & Gorley 2015), as well as RDA using the PC-ORD v. 7.0 (McCune & Mefford 2016).
Results
Post-fire differences in floristic composition. Our sampling effort yielded the presence of 82 % of the expected species in the study area. In the woody stratum, 19 species across 16 genera and 12 families were recorded (Appendix 2). The families with the highest species richness were Asteraceae (4), followed by Fabaceae (3), Ericaceae (2), and Fagaceae (2) (58 %). The remaining families were represented by a single species (42 %). Regardless of topographic condition (TC) and time since the last fire (TSF), the most frequently occurring woody species along the chronosequence included Quercus resinosa (21 %), Pinus oocarpa (20 %), Verbesina fastigiata B.L. Rob. & Greenm. (16 %), Quercus viminea (1 %), and Agarista mexicana (Hemsl.) Judd (8 %), which showed resprouting structures (Table 1A). In the herbaceous stratum, 154 species from 108 genera and 40 families were recorded (Appendix 2). In addition, 11 morphospecies were identified at the family level, six Poaceae and five Asteraceae. The families with the highest species richness were Asteraceae (38), Poaceae (32), and Fabaceae (19) (57 %), while the others had < 8 species (43 %). The genera with the highest number of individuals were Paspalum (2,303 ind.), Sporobolus (2,120 ind.), and Bulbostylis (1,795 ind.) (Table 1B). The most frequently encountered herbaceous species between the post-fire sites included Trachypogon spicatus (L. f.) Kuntze, Paspalum conjugatum P.J. Bergius (5 %), Eriosema grandiflorum (Schltdl. & Cham.) G. Don, Ageratina oligocephala (DC.) R.M.King & H.Rob., and Pteridium caudatum (L.) Maxon (3 %) (Table 1B).
Table 1 Number of individuals (Num. Ind.), relative abundances (ABUN), frequency (FREQ), dominance (DOM), coverage (COV) and the importance value index (IVI) for the seven most frequent species in the woody (A) and the herbaceous (B) stratum of sites with 3, 9, and more than 20 years since the last high-severity fire (3 TSF, 9 TSF, and ≥ 20 TSF) in “La Primavera" Flora and Fauna Protection Area, western Mexico. Species are arranged in descending order based on IVI regarding ≥ 20 TSF. Species marked with an asterisk (*) were observed in the field with capacity of resprouting.
| Species | 3 TSF | 9 TSF | ≥ 20 TSF | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A) Woody stratum | Num. Ind. | ABUN (%) | FREQ (%) | DOM (%) | IVI (%) | Num. Ind. | ABUN (%) | FREQ (%) | DOM (%) | IVI (%) | Num. Ind. | ABUN (%) | FREQ (%) | DOM (%) | IVI (%) |
| Quercus resinosa* | 108 | 28.65 | 21.66 | 33.06 | 27.79 | 92 | 20.77 | 20.13 | 52.48 | 31.13 | 379 | 68.04 | 68.04 | 65.38 | 67.15 |
| Pinus oocarpa* | 34 | 9.02 | 13.38 | 15.82 | 12.74 | 175 | 39.50 | 24.03 | 11.46 | 25.00 | 155 | 27.83 | 27.83 | 30.75 | 28.80 |
| Quercus viminea* | 54 | 14.32 | 14.65 | 43.96 | 24.31 | 29 | 6.55 | 5.84 | 19.87 | 10.75 | 20 | 3.59 | 3.59 | 3.84 | 3.67 |
