1. Introduction
Reinforced concrete structures such as buildings, bridges, etc. are designed to last a long time - it is not uncommon for bridge structures to have a design life of 100 years or more.
By having the correct concrete cover with an appropriate mix design, the concrete itself generally provides the necessary corrosion protection for the embedded steel reinforcement bars.
During hydration, cement generates hydrated lime that produces a highly alkaline environment in the pore liquid of the cement matrix. In this way, the steel reinforcing bars are held in a passivated condition, as stable iron oxides are formed on the steel surface.
However, due to the natural process of carbonation, the concrete can progressively lose its high alkalinity, or due to the localized action of chlorides the stable iron oxides can be broken down, meaning that the steel reinforcing bars are no longer in a passive environment, and they can start to corrode.
These issues happen when for example, the concrete cover is less than specified, or during construction there has been a lack of compaction, or inadequate concrete curing.
The corrosion of steel reinforcing bars embedded in concrete behaves in the same way as the corrosion of a metal in electrolyte solutions, corrosion always occurs at the anodes as described in the figure 1.
Conditions required for steel corrosion:
Loss of alkaline passivity (due to carbonation, or the presence of chloride).
Moisture in the concrete pores (electrolyte).
The presence of Oxygen near the reinforcing bars.
All three criteria must be present for the steel to corrode.
1.1 Carbonation induced corrosion
When atmospheric carbon dioxide meets hydrated lime in the pore liquid of non-carbonated concrete, a carbonation reaction occurs, and the highly alkaline calcium hydroxide (Ca(OH)2 with a pH ~13), from the hydrated lime, is converted into low alkaline (pH ~9), relatively insoluble calcium carbonate as shown in the equation 1:
Figure 2 Adapted from Angst. The anode and cathode are of similar size in carbonation-induced corrosion.
Corrosion initiating from carbonation generates a series of microcell anodic / cathodic areas (figure 2), which lead to what can be seen as widespread corrosion, but which progresses rather slowly, typically a 1/100 to 1/10 mm reduction of the rebar per year (Angst et al, 2020). This type of corrosion frequently affects large areas of reinforcement near exposed concrete surfaces in zones of low or inadequate concrete cover, such the building façade wall shown in the figure 3.
1.2 Chloride induced corrosion
Even in highly alkaline conditions of non-carbonated concrete, when there is the ingress of chloride ions, e.g., in marine atmospheres or when de-icing salts etc. are applied, these reach the surface of the embedded reinforcement, there is a localised generation of acid, which attacks the steel causing pitting corrosion (Silva, 2013), with the formation of local anodic areas, as shown in figures 4 and 5 below:
The rate of chloride induced corrosion can be fast, very local and may produce no externally visible signs, until the concrete cracks and cover delaminates as shown in figure 6. The sudden devastating collapse of structures due to this local loss of reinforcement cross section can happen, without prior warning signs.
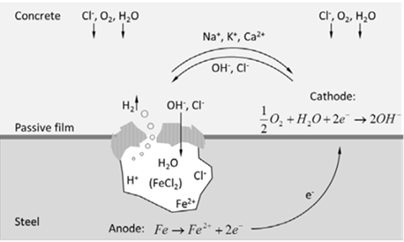
Figure 6 Adapted from Silva (Silva, 2013). Schematic representation of chloride-induced pitting corrosion.
1.3 Corrosion management systems
Different types of corrosion management systems are available to address the problem of corrosion in reinforcing steels:
Concrete repair mortars
active and passive corrosion inhibitors
Anti-carbonation coatings
Induced current cathodic protection
Galvanic anodes (embedded, discrete and surface-applied)
Other available systems are mentioned in the European standard EN 1504, however, they are not described in this article.
The aim of this paper is to carry out a documentary analysis of some of the most widely used corrosion management systems mentioned above.
2. Corrosion management systems
2.1 Concrete repair mortar
Repair mortars are used to provide concrete cover when this is below the designed thickness, and / or to replace concrete spalling due to steel reinforcement corrosion.
Corrosion of steel reinforcement adversely affects many concrete structures. Patch repair is a common technique that involves the removal of physically deteriorated concrete (e.g., mechanically with chisels, or by hydro demolition), cleaning the exposed steel surfaces, and then restoring the original profile with a suitable repair mortar(s).
This process renders the steel within the repair area to a passive condition (Page & Sergi, 2000).
