Introduction
In recent years, excessive use of antimicrobial agents has caused the emergence of microbial resistance and mutations in antibiotics, thus causing major burdens in the medical sector and affecting the patient’s life. (Alvarracin-Baculima et al., 2021; Dicastillo et al., 2020). Currently, there is an indiscriminate administration of commercially available antimicrobial chemicals that is associated with numerous bottlenecks that cause cytotoxicity in tissues (Eslami et al., 2021). For example, chlorhexidine is the gold standard in dentistry as an antimicrobial agent that can be adsorbed on dental surfaces, destabilize the cell membrane, and osmosis, leading to microbial cell lysis. At low concentrations, it is bacteriostatic, whereas at high concentrations it is bactericidal (Moaddabi et al., 2022; Panpaliya et al., 2019). However, prolonged use can cause tooth discoloration, deterioration of the mechanical properties, detrimental effects on the oral microflora, and bad taste, which can cause xerostomia (Bianchi et al., 2020).
For this reason, nanotechnology has been chosen because nanoparticles are currently used in various branches of dentistry seeing that they provide excellent mechanical characteristics and antimicrobial properties comparable to those of conventional materials (Kaladhar et al., 2018; Kochan et al., 2022). Nanoparticles (1-100 nm) have completely different mechanisms of bactericidal action than antibiotics, and it is easier for them to enter microorganisms by damaging the cell wall with greater interaction with microorganisms, where they can release metal ions (Besinis et al., 2014).
Metal oxide NPs are often synthesized and investigated for various applications, from biomedical to environmental, owing to their greater reactivity and efficiency. Cerium oxide, zinc oxide, aluminum oxide, TiO2, magnetite, maghemite, and silicon dioxide are frequently synthesized as they exhibit exceptional characteristics when compared to metallic NPs (Foong et al., 2020).
Recently, nanometer-sized antibacterial materials have attracted a lot of attention in the field of dentistry, specifically metal oxide nanoparticles, because of their strong and broad-spectrum antibacterial activity against different types of microorganisms, strengthening the mechanical properties of the materials, and preventing the development of caries (Nizami et al., 2021). This is because of the release of metal ions that induce oxidative stress and non-oxidative mechanisms. For this reason, several studies have used metal oxide NPs to develop antimicrobial dental materials for resin restoration, endodontic therapy, and orthodontic treatment, such as implant surface repair and removable prostheses, and have found that the antibacterial properties of nano-sized materials are significantly enhanced, and their production is easily scalable (Wang et al., 2020).
Metal oxide nanoparticles (NPs) have gained increasing attention in dentistry, and one of the most interesting NPs is TiO2. TiO2 NPs are considered outstanding antimicrobial compounds because of their chemical stability, non-cytotoxicity, and use of inexpensive precursors for synthesis (Ch-Th et al., 2021). Generally, TiO2 NPs are recognized as safe, with good erosion resistance, corrosion, high refractive resistance, easy control, and excellent surface morphology (Khan et al., 2002). Furthermore, several studies have also revealed that it demonstrates excellent antibacterial properties against various strains of microorganisms, such as Gram-positive and Gram-negative bacteria, as well as antifungal properties (Giti et al., 2022).
Hypochlorous acid (HOCl) is a non-antibiotic agent used to inhibit infections, decrease inflammation, and stimulate wound healing with minimal unfavorable effects and is effective against a wide range of microorganisms (Lafaurie et al., 2018). It is an ideal disinfectant and sanitizer, which is non-cytotoxic, low-cost, and ideal for disinfecting surfaces. As it is non-corrosive in nature, it is effective in various ways, and low-cost, as HOCl is an endogenous substance in all mammals and is effective against a wide range of microorganisms, in addition to being a powerful oxidizing/deproteinizing agent in aqueous solutions. It also has both pro-inflammatory and anti-inflammatory properties that appear to play an important role in the immune system, as they are classified within the group of reactive oxygen species (ROS) that are produced by cells of the immune system, such as neutrophils and macrophages, during an immunological and biological stage known as “respiratory burst” (Block & Rowan, 2020).
HOCl is used to prevent disease, decrease inflammation, and enhance wound healing, with fewer adverse side effects. To date, there have been numerous reports on the microbicidal activity of HOCl in environmental disinfection and antisepsis. In vitro studies have also shown that HOCl is an excellent non-antibiotic antimicrobial agent with significant effects on the following oral pathogens: Streptococcus mutans, Streptococcus sanguinis, Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, and Campylobacter rectus, which live in dental biofilms from teeth and dental implants (Tazawa et al., 2023).