| Agarista mexicana* | 58 | 15.38 | 11.46 | 5.10 | 10.65 | 15 | 3.39 | 3.90 | 9.26 | 5.51 | 0 | 0.00 | 0.00 | 0.00 | 0.00 |
| Clethra rosei* | 10 | 2.65 | 3.18 | 0.37 | 2.07 | 15 | 3.39 | 5.84 | 4.46 | 4.56 | 1 | 0.18 | 0.18 | 0.03 | 0.13 |
| Vachellia pennatula* | 2 | 0.53 | 0.64 | 0.01 | 0.39 | 2 | 0.45 | 0.65 | 0.01 | 0.37 | 2 | 0.36 | 0.36 | 0.01 | 0.24 |
| Verbesina fastigiata* | 77 | 20.42 | 19.75 | 1.37 | 13.85 | 71 | 16.03 | 23.38 | 1.77 | 13.72 | 0 | 0.00 | 0.00 | 0.00 | 0.00 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| B) Herbaceous stratum | Num. Ind. | ABUN (%) | FREQ (%) | COV (%) | IVI (%) | Num. Ind. | ABUN (%) | FREQ (%) | COV (%) | IVI (%) | Num. Ind. | ABUN (%) | FREQ (%) | COV (%) | IVI (%) |
| Trachypogon spicatus* | 169 | 1.37 | 2.99 | 6.88 | 3.75 | 150 | 1.43 | 1.53 | 2.86 | 1.94 | 315 | 24.78 | 31.86 | 48.21 | 34.95 |
| Eriosema grandiflorum* | 171 | 1.39 | 2.18 | 2.22 | 1.93 | 121 | 1.15 | 1.81 | 1.48 | 1.48 | 195 | 15.34 | 12.19 | 6.48 | 11.34 |
| Pteridium caudatum | 218 | 1.77 | 3.05 | 4.45 | 3.09 | 278 | 2.65 | 2.58 | 5.57 | 3.60 | 88 | 6.92 | 4.99 | 1.64 | 4.52 |
| Ageratina oligocephala | 1228 | 9.96 | 5.97 | 7.32 | 7.75 | 22 | 0.21 | 0.56 | 0.40 | 0.39 | 0 | 0.00 | 0.00 | 0.00 | 0.00 |
| Aristida ternipes* | 101 | 0.82 | 1.62 | 3.09 | 1.84 | 274 | 2.61 | 3.55 | 7.77 | 4.65 | 0 | 0.00 | 0.00 | 0.00 | 0.00 |
| Paspalum conjugatum* | 1327 | 10.76 | 5.16 | 4.90 | 6.94 | 946 | 9.00 | 4.88 | 6.91 | 6.93 | 0 | 0.00 | 0.00 | 0.00 | 0.00 |
| Sporobolus macrospermus | 1770 | 14.35 | 4.29 | 4.53 | 7.72 | 348 | 3.31 | 1.95 | 1.95 | 2.40 | 2 | 0.16 | 0.28 | 0.07 | 0.17 |
The NMDS ordination was adjusted to a two-dimensional solution, with a final stress of 0.06 (Supplementary material, Figure S1). In general, there was a marked differentiation between sites with ≥ 20 TSF from 3 TSF and 9 TSF. These differences were further stratified by TC. The ANOSIM test results confirmed significant dissimilarity in species composition and abundance, as influenced by TSF and TC (Global test R = 0.998, P < 0.001).
Regardless of TC, the importance value index (IVI) of Q. resinosa was 2.4 and 2.2 times higher at sites with ≥ 20 TSF, as compared to sites with 3 TSF and 9 TSF, respectively (Table 1A). Similarly, the IVI for P. oocarpa was 2.3 and 1.2 times higher at sites with ≥ 20 TSF, as compared to sites with 3 TSF and 9 TSF, respectively. In contrast, the IVI of Q. viminea was 6.6 and 2.9 times higher at sites with 3 TSF and 9 TSF, as compared to sites with ≥ 20 TSF, respectively. Agarista mexicana and V. fastigiata were not recorded at sites with ≥ 20 TSF (Table 1B). For the herbaceous stratum, the IVI of T. spicatus was 9.32 and 18 times higher at sites with ≥ 20, TSF compared to sites with 3 TSF and 9 TSF, respectively. Eriosema grandiflorum was 5.9 and 7.7 times greater at ≥ 20 TSF, as compared to sites with 3 TSF and 9 TSF, respectively. Ageratina oligocephala, Aristida ternipes, and Paspalum conjugatum were not recorded at sites with ≥ 20 TSF (Table 1B).