In a significant number of cases, subsequent corrosion-induced damage has been observed in what had seemed to be sound concrete in the immediate area around the patch repairs as seen in the figures 7 & 8. This has sometimes been within a few months following completion of the patch repair process (Qian, et al 2006). This phenomenon is known as incipient or ring anode formation, or the halo effect (Bertolini, et al 2004).
The concept that macrocell activity (the formation of spatially separated anodes and cathodes) causes the incipient anode effect was first introduced by Page and Treadaway (Page & Treadaway, 1982). They suggested that the redistribution of anodic and cathodic sites following concrete repair affects future corrosion risk. Christodoulou (Christodoulou, 2012) shows a widely held view that the cause of incipient anodes is the loss of natural cathodic protection provided by the corroding steel to the steel in the parent concrete adjacent to the patch repair.
The Conrep Project (Tilly et all, 2007) indicates that 20% of repair works fail within 5 years & 55% within 10 years. The paper also indicates that only 30% of patch repairs were successful when used as stand-alone, while this percentage rose to 50% when combined with a protective surface coating.
We can therefore summarize the incipient anode process as follows:
Spalling due to reinforcing steel chloride induced corrosion occurs in the anodic zones.
Removal of the concrete is done in these affected areas.
Repair is carried out with proprietary cement-based repair material that is highly alkaline.
The freshly repaired zone is now changed to a cathodic zone (due to the high alkalinity of the repair mortar).
The cathodic zones that were surrounding the anodic zones (spalled areas) are now turned to anodic zones as they are less alkaline than the freshly applied repair mortar and most likely they already contain some chlorides.
The reinforcing steel that was previously in the protected cathodic zones is now no longer in a passivating environment as this zone has become an anode.
Acceleration of the corrosion occurs then in these newly formed anodic zones (surrounding the patch repair areas) - refer to figures 7 & 8.
Therefore, particularly in case of chloride induced reinforcement corrosion, concrete repair with patch repair mortars alone, might not provide the desired long-term efficacy of the repair.
As a result, patch repair work needs to be combined with suitable systems to avoid formation of incipient anodes.
2.2 Corrosion Inhibitors
An inhibitor is a substance that either delays or retards the rate of a chemical reaction. A corrosion inhibitor is defined as a substance that delays the onset of corrosion or reduces the rate of existing steel corrosion.
Corrosion inhibitors for reinforced concrete, are available as admixtures that can be mixed with concrete, repair mortar or replacement concrete, or as surface applied impregnating products; the latter being the type most commonly used for concrete repair works.
There are 2 main types of corrosion inhibitor available in the market:
Active corrosion inhibitors that require the active component to penetrate and reach the reinforcing bars to be able to provide a continuous film on the surface, which protects the steel bars from corrosion.
Passive corrosion inhibitors, which act in different way, which is by preventing liquid water to penetrate and migrate through the concrete, whilst allowing the evaporation of entrapped moisture by vapor diffusion. This increases the resistivity of the concrete surrounding the reinforcing steel. An advantage of this technique is that it also prevents future chloride ingress to the structure.
2.2.1 Active corrosion inhibitor
There are also different active corrosion inhibitor technologies available in the market
Anodic corrosion inhibitors that suppress the anodic reaction - typical product is nitrite-based inhibitor. Their use can be critical if their concentration is not high enough, accelerated corrosion may occur.
Cathodic corrosion inhibitors that either slow the cathodic reaction itself or selectively precipitate on cathodic areas to increase the surface impedance and limit the diffusion of reducible species to these areas - Typical products are zinc compound (precipitation of oxide forming a protective film on the rebar) or sodium sulphite acting as an oxygen scavenger. These are considered as safe, but they are less efficient than anodic inhibitors
Ambiodic (mixed) corrosion inhibitors that act simultaneously on both the anodic and cathodic zones. This class of inhibitor has a synergistic effect, combining the benefits of both anodic and cathodic types, even at low dosage. They are safe at a low dosage, no corrosion acceleration has been found, only a lowering effect.
Ambiodic inhibitors are typically based on a mixture of an amino alcohol and its amino acid salt. These molecules are very small and very volatile. They do not react with cement, and so they are able to migrate freely within the cement matrix (Tritthart, 2002).
