Phosphorus is the limiting macronutrient in most continental soils, mainly because of its mineral origin and poor diffusion in soils (Johan et al. 2021). Unlike nitrogen, the processes that regulate the phosphorus cycle are mostly abiotic. Some of the key factors that limit phosphorus availability to plants are those that determine phosphorus adsorption and desorption from the mineral matrix, particularly in the presence of iron and aluminum compounds that limit the presence of this element in soluble forms (Hekstra 1996). Phosphorus deficiency can also be exacerbated by some management practices. For example, nitrogen fertilizers can increase soil acidity, which favors insoluble forms of phosphorus (García-Oliva 2005) and inhibits symbiotic relationships (Grant et al. 2005). At the same time, mycorrhizal fungi can establish symbiotic relationships with many different plant species, van der Heijden et al. (2015) estimate that there are close to 50,000 fungal species that form mycorrhizal associations with up to 250,000 plant species. Several studies have shown that arbuscular mycorrhiza fungi contribute up to 90 % of plant P uptake (van der Heijden et al. 2015, Strullu-Derrien et al. 2018). Additionally, mycorrhizae improve the formation and stabilization of soil aggregates (Tisdall 1994, Bearden 2001). This in turn leads to major changes in plants’ physiology and responses to the environment (Ferrera Cerrato & Alarcón 2001) and increases soil bacterial diversity (Álvarez 2009). However, there is apparently some initial cost to plants of establishing these symbiotic relationships, as plants have been repeatedly shown to exhibit early growth depression during the initial stages of colonization by mycorrhizae (Bethlenfalvay 1982, Li et al. 2005, Choi et al. 2005, Smith & Read 2008, Barroetaveña et al. 2012, Gómez-Romero et al. 2013). Plants found in soils with low phosphorus content may have a mycorrhizal dependency. Even though has been documented with arbuscular mycorrhizal fungi, that colonization will be variable, depending on phosphorus concentrations in the soil. In this sense, the application of phosphorus can reduce mycorrhizal colonization. In general, plant species with a lower ability to take up phosphorus are recognized as dependent on mycorrhiza. This is why when phosphorus levels in the soil increase, mycorrhizae may not benefit (Graham et al. 1991, Janos 2007).
Organic acids are part of the soluble fraction of the rhizosphere and influence the availability of nutrients, including phosphorus (Adeleke et al. 2017, Baltazar et al. 2021). Plant roots can exude some organic compounds that favor the solubilization of phosphorus from iron or aluminum minerals, thus increasing phosphorus availability (Adeleke et al. 2017). Plants can release organic acids that can bind to cations and in the process release phosphorus into the soil without ectomycorrhizal symbiosis (Ae et al. 1990, 1991). However, when ectomycorrhizal symbionts are present, their mycelium increases plants’ phosphorus uptake considerably (Chuyong et al. 2000) through several mechanisms: 1) an increase in the absorption surface, which allows more efficient exploration of the soil volume; 2) increased surface area of contact with soil particles; 3) formation of polyphosphates; and 4) release of organic acids and phosphatases that solubilize phosphorus by binding to cations in the soil (Marschner & Dell 1994, Bücking 2004). After a thorough review of the knowledge on mycorrhizal ecology and evolution, van der Heijden et al. (2015) conclude that paleontological and phylogenetic evidence indicate that the mutualistic relationship is very old (ca. 450 million-yr-old), and that it probably allowed plant transition from water to land.