Post-fire differences in plant community structure. Woody stratum characteristics exhibited significant variations as a function of TSF and TC interactions (Table 2A). The richness of woody species was not affected by TC, and there was no interaction between this factor and TSF. However, species richness was 1.7 and 1.5 times higher at sites with 3 TSF and 9 TSF, respectively, as compared to sites with ≥ 20 TSF (Table 2a and Table 3A). Sites with ≥ 20 TSF harbored 1.3 and 1.5 times more individuals established in hillside sites, as compared to sites with 3 TSF and 9 TSF within the same TC, respectively (Table 2b and Table 3A). No statistically significant interaction was found between TSF and TC concerning the basal area of established trees (Table 2c and Table 3A). Nevertheless, sites with ≥ 20 TSF recorded tree individuals with the largest basal area (Table 3A). Tree height was 2.7 and 3.1 times greater in flat sites with ≥ 20 TSF than in the same TC of sites with 3 TSF and 9 TSF (Table 2d and Table 3A). Similar results were observed for DBH of trees established on hillside sites (χ2 = 64.4, df = 5, P < 0.001, Table 3A). The diameter distribution of woody species showed an inverted J-trend in the recently burned sites (3 TSF and 9 TSF) (Figure 3). In these sites, the woody stratum was mostly composed by individuals < 11 cm DBH, whereas individuals in the woody stratum at sites with ≥ 20 TSF had 2.5-35 cm DBH. Notably, recently burned sites exhibited a heightened frequency of seedlings and juvenile individuals (< 5 cm DBH, including resprouting) of Q. resinosa, as compared to P. oocarpa (Figure 3). Additionally, a greater establishment of P. oocarpa seedlings (1 cm DBH) was found in sites with 9 TSF, in contrast to those with 3 TSF and ≥ 20 TSF. Furthermore, Q. resinosa juveniles were established more frequently on sites with ≥ 20 TSF (Figure 3). The number of resprouting individuals varied significantly between species in interaction with TSF-TC (F 6, 22 = 2.8, P = 0.03). On average, the number of resprouting individuals of P. oocarpa was 7 and 13 times higher in sites with 9 TSF from flat and hillside sites, respectively, as compared to the Quercus species and sites with 3 TSF (Appendix 3). In sites with 3 TSF, Q. resinosa was the species with the highest number of resprouting individuals in the hillside sites (27.33 ± 5.81 ind.). No resprouting individuals were recorded at sites with ≥ 20 TSF (Appendix 3).
Table 2 Deviance table for Generalized Linear Mixed Models that evaluate differences in the characteristics of the woody (A) and herbaceous (B) stratum in sites with 3, 9, and more than 20 years since the last high-severity fire (TSF), situated in two topographic conditions (TC: flat and hillside sites) in “La Primavera” Flora and Fauna Protection Area, western Mexico. Numerator degrees of freedom = DF num.; Denominator degrees of freedom = DF den.; Number of individuals = Num. Ind.
| Response variables | DF num. | DF den. | F | P |
|---|---|---|---|---|
| A) Woody stratum | ||||
| a) Richness | ||||
| TSF | 2 | 128 | 15.94 | < 0.0001 |
| TC | 1 | 128 | 1.71 | 0.193 |
| TSF:TC | 2 | 128 | 2.98 | 0.054 |
| b) Num. Ind. | ||||
| TSF | 2 | 128 | 4.98 | 0.008 |
| TC | 1 | 128 | 3.80 | 0.053 |
| TSF:TC | 2 | 128 | 8.06 | 0.0005 |
| c) Basal area | ||||
| TSF | 2 | 116 | 45.35 | < 0.0001 |
| TC | 1 | 116 | 4.07 | 0.046 |
| TSF:TC | 2 | 116 | 1.94 | 0.148 |
| d) Height | ||||
| TSF | 2 | 116 | 96.03 | < 0.0001 |
| TC | 1 | 116 | 12.83 | 0.0005 |
| TSF:TC | 2 | 116 | 6.40 | 0.002 |
| B) Herbaceous stratum | ||||
| e) Richness | ||||
| TSF | 2 | 140 | 234.94 | < 0.0001 |
| TC | 1 | 140 | 4.36 | 0.038 |
| TSF:TC | 2 | 140 | 14.47 | < 0.0001 |
| f) Num. Ind. | ||||
| TSF | 2 | 140 | 191.80 | < 0.0001 |
| TC | 1 | 140 | 4.70 | 0.032 |
| TSF:TC | 2 | 140 | 22.22 | < 0.0001 |
| g) Coverage | ||||
| TSF | 2 | 140 | 51.89 | < 0.0001 |
| TC | 1 | 140 | 1.46 | 0.228 |
| TSF:TC | 2 | 140 | 5.24 | 0.006 |
| h) Height | ||||
| TSF | 2 | 140 | 89.93 | < 0.0001 |
| TC | 1 | 140 | 23.88 | < 0.0001 |
| TSF:TC | 2 | 140 | 32.88 | < 0.0001 |
Table 3 Characteristics of the woody (A) and the herbaceous (B) stratum in sites with 3, 9, and more than 20 years since last high-severity fire in an oak-pine forest in “La Primavera” Flora and Fauna Protection Area, western Mexico. Time since last fire = TSF; Topographic condition (flat and hillside sites) = TC; Number of individuals = Num. Ind.; Diameter at breast height = DBH. Mean ± Standard error per site (0.1 ha). Different letters indicate statistically significant differences.