In summary, ambiodic corrosion inhibitors:
Penetrate the concrete in both liquid & vapor phases
Displace hydroxides on the steel surface in carbonated concrete
Displace chlorides on the steel surface (in certain conditions)
Form an adsorbed chemical layer 100-1000 angstrom thick on the surfaces of the steel reinforcement
Reduce iron dissolution at the anode
Reduce oxygen access at the cathode
However, in many countries surface applied corrosion inhibitor technology has only found limited acceptance, and indeed there can be significant limitations with regards to their use and efficacy:
The first limitation is their ability to migrate in sufficient quantity to be effective. If the concrete is of high quality and/or the cover is relatively thick, then the inhibitor molecule’s ability to migrate deep enough in sufficient quantity at the level of the reinforcing bars, is limited. This situation is most likely to arise in civil engineering structures.
The second major limitation is when there are already chlorides present in the concrete. Based on experience and following intensive research such as in the SAMARIS project (SAMARIS, 2003-2005), , these inhibitors are not effective if certain level of chlorides is already present near the reinforcing bars.
In summary, for marine, or civil engineering structures exposed to de-icing salts, active corrosion inhibitors are not the optimum solution to mitigate existing corrosion.
However, there are some positive results for the use of these inhibitors in chloride induced corrosion - the SAMARIS project also presents one of them - Fleet Flood Bridge, where the inhibitor was successfully used to address the issue of incipient anode corrosion.
As shown by Heiyandtuduwa (Heiyantuduwa, 2006) and Taché (Taché, 2000) (refer to the figure 9 where a strong reduction of corrosion is noted with the effect of inhibitors that are applied before or after accelerated carbonation), this technology works best in reinforced concrete with carbonation-induced steel corrosion for three main reasons:
Carbonation induced corrosion is often associated with low concrete cover. Hence it is easier for the inhibitor to reach the reinforcement steel
Carbonation occurs mainly in concrete of lower quality - hence it is of lower density, and respectively there is better penetration (depth and quantity) of the inhibitor.
Corrosion rates associated with carbonation are relatively slow, so it is easier for the inhibitor to be effective.
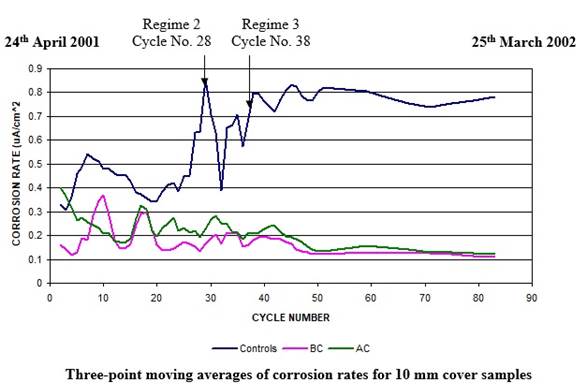
Figure 9 Carbonated concrete - effect of active inhibitor (applied before and after carbonation). (Heiyantuduwa, 2006)
When suitable, active corrosion inhibitor technology is a very cost-effective technique.
Typically, active corrosion inhibitors will be more efficient at reducing carbonation induced corrosion in buildings, rather than to mitigate chloride induced corrosion in civil engineering or marine structures.
2.2.2 Passive Corrosion Inhibitors
As indicated above, passive corrosion inhibitors work by significantly increasing the resistivity of the concrete at the level of the reinforcing bars.
Concentrated silane passive corrosion inhibitors with an active content around 80% for cream types, or more than 90% for liquid types, are effective solutions to reduce water penetration into dense concrete structures. Numerous field reports also attest their long performance. Christodoulou (Christodoulou, et al 2012) has shown that “Treatments carried out 20 years ago can still provide a residual protective effect”.
These passive corrosion inhibitors are very efficient at preventing chloride migration in concrete Eva Rodum (Rodum, 2012 has carried out tests on existing structures and shown that the hydrophobic impregnations used are very effective in preventing chloride migration, even 10 years after their application (refer to the figure 10) where the reference concrete has a very high chloride content while the concretes treated with the different hydrophobic treatments have chloride levels close to zero. Studies over a 11-year period carried out by M. Brem (Brem, et al 2022) show that corrosion rate and the concrete electrical resistivity correlate very well, confirming that that the corrosion reaction is mainly controlled by the moisture content of concrete at the level of the steel reinforcement. In this study, a cream silane was applied to the concrete leaving an unapplied part as a reference. Monitoring systems have been installed to measure the resistivity at different depths in the concrete and the corrosion activity of the reinforcement. After 11 years, there is no moisture penetration in the treated area (in blue in figure 11) with almost no corrosion activity. On the other hand, there are high corrosion activity and high moisture in the untreated area (black in figure 11). This study also shows the positive advantage of this silane treatment in terms of longevity and corrosion protection.