The mutualism between gymnosperms and ectomycorrhizal fungi have been thoroughly studied, and many fungal genera are commonly used to improve gymnosperm growth (Pera & Parladé 2005). Pisolithus arhizus (previously known as Pisolithus tinctorius) is a gasteroid, globose, ectomycorrhiza that has been used with different trees and shrubs to increase their overall growth and root length and volume, as well as phosphorus nutrition, especially in soils with phosphorus deficiency (Gómez-Romero et al. 2015, Becerril-Navarrete et al. 2022). The inoculation with P. arhizus has been reported with forest plants from the five continents (Cairney & Chambers 1997) including Pinaceae family (Pérez-Moreno & Read 2004). Pisolithus arhizus is a cosmopolitan species, distributed in all regions of the country. Widely cited in the states of north, center and south (Chihuahua, Coahuila, State of Mexico, Hidalgo, Jalisco, Nuevo León, Oaxaca, Sonora and Veracruz; Bautista-Hernández et al. 2018). On the other hand, this species of ectomycorrhiza provides resistance to water stress in Pinus pseudostobus. The conditions at the substrate collection site are a highly degraded site with serious drought problems, so P. arhizus is of vital importance at the site (Gómez-Romero et al. 2015). The objective of the present study was therefore to explore the effect of different organic acids, in non-inoculated and inoculated (with Pisolithus arhuizus) Pinus pseudostrobus plants under controlled conditions. We hypothesized that organic acids would improve plant performance for both non-inoculated and inoculated plants, and that adding organic acids would reduce the initial cost of the ectomycorrhizal symbiosis in small plants.
Materials and methods
Locally collected seeds (Nuevo San Juan Parangaricutiro, Michoacán) of Pinus pseudostrobus were cold stratified for 15 days at 4 °C in Petri dishes lined with moistened Whatman filter paper (No. 1), after being superficially sterilized with 20 % sodium hypochlorite (NaClO 1:5 H2O). The seeds were then placed in a growth chamber at 25 °C with a 12:12 hour photoperiod until they germinated. The germinated seeds were planted in plastic containers with 50 cm3 of a substrate composed of a 1:1 mixture of commercial peat moss and agrolite and kept in the growth chamber for another two weeks. The substrate was previously sterilized in an autoclave at 100 °C for two periods of 20 minutes. The seedlings were then transplanted into 375 cm3/ 600 g containers with sterilized degraded acrisol-like substrate. The substrate used comes from a deforested site that is mostly vegetation devoid, with the presence of numerous gullies. The place is severely eroded, with presence of acrisols. Soil analyzes indicate that the phosphorus content is extremely poor (Table 1).
Table 1 Description of the physical and chemical characteristics of the substrate used in the experiments.
| Physical analysis | Clay-silt-sand | 71.80 -16 -12.20 |
| Clase textural | Heavy clay | |
| Apparent density | 1.20 | |
| Field capacity | 43.16 | |
| Permanent wilting point | 23.45 | |
| Usable moisture | 19.54 | |
| Saturation humidity | 56 | |
| Porosity (%) | 45.6 | |
| Chemical analysis | pH in water | 5.4 Moderately acidic |
| pHCaCl2 | 4.9 Acid | |
| C.E. milimohos | 0.06 Deficient in salts | |
| % O.M. | 0.83 Poor | |
| Organic Nitrogen kg/ha | 20.88 Poor | |
| Ammoniacal nitrogen (ppm) | 8.4 Very poor | |
| Phosphorus kg/ha | Traces Extremely poor | |
| Potassium kg/ha | 36.88 Very poor | |
| Calcium kg/ha | 1148 Very poor | |
| Magnesium kg/ha | 133 Medium | |
| C.E.C. | 28.40 |
In our first series of experiments, we tested the effect of organic acids salts on the performance of P. pseudostrobus plants, with and without inoculation with Pisolithus arhizus. Each experiment consisted of six replicates of each of six dilutions (0, 4, 8, 16, 32 and 64 micromolar) of the sodium salts of citrate, oxalate, acetate, tartrate, succinate, and malate, we used these concentrations as that is has been reported that across a broad range of ecosystems, the concentration of organic acids in soil range from ranges from 0 to 50 μM (Adeleke et al. 2017). To provide macro- and micronutrients, we prepared a hydroponic solution consisting of the