| A) Woody stratum | ||||||
|---|---|---|---|---|---|---|
| TSF | TC | Richness | Num. Ind. | Basal area (m2) | Height (m) | DBH (cm) |
| 3 yrs | Flat | 2.51 ± 0.37 | 5.37 ± 0.71c | 0.017 ± 0.03 | 5.29 ± 0.90c | 9.77 ± 2.11 |
| Hillside | 3.33 ± 0.30 | 8.43 ± 0.95c | 0.019 ± 0.04 | 5.22 ± 1.33c | 10.97 ± 3.84 | |
| 9 yrs | Flat | 2.80 ± 0.41 | 9.60 ± 1.64b | 0.018 ± 0.04 | 4.64 ± 0.53c | 9.10 ± 1.34 |
| Hillside | 2.72 ± 0.12 | 7.14 ± 1.30bc | 0.015 ± 0.03 | 3.41 ± 0.12b | 8.68 ± 1.92 | |
| ≥ 20 yrs | Flat | 1.86 ± 0.07 | 7.89 ± 1.08abc | 0.053 ± 0.05 | 14.60 ± 1.43a | 25.33 ± 2.14 |
| Hillside | 1.87 ± 0.15 | 10.93 ± 0.76a | 0.028 ± 0.03 | 9.92 ± 0.43d | 16.72 ± 1.10 | |
| B) Herbaceous stratum | ||||||
| TSF | TC | Richness | Num. Ind. | Coverage (%) | Height (cm) | |
| 3 yrs | Flat | 4.95 ± 0.29b | 8.28 ± 0.84a | 11.68 ± 0.92 | 19.46 ± 0.90d | |
| Hillside | 7.11 ± 0.61ab | 7.06 ± 0.49b | 11.56 ± 1.18 | 28.20 ± 3.08c | ||
| 9 yrs | Flat | 7.59 ± 0.95a | 8.98 ± 0.65a | 10.98 ± 1.89 | 19.74 ± 1.26d | |
| Hillside | 6.16 ± 1.40ab | 5.48 ± 0.17c | 14.72 ± 2.03 | 37.23 ± 4.26c | ||
| ≥ 20 yrs | Flat | 1.78 ± 0.34c | 3.55 ± 0.22d | 23.95 ± 2.79 | 56.72 ± 5.42a | |
| Hillside | 2.12 ± 0.08c | 3.81 ± 0.26d | 21.32 ± 0.55 | 43.80 ± 0.94b | ||
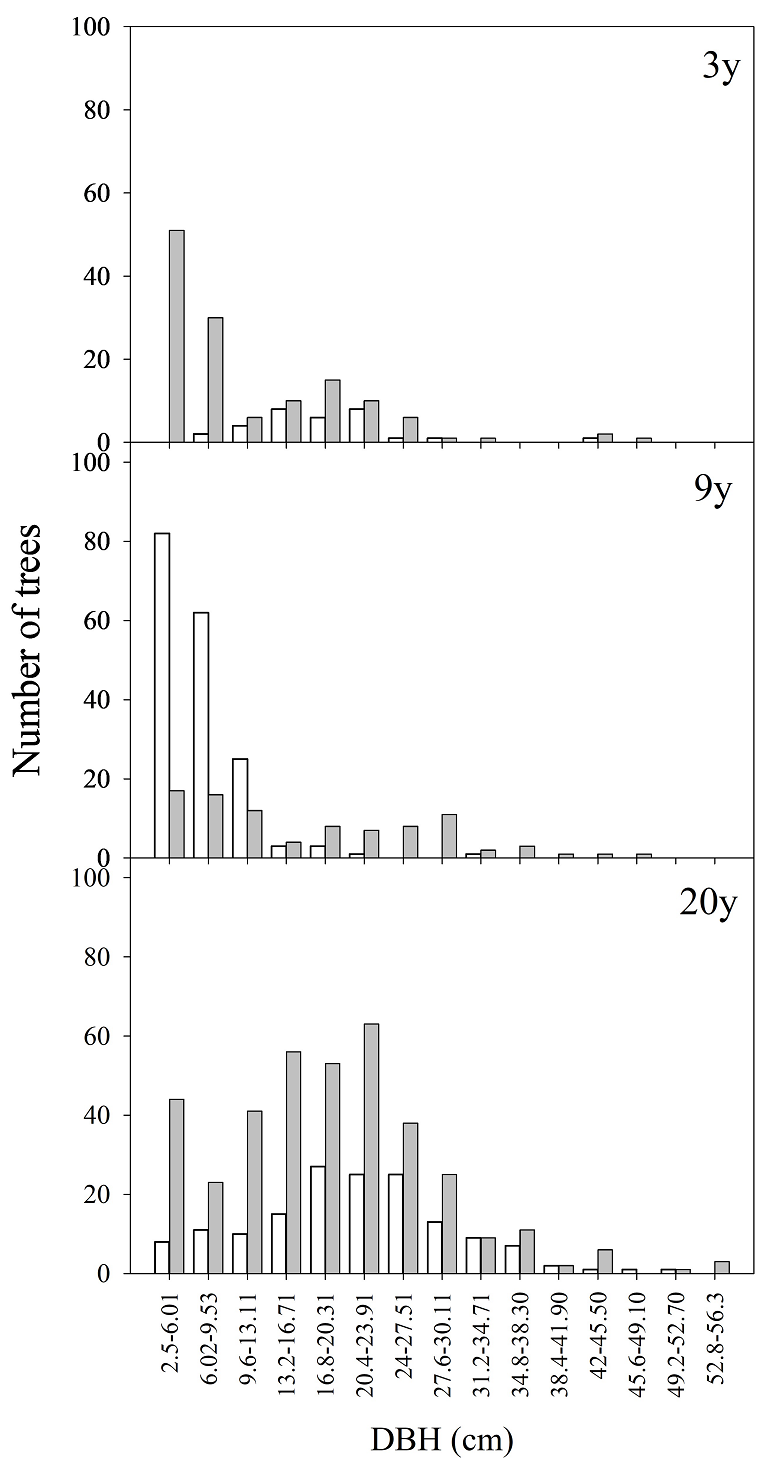
Figure 3 Number of individuals by diametric distribution of Pinus oocarpa (unfilled bars) and Quercus resinosa (gray bars) in sites with 3, 9, and more than 20 years since the last high-severity fire in “La Primavera” Flora and Fauna Protection Area, western Mexico.