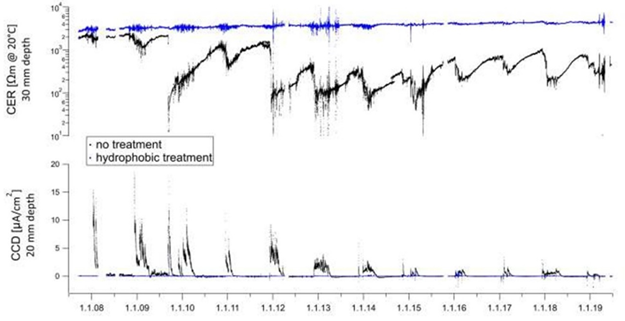
Figure 11 CER Concrete Electrical Resistivity (top) and CCD Corrosion Current Density (bottom) of untreated (black) and treated (blue) surfaces with a cream hydrophobic impregnation from a tunnel ceiling over a period of 11-years (Brem, et al 2022).
In summary, passive corrosion inhibitors are very effective for long-term prevention of chloride induced corrosion.
Their ability to mitigate existing corrosion is more debatable and the efficiency may depend on the level of corrosion, and their ability to penetrate sufficiently enough the concrete surface (not requiring reaching the reinforcing bars). Additionally, the concrete must still allow each-way vapor diffusion for moisture to evaporate and the concrete to dry out at the level of the reinforcement.
2.3 Protective coating
The primary function of protective coating on concrete surfaces is generally to halt the progress of the carbonation front in the cement matrix.
These coatings can be also formulated to be elastic and effectively bridge cracks, even at very low temperatures (down to -20°C).
Depending on the product, surface preparation and application, a typical durability of 10-15 years can be observed for a flexible acrylic-based water dispersion coating, or 15-20 years for a rigid methacrylate resin-based solvent dispersion coating. (Mozaryn, et al 2009). After which a refresher coat may be needed to maintain the protective performance. However, there are examples where premature failure was observed, due primarily to corrosion being too far advanced, excess moisture content, or inadequate surface preparation and application.
After concrete repairs are carried out, protective coatings can be used to stop the future ingress of deleterious elements (e.g., chlorides and CO2), and to provide a homogeneous visual appearance of the substrate by hiding differences in color due to the patch repair works.
Breathable protective coatings will provide corrosion mitigation in the same way as hydrophobic impregnations, by preventing further ingress of deleterious agents (e.g., Chlorides, and CO2) and by allowing the concrete to dry out.
But if the corrosion is too advanced and/or if non-breathable coatings are used, there is a risk of entrapped moisture with all the ingredients present in the concrete for corrosion to proceed.
2.4 Cathodic protection
There is a recently produced updated European Standard which applies: Cathodic protection of steel in concrete (ISO 12696:2016). This is valid for both induced current cathodic protection and galvanic protection.
2.4.1 Induced current cathodic protection
The corrosion protection is providing by placing an anode, made from Titanium mesh for example, at the surface of the concrete and connected directly to the reinforcement network (refer to the figure 12).
A current is then drawn through this system that maintains the rebars in the cathodic zone, meaning no corrosion can occur in this area, even in the presence of high chloride contents.
When installed properly, this is the only system available that completely arrests corrosion activities. However, it requires high skill levels to design and install such systems. It also requires continuous provision of the electrical current, and continuous monitoring through the entire service life, to ensure that the system is running properly. Any lack of such service might eventually result in destructive effects from induced current in the structure
The National Cooperative Highway Research Program of USA in their synthesis 398 (National Cooperative Highway Research Program, 2009), suggests that insufficient or no monitoring and maintenance is being performed by many agencies and this is the most important reason for the disappointing performance of many such systems.
In summary, induced current cathodic protection is a very effective system to stop corrosion activities, but it is very complex to design and install, as well as being very costly to install, continuously supply and monitor throughout its service life.
Additionally, this technique can only be used on prestressed concrete structures with additional measures as a precaution due to the risk of hydrogen embrittlement.