aqueous solution of the following salts: KNO3 (202 mg/l), Ca(NO3)2 (236 mg/l), MgSO4 (493 mg/l), NH4NO3 (80 mg/l), H3BO3 (2.8 mg/l), MnSO4 (2.07 mg/l), ZnSO4 (0.22 mg/l), CuSO4 (0.051mg/l) and (NH4)6MO7O24 (0.09 mg/l). (Modified from Salisbury & Ross 1994). The source of phosphorus was Iron (III) phosphate dihydrate, an insoluble form of this element; 1 g was mixed into the substrate of each plant. The organic acid salts were added twice per week and the hydroponic solution three times per week. Lixiviates were collected and analyzed using the molybdenum blue method (Frank et al. 1998). For the inoculation of experimental plants with the mycorrhiza, each plant was treated with ca. 500,000 spores of Pisolithus arhizus obtained from the company Biosyneterra Solutions Inc. (L’Assomption, Quebec, Canada). The spores were added to 0.5 g of micronized peat as a vehicle, which was applied directly to a portion of root that had been exposed by moving the substrate. Height, stem diameter and canopy cover were measured every month for six months. During the seventh month, many of the plants were destroyed by insect herbivores, therefore only six-month data was analyzed as early performance response of the plants. A second set of experiments was conducted as described above, but using only non-inoculated plants and quantifying height, stem diameter, canopy cover, shoot biomass and root biomass for a longer period: 12 months.
Experiments were performed in a shade house (35 % shade) whose conditions were very similar to those in the habitat where seeds were collected. The temperature in the shade house ranged between 13 and 29 °C depending on the month, humidity was not controlled since the shade house is open, but all plants were subject to the same irrigation regimes during both experiments. For statistical analyses of the first experiment, we used ANOVA to test effects of presence-absence of mycorrhiza and organic acid salt identity. For both experiments we used linear models to test the effects of the organic acid salts concentration on plant response variables. For all tests we verified the fulfillment of the assumptions of the procedures. We included both linear and polynomial fits and retained quadratic polynomial variables whenever this improved the overall fit of the model (i.e., increased the model’s adjusted R 2 value). All statistical analyses were done in R (R Core Team 2020).
Results
The results of the first set of trials after 6 months showed significant differences on plant performance for the presence or absence of mycorrhiza, for: height (F (1,636) = 89.7; P < 0.0001), stem diameter (F(1,635) = 105.9; P < 0.0001) and canopy cover (F (1,635) = 4.8; P = 0.03). For height, plants without mycorrhiza were taller than plants with mycorrhiza (7.06 ± 1.7 and 5.9 ± 1.3 cm, respectively), stem diameter was larger for plants without mycorrhiza than for plants with it (0.9 ± 0.2 and 1.2 ± 0.4 mm, respectively), and for canopy cover plants had greater cover without mycorrhiza than for plants with it (22.6 ± 8.3 and 21.0 ± 9.3 mm2, respectively). The effect of organic acid salt identity was significant for stem diameter (F (5,635) = 27.1; P < 0.001), and canopy cover (F (5,635) = 4.3; P < 0.001).
When considering the effect of each organic salt concentration in the first experiment, for plants without mycorrhizal inoculation (Table 2), increasing concentration of organic acid salts had non-significant or negative effect on response variables. This was the case of citrate (for height, stem diameter, and canopy cover), of tartrate (for height and stem diameter) and for oxalate and succinate (both for canopy cover). In plants inoculated with mycorrhizae, there were negative effects of acid concentration of citrate on stem diameter, tartrate on stem diameter and malate on canopy cover.
Table 2 Effect of organic acid addition on different Pinus pseudostrobus performance variables in experiments with and without the ectomycorrhiza Pisolithus arhuizus. Experiment 1 had only un-inoculated plants. Only significant effects are shown. Significance codes: 0 '***', 0.001 '**', 0.01 '*' specifying when the relationship was significantly positive (+), negative (-) or polynomial (polynomial). N/E: not evaluated.