The herbaceous stratum differed significantly as a function of TSF and TC interactions (Table 2B). In general, the highest number of herbaceous species was found on sites with 3 TSF and 9 TSF relative to sites with ≥ 20 TSF (Table 3B). Specifically, this diversity was 1.5 times higher at flat sites with 9 TSF, and hillside sites at 3 TSF (Table 3B). The highest number of herbaceous individuals was recorded in flat sites at 3 TSF and 9 TSF, while sites with ≥ 20 TSF showed a similar number of individuals across TC (Table 3B). Regardless of TC, herbaceous cover was 3 and 2.7 times higher at sites with ≥ 20 TSF relative to sites with 3 TSF and 9 TSF (Table 3B). Regarding plant height, hillside sites presented taller plants at 3 TSF and 9 TSF conditions, whereas plant height at ≥ 20 TSF was greater in flat sites (Table 3B).
Microenvironmental variables associated with post-fire plant communities. Most soil attributes varied significantly as a function of TSF and TC (Appendix 4). The OM, BD, and Na levels reached highest values at sites with ≥ 20 TSF (Appendix 5). Conversely, recently burned sites (3 TSF and 9 TSF) recorded higher K, Ca, and Mg values. Similarly, sites with different TSF varied concerning microenvironmental variables (Appendix 6). In contrast to sites with ≥ 20 TSF, recently burned sites (3 TSF and 9 TSF) had light values (PAR) 5 times higher, SMC up to 2 times lower, and higher ST (Appendix 7).
The RDA analysis extracted 77 % of the total variance explained in the first three axes. The first axis extracted 40 %, the second axis 25 %, and the third axis 12 % (Figure 4). The first axis revealed that canopy cover (CC) and soil bulk density (BD) were distinctive characteristics of sites with ≥ 20 TSF. In contrast, light (PAR) and herbaceous cover (Cher) dominate recently burned sites (Figure 4). In these latter environments, hillside sites had higher calcium (Ca) and electrical conductivity (EC) levels (Figure 4). On Axis 2, a gradient strongly marked by TC was reflected in sites that experienced recent fires (3 TSF and 9 TSF, 25 % variance explained).
