1. Introduction
Global cement production was estimated to be 4.0 billion tonnes in 2013 owning to the fact that concrete is the second most consumed material on earth after water (U.S. Geological Survey 2014, European Federation for Precast Concrete 2014). Cement production has a significant environmental impact as it is responsible for 7% of worldwide manmade CO2 emission (Benhelal et al. 2013). This is due to the fact that one tonne of cement produces approximately 900 kg of CO2 of which 450 kg is produced from the decomposition of raw materials and 360 kg from burning fuel (Benhelal et al. 2013). Replacing cement with supplementary cementing materials or fillers such as limestone filler (LF) has been one approach to reduce the negative environmental impact of concrete (Mohammadi and South 2016) In addition, LF can reduce the cost of cement production. This is mainly due to the relatively lower cost and hardness of LF compared to cement clinker (Celik et al. 2015).
LF has been accepted as a cement replacement in many standards around the world. For example, the use of interground limestone as a cement replacement has been accepted in many standards in Europe since 1960, Canadian Standard Association (CSA) in 1983, and ASTM C150 in 2004. However, all of these standards have set a maximum interground limestone content which ranges from 5% to 15% (Tennis et al. 2011, Hooton et al. 2007).
When replacing cement, LF influences the behavior of cement through physical and chemical effects. The physical effect is caused by (i) modification of particle size distribution, (ii) dilution and (iii) heterogeneous nucleation. Modification of particle size distribution and heterogeneous nucleation can improve the properties of concrete whereas dilution has adversely effect. The chemical effect of LF is caused by the chemical reaction between LF with monosulfate and calcium aluminate hydrate in the hydrated cement system.
1.1 Physical effect of LF
(i) Modification of the particle size distribution due to the presence of LF is primarily attributed to its relatively lower hardness compared to cement, and so when ground it yields a wider particle size distribution (Gao 2012, Sellevold et al. 1982). This allows LF to improve the particle size distribution when added to the cement (Sellevold et al. 1982). Furthermore, LF can decrease the water demand by replacing some of the water in the voids. This water provides additional reduction in the friction between solid particles and thus improves the workability (Hawkins et al. 2003). However, this effect could be masked by the higher water adsorption when LF fineness increases (Schmidt 1992). When LF particles are finer than cement, LF can reduce the bleeding of concrete through water adsorption at replacement levels greater than 5%. At a replacement level less than 5%, the bleeding is only influenced by the surface area of the cement (Moir and Kelham 1993).
(ii) Dilution effect occurs when the cement content is reduced due to cement replacement by LF (Irassar 2009). The reduction in the cement content decreases the hydration products and thus adversely affects the compressive strength at early and later ages, porosity and permeability of concrete. The effect of dilution masks any other LF effects at replacement level higher than 5%. Below 5%, the dilution effect is minimized (Tsivilis et al. 2003). Although dilution influences the properties of the cement system at all ages it is mainly observed after 3 days (Kenai et al. 2004). Before 3 days, a portion of the dilution effect is compensated for by the heterogeneous nucleation effect of LF.
(iii) Portion of the hydration products precipitates on the surface of LF particles (Irassar 2009). This effect depends mainly on the fineness of LF. The increase in LF fineness increases the nucleation sites for the precipitation of the hydration products (Ezziane et al. 2010). This accelerates the cement hydration process and results in faster early age strength gain (Irassar 2009). In addition, the surface area of LF will accommodate some of the hydration products, which reduces the thickness of the hydration products coating unhydrated cement particles (Lin and Meyer 2009). This allows the inner part of unhydrated cement particles to hydrate sooner and thus accelerate the hydration process.
1.2 Chemical effect of LF
The chemical interaction between LF and other hydration products was debated. However, research work in the past 20 years proved that LF is not a chemically inert material but rather a partially reactive material (Hooton et al. 2007, Hawkins et al. 2003). LF reacts with monosulfate ((CaO)3(Al2O3)·CaSO4·12H2O) and calcium aluminate hydrate ((CaO)3(Al2O3)·6H2O) to form calcium mono-carboaluminate (3CaO·Al2O3·CaCO3·11H2O) as presented in Equations 1 and 2 (Kakali et al. 2000, Bentz 2006, Kuzel et al. 1996). The reactions between LF and monosulfate and calcium aluminate hydrate take place after the exhaustion of sulfate ions in the system (Wang 2010). In addition, the fineness of LF influences these reactions; the higher the fineness of LF the more LF is consumed in these reactions (Hooton et al 2007).