Thus, TiO2 NPs and HOCl have an oxidizing effect and are capable of producing molecules called reactive oxygen species (ROS), whereby HOCl is obtained by means of the cells of the immune system (neutrophils and macrophages) through an immunological process known as “respiratory burst,” during the process of phagocytosis of antigens (Castillo et al., 2015; Choi et al., 2009). In contrast, the antimicrobial action mechanism of TiO2 NPs is linked to their ROS, which damages bacterial cells and leads to their apoptosis (Huang et al., 2016). Therefore, the combination of two antimicrobial agents with these particularities may have a broad-spectrum activity against Gram-negative, Gram-positive bacteria and fungi, which will be of great importance in overcoming MDR and microorganism mutations (Vatansever et al., 2013).
Biomedical and dental applications require nanoparticles to be in their colloidal form in water or liquid media because they allow much better interaction with a biological interface. Their colloidal form allows them to be more accessible to remote targets such as organs, cells, and subcellular compartments, and is easy to use (Jana, 2019). Therefore, very few studies have reported the production of nanodispersions based on metal oxides and HOCl to decrease the microbial load without being cytotoxic (Shirkavad & Moslehifard, 2014).
Currently, there is no line of research on HOCl solutions with TiO2 NPs; instead, there are similar products or studies that use HOCl, but with different types of NPs that can be metallic or metallic oxides in different types of microorganisms, as well as with different applications. Büyükünal, et al. 2022 made a dispersion of HOCl with NPs of CuO, ZnO, AgO, which is of size 100 nm and evaluated with an agar-diffusion assay for the following microorganisms: Salmonella Typhimurium, Salmonella Enteritidis, Salmonella Dublin, Salmonella Infanti as a surface disinfectant. Kuwabara et al. (2020) used AgNPs with an average size of 5.17 ± 1.92 nm with the HOCl solution compounded and tested in an in-vivo model of diabetic mice against the bacterium Pseudomonas aeruginosa. In addition, there is a commercial product named GS Nano Silver + HAW https://product.statnano.com/product/12471/gs-nano-silver-haw-(hypochlorous-acid-water) that have an antibacterial, antiviral effect can be used as a disinfectant. Based on the aforementioned research and product, it gives us the guideline to follow a similar line of research but following an approach in the field of dentistry using TiO2 NPs and HOCl due to their properties and antimicrobial effect.
In this study, we used a nanodispersion of TiO2 NPs in HOCl and characterized it by UV-visible spectroscopy and scanning electron microscopy. Later, it was evaluated as an antimicrobial agent against three bacterial and one fungal pathogen, which are very common in the oral cavity. Cytotoxicity was assessed using SCAPs.
Materials
List of chemicals and reagents
Titanium chloride IV (Fluka, Analytical Monterrey, N. L, Mexico)
Ethyl alcohol (Karal, Leon, Gto., Mexico)
Hypochlorous acid, COT brand (Translational Dental Consortium, Toluca, State of Mexico)
Chlorhexidine (FGM, Joinville, Santa Catarina, Brazil)
Sterile Injectable Water (PISA Laboratories, Guadalajara, Jalisco, Mexico)
Microbial strains & cell line
Streptococcus mutans (S. mutans) - clinical isolate
Enterococcus faecalis (E. faecalis) - clinical isolate
Staphylococcus aureus (S. aureus) - clinical isolate
Candida albicans (C. albicans) - ATCC 90028
SCAPs cells - clinical isolate
Cell culture supplies
Supplemented culture medium Minimum essential medium Eagle (MEM), 10% fetal bovine serum, 1% penicillin-streptomycin, and 1% glutamine were obtained from Sigma-Aldrich. Louis, MO, USA). Louis, MO, USA).