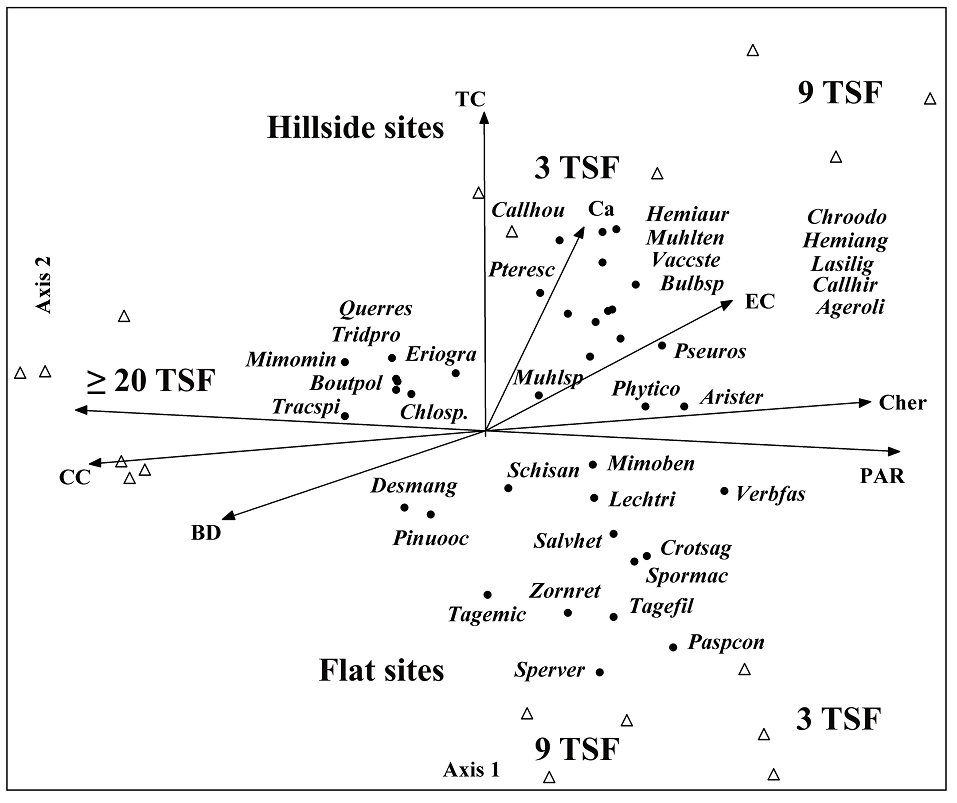
Figure 4 RDA ordination displays species scores from 18 sites with 3, 9, and more than 20 years since the last high-severity fire (3 TSF, 9 TSF, and ≥ 20 TSF) in two topographic conditions: flat and hillside sites, plotted relative to the significant explanatory variables, with fitted vectors indicating variable direction and strength. TC = topographic condition; EC = electrical conductivity; BD = soil bulk density; Ca = calcium; PAR = photosynthetically active radiation available to the plants; Cher = average herbaceous cover; CC = average canopy cover. Triangles represent sites. Black dots represent species. Species are encoded using their scientific name, comprising the initial four letters of the genus and the final three letters of the specific epithet.
Species associated with ≥ 20 TSF sites were Q. resinosa, Bouteloua polymorpha, Chloris sp., Mimosa minutifolia, and T. spicatus (Figure 4). In recently burned areas, the species associated with hillside sites were Calliandra houstoniana (Kunth) Barneby, C. hirsuta (G. Don) Benth., Hemionitis angustifolia (Kunth) Christenh., Chromolaena odorata (L.) R.M. King & H. Rob., P. caudatum, and A. oligocephala (Figure 4), while the species associated with flat sites were P. conjugatum, Spermacoce verticillata L., Crotalaria sagittalis L., Zornia reticulata Sm., Tagetes filifolia Lag., and T. micrantha Cav. These latter species seem to be associated with higher light levels (Figure 4). In both flat and hillside sites, woody species, such as Q. resinosa, P. oocarpa, and the herbaceous species T. spicatus, had higher abundances in sites with ≥ 20 TSF.
Discussion
Post-fire regeneration in a seasonally dry oak-pine forest. The seasonally dry oak-pine forest (OPF) affected by high-severity fires shows a successional tendency toward communities with lower floristic richness, mainly of herbaceous species. This decline in the number of individuals and species richness across all life forms during the recovery of forest communities is a typical trend observed in various fire-prone ecosystems (Capitanio & Carcaillet 2008, Freestone et al. 2015, Heydari et al. 2020). The herbaceous stratum composition along the post-fire chronosequence is mainly represented by species from the families Asteraceae, Poaceae and Fabaceae, which are recognized as widely distributed species in Mexico (Villaseñor & Espinosa-Garcia 2004, Villaseñor 2016, 2018).
Our first hypothesis is partially fulfilled for the herbaceous stratum. Although abundance and richness are influenced by soil and microenvironmental characteristics associated with the TC, they vary as a function of TSF. Recently burned environments in flat sites in (3 TSF and 9 TSF), with lower canopy cover and higher levels of K, Na, and OM, may favor greater richness and larger numbers of individuals in the herbaceous stratum individuals within the OPF. However, no clear trends are observed between TC along the different TSF. Previously, in the APFFLP was reported that exchangeable cations (K, Na, Ca, Mg) undergo changes depending on the intensity of the fire, differentially affecting natural regeneration (Cadena-Zamudio et al. 2020). Under such conditions, the presence of perennial grass species, such as Paspalum conjugatum, Sporobolus macrospermus, and Schizachyrium sanguineum, which exhibit resprouting tendencies and tolerance to fire (e.g., Everson et al. 2021), becomes more pronounced. In recently burned areas of the OPF, pioneer species such as those of the family Asteraceae, characterized by their prolific production of small and wind-dispersed seeds, facilitate the establishment of various plant species, which could be associated with a broad tolerance to open canopy conditions (e.g., Heydari et al. 2016, 2020).