2.4.2 Galvanic protection
Galvanic corrosion protection of steel in concrete is based on the formation of a galvanic element if a metal less noble than steel (refer to the figure 13), in direct contact with the concrete overlay, is electrically connected to the steel reinforcement bars. The reinforcing steel is protected from corrosion as long as sufficient galvanic current flows between the galvanic element (acting as anode) and the steel reinforcement (acting as cathode). Most commonly, zinc is used as the sacrificial element / anode material. The galvanic cell that is formed corresponds to a conventional zinc/air battery. The first known application of a galvanic corrosion protection system for reinforced concrete, was on a bridge deck in Illinois in 1977.
The efficiency of galvanic corrosion protection depends on the lasting activity and durability of the zinc anode. However, passivation of the zinc anode by formation of a passive layer that reduces current flow on the zinc surface can be caused by:
Deposition of anodic (zinc corrosion) products on the zinc element surface
Contact with alkaline calcium hydroxide in concrete pore solution.
The first galvanic systems applied on reinforced concrete structures suffered from rapid passivation of the zinc anodes and therefore lost their protective effect after a short time. This passivation had to be addressed by the introduction of suitable activation agents - which could also result in excessive self-corrosion, consuming up to 70% of the zinc without producing the necessary protective current.
Critical reports about mixed results from early applications created resistance to the use of galvanic systems, which has continued up to now in some countries. Huge research and development effort was put into development of better galvanic anodes with balanced activation for long lasting effectiveness. All the known successful approaches developed to date, are extensively protected by patents. However, there is also now evidence of a useful proprietary system service life of more than 20 years.
Various systems of galvanic protection are available:
Embedded anodes in the patch repaired area
Discrete anodes installed at the periphery of the patch repaired area
Typical advantages of galvanic systems over impressed current cathodic protection are:
No need for external hard wiring the anodes (no risk of copper wire thefts)
Simple installation, relatively low cost
No risk of hydrogen embrittlement in prestressed or post-tensioned tendons
Self-adjusting current density
No continual service or monitoring required (although monitoring is always recommended when a design life of more than 15 years is required)
2.4.2.1. Incipient anodes mitigation
These anodes are placed and embedded in the patch repair zone (Figure 14) and need to use a mortar with a specific resistivity to fill the patch (Lozinguez, et al 2018; Christodoulou, et al 2014), or within the concrete surrounding the patch repair zone (Figure 15). The efficiency of these latest anodes does not depend on the resistivity of the mortar used to fill the patch (Lozinguez, et al 2018; Christodoulou, et al 2014) to solve the problem of incipient corrosion of the anodes.
Galvanic anode technology for corrosion protection of reinforced concrete has been available for more than 20 years.
Sergi George (George et all, 2010) has shown, from several field experiments, the long-term durability of these anodes, and a good correlation between their estimated life span (from the anode consumption) and the actual measurement of residual zinc remaining in the puck after 10 years.
The anodes placed in the repair patch areas, require a repair mortar to be used with a low resistance, which unfortunately also limits the quality level of the mortar
When anodes are placed in the original concrete outside the repair patches, e.g., outside but in their vicinity, the repair work can be carried out using high quality mortar, as there is no limitation in the resistivity of the mortar. In Cancun, Mexico, a field test was carried out, installing anodes inside the host concrete to protect the reinforcing steel against the incipient effect of anode corrosion. In situ measurements showed the level of protection of the installed anodes (figure 16 ).

Figure 16 Installation of anodes inside host concrete with installation verification in Cancun, Mexico.
Independent papers (Lozinguez, et al 2018; Christodoulou, et al 2014) have shown the importance of placing the anodes in the surrounding parent concrete and not in the patch areas.
This is a relatively simple but effective system to prevent the development of incipient anodes, even in the presence of high chloride contents in good quality sound concrete. If the anodes are installed inside the patch, the repair mortar to fill the patch must have a specified resistivity. On the contrary, if anodes are placed around the patch, a high-quality mortar can be used.
2.4.2.2. Corrosion prevention with discrete anodes
These anodes are placed in healthy but contaminated concrete and then linked together to produce the galvanic current as can be seen in the photo in figure 17, which shows the preparation for installing these anodes and linking them together.
Some issues (Holmes et al, 2011) have been raised regarding the efficiency of this system especially in concrete structures with heavy existing corrosion. When running purely in galvanic mode, in the presence of high chlorides, it has been found that the anode current output is the same as the current output of anodes placed in concrete with no chlorides present. This means that the effectiveness of discrete anodes might be limited if used to stop on-going corrosion in area of high chloride induced corrosion.
The use of galvanic anode is a simple system, but with some limitations in areas of high chloride-induced corrosion as the level of galvanic current released by the anodes may not be sufficient to remove the chlorides and consequently repassivate the steel.