The influence of LF on the concrete properties cured at ambient temperature (i.e., 23°C) have been fairly reported in the literature (Hooton et al. 2007, Hawkins et al. 2003, Irassar 2009). However, the results in the literature often vary and in many cases contradict. This contradiction is evident in the workability, mechanical properties and durability performance results (Tennis et al. 2011, Ramezanianpour and Hooton 2013, Sirisawat et al. 2014). Furthermore, information on the influence of LF when the concrete is steam cured is limited. While the influence of LF is caused by a combination of physical and chemical effects no elaboration on the influence of each effect has been reported. Therefore, it is essential to identify the influence of each effect to understand how LF interacts in the cement system and to optimize the use of LF for the precast/prestressed applications.
The aim of this paper is to decouple the physical and chemical effects of LF on paste and mortar systems. This was achieved by using LF and an inert filler (brucite, Mg(OH)2 which will be referred to as Mg) with similar particle size distribution and fineness. The concept of using inert material to evaluate the effect of a reactive material is not new. However, no research work has been done to utilize this concept to decouple the physical and chemical effects of LF and quantify the contribution of each effect separately.
Mg is an inert material by nature but could chemically react with the amorphous silica in fly ash under sulphate rich environment (Zhang et al. 2014, Moore et al. 2009). However, this condition at which Mg can chemically react does not apply in this study and therefore, Mg was considered an inert material. Mg was used to evaluate and measure the combined physical effects of LF while LF was used to measure the combined physical and chemical effects. The difference in performance between LF and Mg mixtures is attributed to the chemical reaction of LF.
The physical and chemical effects of LF on the heat of hydration, chemical composition, and cube compressive strength, were evaluated. The heat of hydration of cement pastes was measured at 23°C and 55°C for a duration of 72 hours using Isothermal Calorimetry. The chemical composition of cement pastes was measured at 16 hours (following steam curing) and at 28 days using thermal analysis. The cube compressive strength of mortars were evaluated at 16 hours and 28 days.
2. Experimental program
2.1. Materials
CSA type HE cement with no interground limestone was used. The cement was supplied by Lafarge Canada Inc. The physical and chemical properties of cement are presented in Table 1. LF and Mg were supplied by Omya Canada Inc. and Aldon Corporation, respectively. The selection of Mg was based on the chemical reactivity and hardness. Mg is an inert material and has similar Mohs Hardness (i.e., 3) compared to LF (Moore et al. 2009, Santhanam 2013). The hardness of Mg and LF should be similar to avoid introducing a new variable in the compressive strength results (Zhange et al. 2011). LF had a Blaine fineness of 1125 m2/kg, median particle size of 3µm and specific gravity of 2.7. The supplied Mg had a Blaine fineness of 1450 m2/kg, median particle size of 4µm and specific gravity of 2.4. Since the particle size distribution and the Blaine fineness of the supplied LF and Mg were different, both materials required modification in particle size distribution to achieve similar particle size distribution and Blaine fineness. This modification consisted of sieving LF and Mg using 10µm, 7µm, 5µm and 2µm sieves and using equal proportion retained on each sieve. The sieving was conducted to ensure similar particle size distribution of LF and Mg. In addition, the portion of LF passing 2µm sieve was ground so that the final LF product has similar Blaine fineness compared to Mg namely, 1450 ± 30 m2/kg. The particle size distribution of cement, LF and Mg is presented in Figure 1. The fine aggregate (natural sand) and coarse aggregate (crushed limestone) were supplied by Dufferin Aggregates. The specific gravity of the sand was 2.72 and the fineness modulus was 2.84. Plastol 6400, a high-range water reducer (HRWR), supplied by Euclid Chemical was used.