We identified TSF, not TC, as an essential driver of post-fire natural regeneration of woody plants of the OPF. The general pattern of succession after high-severity fires in the OPF implies an increase in the IVI of the woody stratum in detriment of the herbaceous stratum. The woody species more representative of Mexican OPF include the families Asteraceae, Ericaceae, Fabaceae, Fagaceae, and Pinaceae (Tellez et al. 2020). These families are key structural components of OPF landscape after fires (Alanís-Rodríguez et al. 2012, 2020). However, the low woody species richness in sites with higher TSF of the OPF contrasts with that reported in similar secondary mixed pine-oak forests (González-Espinosa et al. 1991, Almazán-Núñez et al. 2016). This variation may be attributed to the higher moisture content and lower latitude characteristics of the OPF in Chiapas and Sierra Madre del Sur, as compared to our study site.
Our second hypothesis was fulfilled, as Q. resinosa establishment is favored, while P. oocarpa regeneration decreases, on sites with major time elapsed since the last fire, a trend observed in studies of competition between Quercus and Pinus (Retana et al. 2002, Torres et al. 2016, Barton & Poulos 2018). Environmental filters associated with site conditions, resprouting capacity, seed banks, and dispersal mechanisms are probably the most influential factors in the success of establishment and regeneration of oaks and pines (González-Espinosa et al. 1991, Rodríguez-Trejo & Fulé 2003, Rodríguez-Trejo & Myers 2010, Alfaro-Reyna et al. 2019). Although P. oocarpa shows higher number of resprouts than Quercus on recently burned sites (3 TSF and 9 TSF), it appears that most of these resprouts fail in persisting through time (Fulé & Covington 1998). This response could be related to the ability of Q. resinosa to tolerate water stress (e.g., Carnicer et al. 2014, del Castillo et al. 2016, Torres et al. 2016, Arenas-Navarro et al. 2023). As other authors have pointed out, drier and warmer conditions would favor the regeneration and persistence of some oak species over pines (del Castillo et al. 2016, Alfaro-Reyna et al. 2019, Arenas-Navarro et al. 2023). The canopy type and competition for light are other factors associated with the success of the regeneration of oaks and pines (Retana et al. 2002, Alfaro-Reyna et al. 2019). Canopy openness and, consequently, higher light availability can favor the survival and growth of Quercus (González-Espinosa et al. 1991, Quintana-Ascencio et al. 1992, González-Tagle et al. 2008), excluding the regeneration of pines. The pioneer tree species, especially pines, play a critical role in ecosystems, as their heliophilous character and a great capacity for seed dispersal allows them to establish in open sites at a regional scale (Fulé & Covington 1998, Almazán-Núñez et al. 2016, Naudiyal & Schmerbeck 2017). However, P. oocarpa loses importance in sites with long periods since fire, which could be related to the lack of seed dispersal (it has serotinous cones) and the fact that the canopy cover of Quercus prevents its establishment and growth (Rodríguez-Trejo & Fulé 2003, Torres et al. 2016, Rodríguez-Trejo et al. 2019).
Additionally, the succession model based on the “initial floristic composition” (Egler 1954) could explain the natural regeneration pattern of the OPF. In this case, the species from advanced successional stages persist due to legacies associated with their ability to resprout, as was observed in Pinus oocarpa, Q. resinosa, and Q. viminea. Therefore, the capacity to resprout in species of Pinus and Quercus greatly favors the persistence of these species (Rodríguez-Trejo & Fulé 2003, Rodríguez-Trejo & Myers 2010, Alanís-Rodríguez et al. 2012, Gómez-Mendoza & Rodríguez-Trejo 2021) and the resilience of this ecosystem to fires.
Key species for forest restoration. Restoration strategies must be grounded in a prior evaluation of stressors (biological and physical) that limit the natural regeneration of an ecosystem (An et al. 2019, Paz et al. 2022, Souza-Alonso et al. 2022). Our data show that herbaceous species, which produce large amounts of small wind-dispersed seeds, dominate recently-burned sites. These sites also allow regrowth, germination, and establishment of seedlings, as well as the growth of juvenile individuals, of dominant tree species belonging to advanced stages of plant succession. Therefore, if the biological threshold has not been exceeded, actions such as passive restoration and fire protection (Holl & Aide 2011, Souza-Alonso et al. 2022) can favor the natural regeneration of areas affected by high-severity fires.