Microbial culture reagents
Mueller Hinton Broth (Sigma - Aldrich Saint Louis, MO, USA)
Mueller Hinton Agar (Becton Dickins, Cuamatla State of Mexico, Mexico)
Sabouraud Dextrose Agar (Becton Dickins, Cuamatla, Cuautitlan Izcalli, State of Mexico, Mexico)
Enterococcus Selective Agar (Sigma - Aldrich Saint Louis, MO, USA)
RPMI Medium 1640 (Gibco, Grand Island, New York, USA)
MTT & XTT reagents
Tetrazolium bromide (Sigma -Aldrich, Saint Louis, MO, USA)
Dimethyl sulfoxide (MEYER, Tláhuac, Mexico)
XTT sodium salt (Sigma-Aldrich, Saint Louis, MO US)
Phenazine methosulfate (PMS, Sigma-Aldrich, Saint Louis, MO US)
List of equipment
Centrifuge (Beckman, J2-MC, Indianapolis, USA)
Incubator (Binder, Tuttlingen, Germany)
UV-Visible Spectroscopy (Thermo Scientific, Grand Island, New York, USA)
IKA C-MAG HS7 magnetic stirrer (IKA Works, Wilmington, USA)
Analytical balance (Denver Instrument, Colorado, US)
Vortex (Genie 2 Daigger, Vernon Hills, Illinois, US)
Densitometer (Grant-bio, Grant Instruments, Cambridge, United Kingdom)
Shaking incubator (VORTEMP 1550 LABNET, Edison, New Jersey, US)
Autoclave (Tuttnauer, Hauppauge, New York, USA)
Optical microscope (LEICA DM IL LED, Wetzlar, Germany)
Muffle furnace (Thermo Scientific, Grand Island, New York, USA)
Ultrasonic bath (Branson Ultrasonics 2800, USA)
Plastic disposables
Methods
Synthesis of TiO2 NPs
To synthesize anatase-TiO2 NPs, we chose the sol-gel method. The process was as follows: 60 mL of ethyl alcohol was placed in a beaker and then placed on a magnetic stirrer hot plate mixer with continuous stirring at a temperature of 90 ºC for 5 min, after which 6 mL of TiCl4 (Titanium tetrachloride) precursor was added. The light-yellow solution was maintained in the stirrer for 1 h and allowed to gelatinize. The resulting dry gel was calcined in a muffle furnace at a temperature-500-550 ºC for 1 h to eliminate the organic components of the precursor. Finally, the powder obtained during the gelling stage was crushed using a mortar and pestle. The coarse grains of the powder were further finely crushed to obtain anatase anatase-TiO2 NPs. (Zhu et al., 2000).
Dispersion of TiO2 NPs in HOCl - Nanodispersion
To prepare a 1 mg/mL nanodispersion solution, 25 mg of TiO2 NPs were weighed and dispersed in 25 mL of HOCl using a 50 mL Falcon tube. The tube was then vortexed for 15 min until a homogeneous mixture was formed. The tubes were then placed in an ultrasonic bath and sonicated for 1 h. The obtained solution was stored in a refrigerator until further use. Before each experiment, the tube was vigorously shaken and sonicated for 15-30 minutes to ensure that there was no sedimentation of the particles, and a homogeneous solution was formed.
Microdilution assay
To obtain the microbial susceptibility data and determine the minimum inhibitory concentration (MIC) of the different nanodispersion concentrations, we performed microdilution tests in 96-well microplates for each microbial strain and repeated the experiments in triplicate.
Bacterial growth and culture
First, Mueller Hinton agar (MH agar) was used to grow the bacterial cultures (S. mutans and S. aureus), and in the case of E. faecalis, Enterococcus Selective Agar was used. An aliquot from -80 ºC was processed and inoculated in a 6 cm autoclaved agar petri plate and placed in a 37 ºC incubator overnight to obtain a young culture of each strain.
MH liquid broth (100 mL) was prepared, 15 mL of broth medium was added to a 50 mL falcon tube, and three to five bacterial colonies were selected from the young culture of each bacterium. The Falcon tubes containing bacteria were shaken overnight and incubated at a constant temperature of 37 ºC (overnight from 16 to 18 h). The overnight culture was adjusted to a 0.5-McFarland scale for each bacterium. The working solution was prepared at a dilution factor of 1:1000 to obtain a final working solution of 1x104 CFU/mL.
In a 96-well microplate, the nanodispersion solution was used at different concentrations (100, 75, 50, 25, 12.5 & 6.25 µg/ml), with 100 µL of culture added along with positive and negative controls. The plates were then incubated at 37 ºC for 24 h under agitation.
After 24 h of incubation, bacterial inhibition was evaluated using an MTT reagent, which was dissolved in MH broth and studied at 570 nm. The protocol is described in the cytotoxicity assay section.