Table 1 Chemical and physical properties of cement
| Chemical and Physical Properties | HE Cement |
|---|---|
| SiO2 (%) | 19.7 |
| Al2O3 (%) | 5.0 |
| Fe2O3 (%) | 3.3 |
| CaO (%) | 61.8 |
| MgO (%) | 2.5 |
| SO3 (%) | 4.1 |
| Na2Oeq (%) | 0.7 |
| C3S (%) | 54.0 |
| C3A (%) | 8.0 |
| C4AF (%) | 10.0 |
| C2S (%) | 14.0 |
| LOI at 1150 °C (%) | 0.9 |
| Blaine (m2/kg) | 505 |
2.2. Mix design
Three mix designs were evaluated. For each mix design, cement paste, and mortar were prepared. The details of the mixtures are presented in Table 2 for cement paste and mortar mixes. LF and Mg were used to replace 15% by weight of the cement. The water-to-cement ratio (w/c) was kept constant in the paste, mortar and concrete at 0.34. LF and Mg were not considered as cementing materials in the w/c ratio calculation. This was done in accordance with the Canadian Standards Association CSA A23.1-14. The use of 0.34 w/c ratio was to represent a typically used w/c in self-consolidating concrete (Esmaeilkhanian et al. 2014, Celik et al. 2015). No HRWR was used in cement pastes to prevent any variation in the heat of hydration or thermal analysis results. The sand-to-cement ratio in the mortar mixtures was 2.
2.3. Curing regime
Paste, and mortar specimens were steam cured at 55°C and 95% relative humidity (RH) for 16 hours as presented in Figure 2. A 0.45 m3 Cincinnati Sub-Zero environmental chamber was used. A maximum curing temperature of 55°C was used in order to prevent any formation of delayed ettringite (Brunetaud et al. 2006). Following steam curing, the specimens were placed in limewater at 23°C until tested.
2.4. Test methods
Cement paste specimens were used for the heat of hydration, and thermal analysis. Mortar specimens were used for cube compressive strength.
2.4.1 Heat of hydration
For each paste mixture, the heat of hydration was measured at 23°C and 55°C over a period of 72 hours in accordance with ASTM C1702-09 Method B. Three samples were tested for each paste mixture. Pastes cured at 23°C were tested using an isothermal calorimeter (TAM Air) manufactured by Thermometric. At 55°C, I-Cal 8000 isothermal calorimeter manufactured by Calmetrix was used. Before mixing the cement pastes, all materials were preconditioned to a temperature within ± 2°C of the isothermal calorimeter testing temperature. This was done by placing the materials in the environmental chamber set at ± 2°C of the isothermal calorimeter testing temperature for 2 hours.
2.4.2 Thermal analysis
Calcium hydroxide (Ca(OH)2), calcium carbonate (CaCO3) and magnesium hydroxide (Mg(OH)2) contents were measured at 16 hours and 28 days using Thermal Gravimetric /Differential Thermal Analysis (TG/DTA). For each mix design, two TG/DTA tests were conducted. The tests were conducted using Netzsch SA Simultaneous Thermal Analyzer with a maximum temperature of 1100°C and 10°C/min heating rate. Ca(OH)2 content was used to evaluate the hydration products for each mixture. The paste samples were freeze-dried until a constant mass was achieved. In freeze-drying process, the paste samples were frozen in liquid nitrogen to stop the hydration reactions. Thereafter, the paste samples were placed under vacuum at -10°C. Under these conditions, the free water in the cement paste samples is transformed from a solid state to gas state without going through the liquid state. The use of freeze-drying instead of heat drying was to prevent the loss of any chemically bonded water.
CaCO3 content was used to calculate the amount of LF that was consumed in the chemical reaction. The initial CaCO3 content (prior to mixing), expressed in percentage by weight (wt%), was calculated according to Equation 3. The final CaCO3 content was calculated using TG/DTA mass loss at approximately 680 to 800°C as presented in Equation 4 (Maria 2011). The amount of reacted LF was calculated using Equation 5.
Similarly, the initial content of Mg was calculated using Equations 6. Mass loss corresponding to the decomposition of Mg between 350 and 400°C was used to calculate the final Mg content as presented in Equation 7. Ca(OH)2 content was measured using TG/DTA mass loss between 450 to 500°C as presented in Equation 8 (Maria 2011).
2.4.3 Mortar compressive strength
For each mortar mixture, three cubes were tested at 16 hours and 28 days for compressive strength in accordance to ASTM C109-12.