Understanding plant succession (species turnover over time) allows us to identify species that may favor and accelerate ecosystem regeneration (Balaguer et al. 2014) to propose strategies to accelerate this process and restore degraded areas (Prach & Walker 2011, Souza-Alonso et al. 2022). The successful regeneration of the dominant tree species, Q. resinosa and P. oocarpa, in the early stages of succession favors the fire-resilient character of this ecosystem. Therefore, if the objective is to accelerate the regeneration in recently burned sites, an effective restoration strategy could be planting juvenile individuals of Q. resinosa and P. oocarpa in open sites (without tree cover), together with nitrogen-fixing species.
Like other species, Q. resinosa and P. oocarpa present adaptations that allow them to resist fires (Rodríguez-Trejo & Fulé 2003, González-Tagle et al. 2008, Rodríguez-Trejo & Myers 2010). In the case of Quercus, the ability to regenerate vegetatively from dormant buds of stems is elevated, even from their early life-cycle stages (Gracia et al. 2002, Moreira et al. 2012, Clarke et al. 2013). Although both species are post-fire resprouters, P. oocarpa is also characterized by thick bark and serotinous cones (Rodríguez-Trejo & Fulé 2003, Rodríguez-Trejo 2008, Rodríguez-Trejo et al. 2019). Therefore, including these species in restoration programs would favor the resilience of the OPF to high-severity fires. Despite the adaptability of most species to post-fire environments, successful seed germination and establishment of new seedlings, including resprouting stems, rely on favorable climatic conditions after fires. Without these conducive conditions, the development of recruited and resprouting species becomes unattainable following a fire (Chapin et al. 2014, Rodríguez-Trejo 2014, Boucher et al. 2020). It is important to mention that, after high-intensity fires, the success of establishing and growing tree seedlings is negatively affected by the absence of mycorrhizae (Chapin et al. 2014), so the prior evaluation of the conditions of the microbiota and soil nutrients is an essential part of the restoration program.
Our data demonstrates that legumes are frequent species in less fertile sites with higher TSF. Since legumes are nitrogen-fixing species that play a key role as nurse plants (Callaway 2007, Löf et al. 2014), in burned sites they could act as nuclei to accelerate natural regeneration (Goergen & Chambers 2009). In the plant succession within the OPF, the presence of Fabaceae may be linked to an increase in the IVI of tree species on sites with higher TSF. Therefore, mixed planting of woody species, such as Calliandra hirsuta and Mimosa minutifolia, and herbaceous species, such as Crotalaria sagittalis, Desmodium angustifolium, and Eriosema grandiflorum, could favor the growth and survival of Q. resinosa and P. oocarpa individuals, particularly on hillside sites.
The abundance and IVI of the species comprising the post-fire chronosequence allow the identification of key species for the restoration of areas affected by high-severity fires in OPF. Based on abundance and IVI, we can conclude that Q. resinosa may represent a foundational species for recovering the structure and composition of OPF after high-severity fires. Our findings also indicate that flat sites are potential regeneration cores with greater richness and numbers of individuals of herbaceous species, while hillside sites, which display lesser regeneration, require prioritized restoration efforts, possibly involving the introduction of oak species with greater resilience to high-severity fires. However, because of the current regime of high fire incidence and livestock activity within the APFFLP (Huerta-Martínez & Ibarra-Montoya 2014, CONAFOR 2022), establishing successful restoration strategies could be challenging and costly. In this context, the committed participation of government institutions, communal owners (such as Ejidos), scientists and the society, in general, is imperative to achieve feasible goals for restoring and preserving of this type of ecosystem. Despite the value of the APFFLP, information on post-fire regeneration patterns is scarce. The current work fills this gap contributing to the understanding of plant succession in areas affected by high-severity fires, and providing essential information for the design of restoration techniques.
Our findings allow us to propose potential restoration techniques for managing ecosystems facing similar disturbances. The proposed recommendations for ecological restoration in areas impacted by high-intensity fires involve identifying key species across various successional stages and understanding the microenvironmental conditions linked with the presence of these species, abundance and Importance Value Index (IVI). This information could facilitate the utilization of these species in diverse restoration techniques, including seed or seedling clusters, as well as larger-scale plantations.
Supplementary material
Supplemental data for this article can be accessed here: https://doi.org/10.17129/botsci.3440











 nueva página del texto (beta)
nueva página del texto (beta)