2.4.2.3. Corrosion prevention with hybrid systems
More than 15 years ago, a UK based company patented and launched a hybrid system that combines both induced current and galvanic protection.
During the induced current phase (typically used initially for 1 to 2 weeks depending on the voltage used), the steel reinforcement is re-passivated by the formation of hydroxide ions due to the induced current output, whilst chlorides are removed from the surrounding pore liquid. Once the steel is back in passivated form, the current is switched of, and the anodes are then connected so the system can run in pure galvanic mode.
In the previously mentioned study, Holmes (Holmes et al, 2011), figure 18 compared the activity of a zinc anode in concrete containing 2.5% chloride, operating at 100% in galvanic mode, with the same anode that had previously been activated for one week by an external current. These two systems were compared with the activity of the same galvanic anode placed in concrete without chlorides. The study shows that the anode operating in pure galvanic mode generates as much current as the anode placed in concrete without chlorides, demonstrating its ineffectiveness in protecting the steel. Whereas the anode that has been previously activated, is very active due to the presence of these chlorides. It sacrificed itself instead of the surrounding steel (see figure 19).
If required by the consultant, monitoring systems similar to those installed in the eddy current cathodic protection system can be installed using a reference electrode and monitored according to the recommendations of EN ISO 12696.
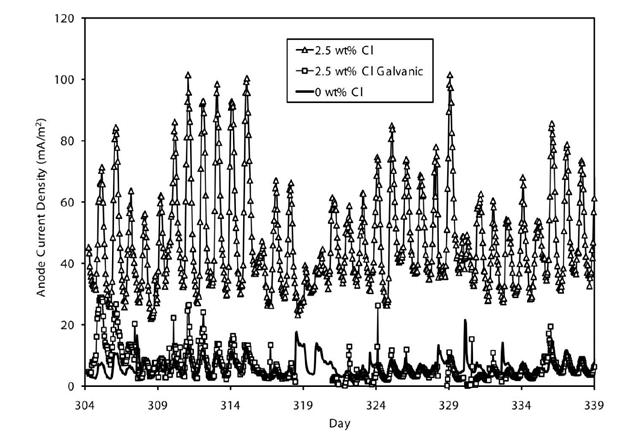
Figure 18 Comparison between the anode (2.5% Cl. galvanic) operating in pure galvanic mode and the anode (2.5% Cl.) being activated by the induced current for a short period of time before operating in galvanic mode. (Holmes et al, 2011).
Installed at the Whiteadder Bridge, Scotland in UK, this hybrid system still responds to the environment long after the installation (Dodds, 2018).
The hybrid system is very attractive, as illustrated in Figure 20, as it provides a certain level of performance warranty, such as those offered by the induced current cathodic protection system, by being less complex and not requiring the same levels of long-term maintenance with constant adjustment of the current input as required in the ICCP.
Additionally, it can also be used in prestressed structures, as the high current output is only provided for a very limited period initially, which is not enough to promote hydrogen embrittlement (Dodds, 2018).
3. Conclusion
Depending on the requirements of the structure, its exposure, the existing corrosion levels, the additional service life extension needed and, of course, the budget, there are different corrosion mitigation techniques available on the market.
To protect structures against carbonation-induced corrosion, active corrosion inhibitor-based protection systems, used alone or in combination with a protective coating that allows the substrate to breathe, but prevents liquid water penetration, are simple to install, but effective and cost-effective in relation to the increased durability they provide.
To protect structures against chloride-induced corrosion, if the corrosion is not very advanced and the substrate has the possibility to dry out, the use of passive corrosion inhibitors is an effective and long-lasting solution.
To avoid purging large amounts of healthy but contaminated concrete and, at the same time, to prevent the undesirable effects of the halo effect, the use of galvanic anodes placed in the concrete outside the area to be repaired is a possible solution.
For the prevention and control of corrosion in healthy but contaminated concrete (especially contaminated with chlorides), cathodic protection is the most effective solution.
The solution with the hybrid system combining induced current and galvanic current is a good compromise between the need for permanent monitoring of impressed current protection systems and the simplicity of galvanic systems.
Close collaboration with all those involved in the renovation project is required to ensure the selection of the most appropriate mitigation technique and systems, as well as their installation and monitoring if necessary.











 texto en
texto en 
