3. Results and discussion
3.1. Heat of hydration
The total heat released during the first 40 hours of hydration from each paste cured at 23°C and 55°C is presented in Figure 3. At curing temperature of 23°C, during the first 12 hours of hydration, mixes made with LF and Mg showed higher total heat released compared to control mixture made of 100% cement. At approximately 14 hours, the total heat released from all mixes were similar. After 14 hours, control mixture made of 100% cement showed higher total heat released compared to mixes made with LF and Mg. At curing temperature of 55°C, mix LF showed higher total heat released compared to mix Mg and control mixture made of 100% cement. Mix Mg showed higher total heat released in the first 18 hours of hydration compared to control mixture made of 100% cement. After 18 hours, mixes made with Mg and 100% cement had similar total heat released. The increase in the total heat released of HE cement paste with the addition of fine particles (i.e., LF and Mg) was due to the acceleration in hydration reaction which is in alignment with the literature (Kumar et al. 2013, Ye et al. 2007, Pera et al. 1999). The precipitation of the hydration products from the pore solution is assumed to be similar on the surface of LF and Mg particles since both materials have similar physical characteristics.
The physical effect of LF (the difference in the results between control mixture made of 100% cement and mix Mg) increased the heat of hydration compared to control mixture made of 100% cement. This increase is caused by the heterogeneous nucleation which causes acceleration in the hydration rate. The chemical effect of LF (the difference in the results between mix LF and mix Mg) showed an additional increase in the heat of hydration. This increase in heat of hydration was caused by the chemical reaction of LF and calcium aluminate which is an exothermic chemical reaction (Chowaniec 2012). The combined effect (physical and chemical) of LF was influenced by curing temperature. This was evident in total heat released after 40 hours where LF reduced the total heat released when cured at 23°C and increased the total heat released at 55°C compared to control mix made of 100% cement.
3.2. Thermal analysis
The thermal analysis was used to measure the amount of reacted LF and to confirm that Mg is chemically inert material. In addition, a relative evaluation of hydration products was conducted using Ca(OH)2 content. The mass loss from TG analysis and the DTA results are presented in Figures 4 and 5, respectively. Figure 4 presents the mass loss with temperature. Figure 5(a) presents DTA results for control mixture made with 100% cement while Figures 5(b) and (c) present DTA results for mixes made with LF and Mg, respectively. Based on the measured data, Ca(OH)2, CaCO3, and Mg contents were calculated using Equations 3 through 8. The results in Figures 4 and 5(b) showed that the addition of LF increased Ca(OH)2 content at 16 hours compared to control mixture made with 100% cement. The content of Ca(OH)2 increased from 7.4 wt% in control mixture made of 100% cement to 8.6 wt% in mix LF. This is expected as the additional surface area provided by LF acts as nucleation sites for the precipitation of the hydration products. This accelerates the hydration process resulting in a higher Ca(OH)2 content in mix LF compared to control mixture made of 100% cement. At 28 days, the Ca(OH)2 content in mix LF and control mixture made of 100% cement were approximately similar (11.9 wt% in control mixture made of 100% cement and 12.2 wt% in mix LF).
The amount of reacted LF in mix LF was 1.4 wt% at 16 hours and 2.5 wt% at 28 days. Dividing the amount of reacted LF by the initial CaCO3 content yields the percentage of reacted LF to the total available LF in the system (11.8% at 16 hours and 21.6% at 28 days). The amount of the reacted LF at 16 hours was approximately 55% of the amount of reacted LF at 28 days. This indicates that the reaction of LF took place early during the hydration process and explains the higher heat of hydration in mix LF compared to mix Mg and control mixture made of 100% cement.
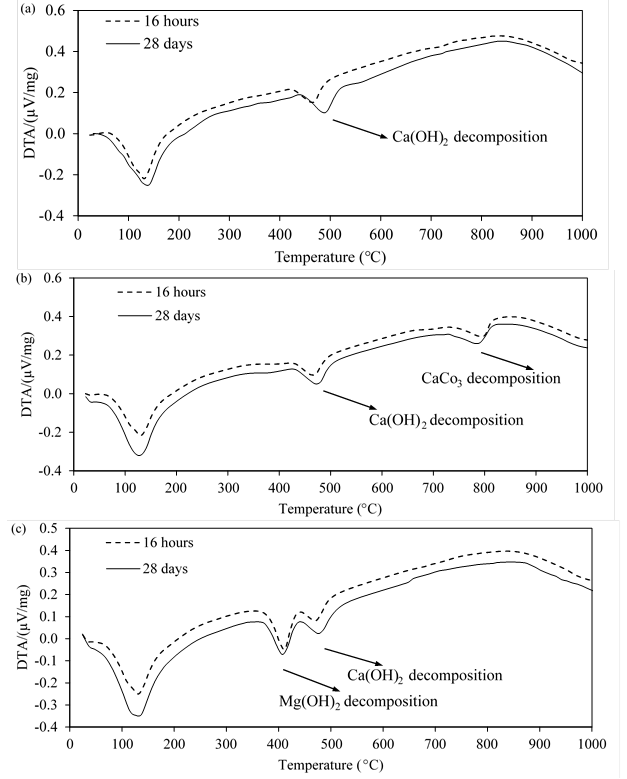
Figure 5 Effect of mixture design on DTA results of cement pastes at 16 hours and 28 days (a) 100% cement, (b) LF and (c) Mg
The addition of Mg also increased Ca(OH)2 content at 16 hours compared to control mixture made of 100% cement as presented in Figures 4 and 5(c). The content of Ca(OH)2 increased from 7.4 wt% in control mixture made of 100% cement to 8.2 wt% in mix Mg. This is due to the additional surface area provided by Mg that acts as nucleation sites. At 28 days, mix Mg and control mixture made of 100% cement showed similar Ca(OH)2 content (11.9 wt%). The initial and final contents of Mg were similar (11.6 wt%) regardless of testing age (i.e., 16 hours or 28 days). This confirms the chemically inert behaviour of Mg.