Fungal growth and culture
Sabouraud Dextrose agar (SD agar) was made for C. albicans fungal culture. The whole process is very similar to bacterial culture; RPMI (Roswell Park Memorial Institute) medium 1640 was used instead. After 24 h, the 2, 3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2 H-tetrazolium-5-carboxanilide (XTT) assay was carried out as described below.
XTT assay
The XTT solution was prepared as follows. Solution A (10 mg) was weighed and dissolved in 10 mL of RPMI medium 1640. Solution B: Phenazine methosulfate (PMS, 3 mg) was weighed and added to 1 mL of PBS. Mix 10 µL of solution B with 4 mL of solution A in a sterile tube and shaken for 30-60 seconds until a homogeneous solution is formed. Then, 100 µL of XTT solution was added to each well, and the microplate was incubated for 3 h at 37 ºC. The absorbance was measured using a spectrophotometer at a wavelength of 510 nm.
Cell culture & cytotoxicity assay
SCAPs cells subculture process
Stem cells from the human dental apical papilla (SCAPs) were sub-cultured in a culture dish at a density of 8x105, which was counted using a Neubauer counter under a light microscope until the SCAPs cells reached a confluence greater than 80%. Subsequently, the culture medium was removed from the 3 cm dish and washed three times with 0.5 mL of PBS (phosphate buffer solution); 0.05% EDTA-2Na was added and incubated for 5 min at 37 ºC, 95% humidity, and 5% CO2. The cells were then transferred to a new culture dish, and 2 mL of supplemented MEM culture medium was added. The cells were then detached using trypsin and the cell number was adjusted to the desired concentration. Finally, 100 µL of the medium containing the cells was placed in a 96-well plate and incubated for 24 h to allow the culture to be well established for inoculation with TiO2 NPs.
Inoculation of NPs with SCAPs
Fresh medium (100 µL) was placed in a 96-well cell culture microplate, and 100 µL of known concentrations of the nanodispersion (0.5, 1.5, 10, 20, 30, 40, 50, and 100 µg/mL) was added to the wells in triplicate. The positive control (only cells) and two negative controls, such as CHx (chlorhexidine digluconate, 2%) and HOCl without NPs were considered. The microplate was then incubated at 37 ºC with 5% CO2 and 95% relative humidity for 24 h.
Cytotoxicity assay
After the indicated time, the relative number of viable cells was determined using the MTT assay [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] by preparing 0.2 mg/mL of stock solution dissolved in culture medium. 100µL solution (0.2 mg/mL) was added to each well and incubated for 3 h at 37 °C with 5% CO2 and 95% relative humidity. After 3 h, 100 µL of DMSO was added to dissolve the formazan crystals, which were analyzed using a spectrophotometer at a wavelength of 570 nm.
Data analysis & plotting
All experiments were performed in triplicate, that is, three independent experiments (n = 9). All graphs were plotted using Origin software, 2018 SR1.
Optical study: UV-Vis spectroscopy
UV-Vis analysis is a widely used technique as it provides a non-invasive and rapid evaluation in real time of the size, concentration, and state of NPs aggregation through absorption. UV-Vis analysis was used to evaluate the nanodispersion of TiO2 NPs in HOCl, as shown in Figure 1. The image shows the optical characterization of HOCl, TiO2 NPs, and the nanodispersion. It can be seen that TiO2 NPs have an absorbance in the entire visible region (400-700 nm) and HOCl has a slight absorption at 285 nm; however, when HOCl is added to the NPs, it forms a peak at 340 nm, which refers to the hypochlorite ion (ClO-), due to the photolysis of chlorine (Kishimoto 2019).
Structural characterization: SEM
For structural realization, SEM microscopy was used, in which a high-energy electron beam is used that generates a variety of signals within the surface of samples that are to be analyzed, in which the signals are derived from the sample-electron interactions that represent the information about the sample that includes the external morphology, such as the chemical composition, crystalline structure, and orientation of the materials that make up the sample. Figure 2 depicts detailed images of the surfaces of NPs, where the NPs have a size of 10-15 nm with negligible aggregation of a spherical morphology.