| Organic Acid | Response Variable | Experiment 1 | Experiment 2 | |
|---|---|---|---|---|
| Without mycorrhiza | With mycorrhiza | Without mycorrhiza | ||
| Sodium citrate | Height | ** (-) R2 = 0.16 |
||
| Sodium tartrate | Height | * (-) R2 = 0.12 |
||
| Sodium citrate | Stem diameter | ** (-) R2 = 0.15 |
* (-) R2 = 0.08 |
* (+) R2 =0.12 |
| Sodium oxalate | Stem diameter | ** (+) R2 = 0.25 |
||
| Sodium succinate | Stem diameter | *** (+) R2 = 0.28 |
||
| Sodium tartrate | Stem diameter | * (-) R2 = 0.08 |
* (+) R2 = 0.13 |
|
| Sodium acetate | Canopy cover | * (+) R2 = 0.27 |
||
| Sodium citrate | Canopy cover | *** (-) R2 = 0.42 |
* (-) R2 = 0.12 |
** (polynomial) R2 = 0.29 |
| Sodium malate | Canopy cover | *** (-) R2 = 0.19 |
** (polynomial) R2 = 0.44 |
|
| Sodium oxalate | Canopy cover | ** (-) R2 = 0.14 |
** (polynomial) R 2 = 0.26 |
|
| Sodium succinate | Canopy cover | * (-) R2 = 0.07 |
** (polynomial) R2 = 0.36 |
|
| Sodium tartrate | Canopy cover | ** (+) R2 = 0.15 |
||
| Sodium citrate | Shoot biomass | N/E | N/E | *** (polynomial) R2 = 0.45 |
| Sodium succinate | Shoot biomass | N/E | N/E | * (polynomial) R2 = 0.24 |
| Sodium tartrate | Shoot biomass | N/E | N/E | ** (polynomial) R2 = 0.30 |
| Sodium oxalate | Root biomass | N/E | N/E | ** (polynomial) R2 = 0.35 |
| Sodium succinate | Root biomass | N/E | N/E | *** (polynomial) R2 = 0.89 |
| Sodium tartrate | Root biomass | N/E | N/E | ** (polynomial) R2 = 0.18 |
For the second set of trials, in non-inoculated plants, the results after 12 months were different. In this case, when organic acids showed a significant effect, those effects were positive (Figure 1). Stem diameter increased as the concentration of citrate, oxalate, succinate, or tartrate salts were increased, the best fit being linear for all (Figure 1A-D). All organic acid salts significantly increased canopy cover as their concentration increased; for acetate and tartrate the best fit was linear, while for the rest of the salts the best fit was quadratic polynomial (Figure 1E- J). Interestingly, when the best fit was a curve, the inflection point of the curve was around the concentration of 40 micromolar. Citrate, oxalate, and succinate salts had a positive effect on shoot biomass, in all cases being the best fit polynomial (Figure 1K-M), with an inflection point close to the concentration of 40 micromolar. Oxalate, succinate and tartrate salts had a positive effect on root biomass, and the best fit was polynomial in all cases (Figure 1N-P). For oxalate and tartrate salts, the inflection point is near 40 micromolar, though this was not the case for succinate.