3.3 X-Ray diffraction
The x-ray diffraction was used to confirm the presence of calcium monocarboaluminate in the hydrated cement paste in mix LF. Figure 6 presents the x-ray diffraction results for the control mix (Figure 6.a) and LF mix (Figure 6.b). The results showed that a peak at approximately 12° 2θ representing calcium mono-carboaluminate was observed in mix LF while monosulfate peak was observed in the x-ray diffraction results of the control mix.
3.4. Compressive strength of mortar
The results of the cube compressive strength of mortars at 16 hours and 28 days are presented in Figure 7. Each column in the figure represents the average of three tests. The coefficients of variation were below 5%. At 16 hours, the addition of LF and Mg increased the cube compressive strength by 7% and 3%, respectively. At 28 days, the strength of all mixes was approximately similar (90 to 94 MPa).
The increase in the 16-hour compressive strength with the addition of Mg (physical effect of LF) was caused by two factors. Firstly, the fine particles of Mg fill the voids between the larger particles which reduces the porosity and increase the strength. Secondly, the increase in hydration rate with the addition of Mg increases the hydration products and thus reduces the porosity and increase the strength. This agrees with the results obtained from the heat of hydration, and thermal analysis. The chemical effect of LF (the difference between mix LF and mix Mg) showed that the production of calcium mono-carboaluminate increases the strength at 16 hours. Although a distinct effect of LF and Mg was observed in heat of hydration, thermal analysis, and compressive strength results at 16 hours, no effect was observed at 28 days. This is due to dilution effect which is in alignment with the literature (Irassar 2009, Tsivilis et al. 2003, Kenai et al. 2004).
3.5. Physical and chemical effects of LF
LF has physical and chemical effects that influence the properties of concrete. These effects occur simultaneously and is difficult to evaluate the contribution of each effect individually. However, by using an inert material such as Mg with similar physical properties to LF, the physical and chemical effects of LF could be decoupled. The thermal analysis confirmed the chemically inert behavior of Mg. The difference in performance between mix LF (physical and chemical effects) and mix Mg (physical effect) defines the influence of the chemical effect of LF (i.e., calcium mono-carboaluminate). In the following discussion, the combined effect of modification of particle size distribution, dilution and heterogeneous nucleation is referred to as the physical effect of LF whereas the chemical reaction of LF is referred to as the chemical effect of LF.
At 16 hours, the physical and chemical effects of LF increased the compressive strength of mortar. At 28 days, the physical effect of LF had a negative impact on the compressive strength of mortar. To the contrary, the chemical effect of LF increased the compressive strength of mortar. Further study is required to examine the interplay between the permeability, sorptivity, and pore distribution.
4. Conclusion
The following conclusions are drawn from the results of this study:
The physical effect of LF increases the compressive strength of mortar at 16 hours. This increase is due to the acceleration in the hydration rate and reduction in the porosity. However, the increase in the compressive strength of mortar was diminished at 28 days due to the dilution effect.
The reactivity of LF and the production of calcium mono-carboaluminate had an important role in enhancing the compressive strength and microstructure of mortar specimens at 16 hours and at 28 days.











 texto en
texto en 