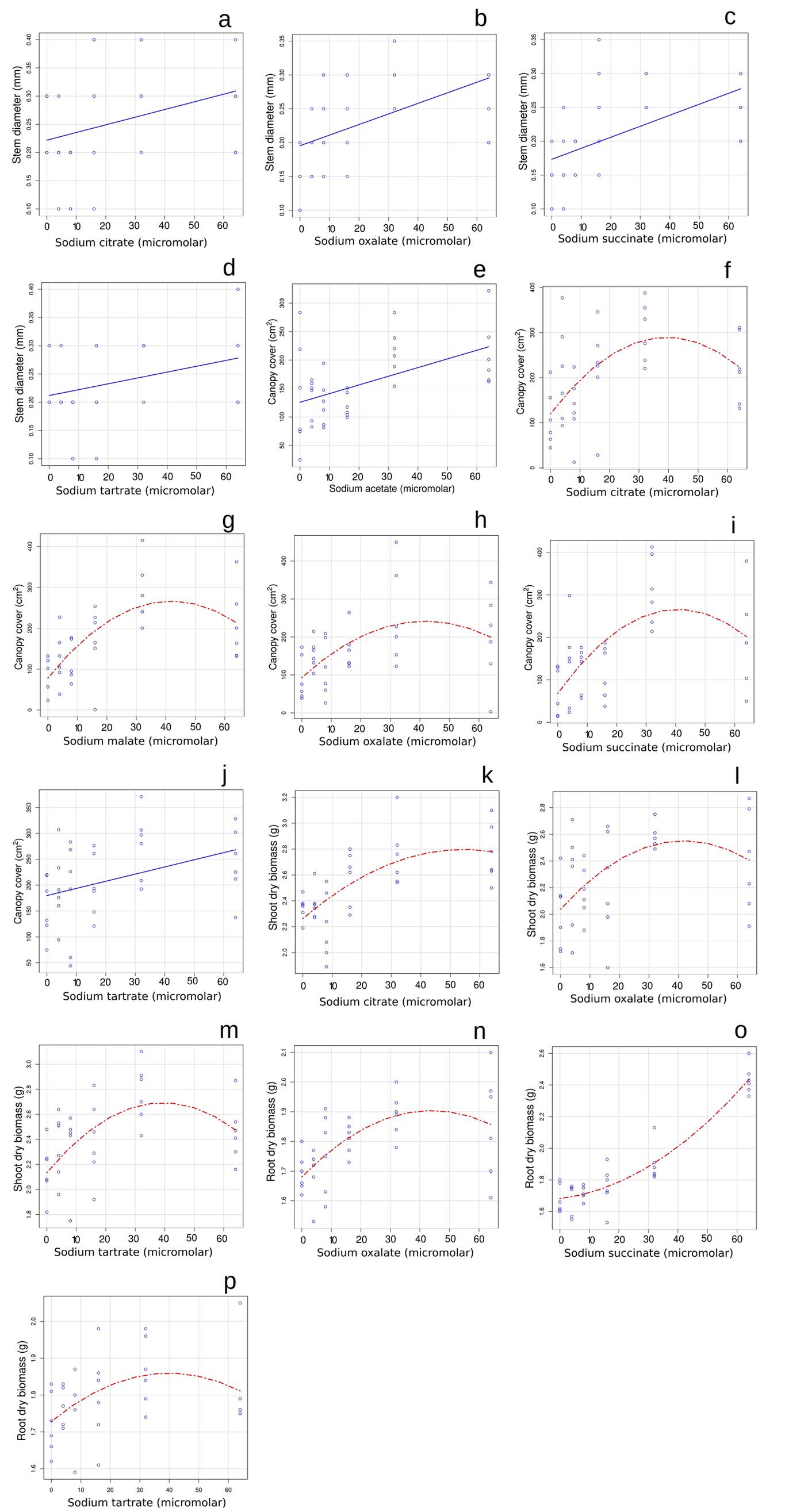
Figure 1 Four plant performance variables for Pinus pseudostrobus to increasing concentrations (0, 4, 8, 16, 32 and 64 micromolar) of organic acid sodium salts after 12 months in plants not inoculated with mycorrhizae (Experiment 2). Effects on Stem diameter: A) citrate, B) oxalate, C) succinate, D) tartrate; on Canopy cover E) acetate, F) citrate, G) malate, H) oxalate, I) succinate, J) tartrate; on Shoot dry biomass: K) citrate, L) oxalate, M) tartrate; on Root dry biomass: N) oxalate, O) succinate and P) tartrate. Blue lines denote significant linear relations while red lines denote significant quadratic relations. We are only showing the variables and treatments that showed significant effects.
Discussion
The process of phosphorus absorption by roots is directly related to aspects of root morphology, which determine the efficiency with which the root system explores the soil. It also depends on chemical processes involving proton and low-molecular-weight organic acid excretion (Junk et al. 1993). Our results indicate that when added directly to the growing medium, organic acids have positive effects on plant performance, but the magnitude and specific growth variables promoted differ. Several studies have shown that plants release organic acids when available phosphorus concentrations in the soil are low (Peñaloza et al. 2000, Tian 2004). For example, there are a handful of studies of the organic acid root exudates of pines (Wang et al. 2007, Johansson et al. 2008, 2009, Meier et al. 2013), and for Pinus radiata, the profile of organic acid exudates has been fully characterized (Shi et al. 2011). That species produces mainly: formate, acetate and malate, and to a slightly lesser degree: lactate, shikimate, succinate and tartrate (Shi et al. 2011). It has been documented that the application of citric acid, can improve growth, biomass accumulation and chlorophyll content (Chen et al. 2020). On the other hand, malic acid increases plant productivity, showing greater growth by alleviating stress from the presence of metals (Zhang et al. 2020). This acid increases biomass in Salix variegata (Zhang et al. 2020) and in Oriza sativa (Sebastian & Prasad 2018). Oxalic acid also increases plant productivity (Guo et al. 2019) and can counteract stress effects, for example in Cicer arietinum (Sakouhi et al. 2022) or in Sedum alfedii (Liang et al. 2021).
Interestingly, in the literature oxalate and citrate are frequently reported as exudates by bacteria and mycorrhiza (Banik & Dey 1982, Gyaneshwar et al. 1998, Ahonen-Jonnarth et al. 2000, Sheng & He 2006, Eldhuset et al. 2007, Vyas & Gulati 2009, Sheng et al. 2011), in our study these organic acids showed a significant effect on four and three response variables (the only variable for which citrate had no effect was root dry biomass). A study by Ohno & Kubicki (2020) on phosphorus availability in the presence of organic acids, including four of the organic acids examined in the current study (oxalic, citric, succinic, and malic acids), showed that phosphorus adsorption to FeOOH was the lowest in the presence of oxalic and citric acids; malic acid also reduced adsorption, but succinic acid did not. These adsorption results are consistent with ours except for succinate, which had a significant effect on 3 response variables suggesting that other mechanisms might be at play, including the presence of exudates by the plants themselves or the pH of the growing medium, that influences the deprotonation of the carboxyl group (Ohno & Kubicki 2020). Some studies have shown that succinate is a key molecule in promoting plant growth (Iyer et al. 2017). Also, tartrate has been shown to play a role in phosphorus solubilization of iron-bound phosphates (Shen et al. 2002) and that malate plays a role in increasing phosphorus assimilation in species such as Lupinus albus (Johnson et al. 1996). For our results, it is interesting that the best fit for increase in plant performance under the different organic acid addition in seven cases was polynomial, with an inflection point of the curve close to 40 micromolar, suggesting that high concentrations of the organic acids may no longer promote plant performance after those concentrations, those concentrations may be detrimental.
Mycorrhizal inoculation did not improve P. pseudostrobus performance in our short experiment, since inoculated plants showed negative effects when salts were added. Mycorrhizal inoculum has been shown in other studies to negatively affect growth when phosphorus concentrations are high (Johnson et al. 1997, Johnson 1998). In particular, plants with citrate and tartrate showed a decrease in stem diameter while the addition of malate, oxalate and succinate affected plant cover. Mycorrhizae impose an initial cost on young plants, reducing their performance in the short-term during the early stages of colonization as other studies have found, but this initial cost is compensated by strong benefits later on (Pera & Parladé 2005). This ontogenic transition warrants further investigation in our study system with P. pseudostrobus.
Addition of organic acids to heavily degraded soils might have a positive direct effect on the plants, as shown by the longer-term responses in our experimental non-inoculated plants. These results are significant since they could be a cheaper and attainable management for restoration purposes. However, the selection of which organic acid to apply is key to having the strongest possible positive effect on plant performance. Therefore, knowledge of the profile of the exudates produced by root systems is desirable. These results could be applicable to restoration efforts in degraded Pinus ecosystems.











 nueva página del texto (beta)
nueva página del texto (beta)