Microdilution assay for antibacterial study: MTT assay
To determine the antibacterial property of the TiO2-HOCl nanodispersion, it was incubated with three different bacterial species and evaluated using an MTT test that was carried out for a period of 24 h. The existence of metabolically active bacteria was verified by measuring the optical density at 570 nm for S. aureus, S. mutans and E. faecalis. Figure 3 (a, b, & c) shows the results for S. mutans, S. aureus, and E. faecalis, respectively. The obtained results demonstrated the significant impact of nanodispersion by inhibiting bacterial growth. However, most interestingly, each bacterial inhibition response towards the nanodispersion was different. For instance, in the case of S. aureus and S. mutans, the effect was dose-dependent as the concentration increased, and growth was not affected. In the case of E. faecalis, we observed more than 50% inhibition at all concentrations.
Microdilution assay for antifungal study: XTT assay
The antifungal effect against C. albicans was studied, and the XTT test was carried out after 24 h to determine whether there were metabolically active fungi after incubation with the NPs. The readings were recorded at an optical density of 510 nm. Figure 4, where the results of the assay are observed, shows very similar results when acting against the fungi. For fungi, nanodispersion enhanced inhibition in a dose-dependent manner. More than 90% of the growth was inhibited within 24 h.
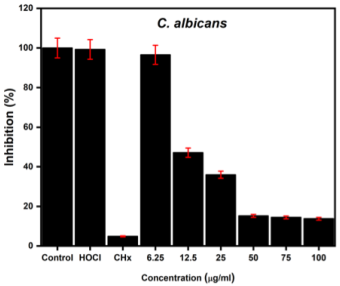
Source: Author’s elaboration.
Figure 4 Antifungal effect of the nanodispersion on C. albicans. (Concentration refers to the nanodispersion solution).
The numerical data from Table 1 show clear evidence of the inhibitory effect of nanodispersion, which is enhanced in the case of fungal species.
Table 1 The percentage inhibition of the antimicrobial effect for each microorganism was evaluated using nanodispersion.
| Nanodispersion concentration (μg/ml) | Microbial inhibition (%) | |||
|---|---|---|---|---|
| S. aureus | S. mutans | E. faecalis | C. albicans | |
| 100 | 64 ± 15.07 | 78 ± 14.4 | 53 ± 8.12 | 13 ± 2.5 |
| 75 | 76 ± 9.6 | 53 ± 17.9 | 57.71 ± 10.14 | 14 ± 1.4 |
| 50 | 43 ± 21.4 | 30 ± 16.8 | 46 ± 13.6 | 15 ±2 .9 |
| 25 | 62 ± 10.4 | 32 ± 12.5 | 45 ± 9.9 | 35 ± 9.8 |
| 12.5 | 51 ± 10.9 | 53 ± 24 | 49 ± 5.2 | 47 ± 10.07 |
| 6.25 | 51 ± 11.6 | 47 ± 3.6 | 48 ± 3.2 | 96 ± 7.5 |
Source: Author’s elaboration.
Cytotoxicity assay
The cytotoxicity of the nanodispersion was evaluated using the MTT assay following the established protocol. In this assay, we observed two important factors: 1. At very low concentrations (0.5 - 10 µg/mL), an increase in cells was observed, that is, cell proliferation was superior to that of the positive control within 24 h. 2. When increasing the concentration, a slight dose-dependent cytotoxicity was observed, but at all concentrations (0.5 - 100 µg/mL), more than 50% cell viability was observed, as shown in Figure 5. From Table 2, it can be seen that the toxicity of TiO2 NPs is size-, synthesis-, dosage-, and cell-dependent.
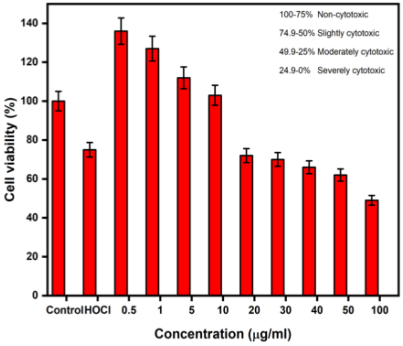
Source: Author’s elaboration.
Figure 5 Cytotoxic effects of the TiO2-HOCl nanodispersion on SCAPs cells.
Table 2 TiO2 NP-based cytotoxic effects of different cell lines and their concentrations.
| Author & year | Synthesis methodology | Size (nm) | Cell line | Cytotoxicity level (L, M & H) |
|---|---|---|---|---|
| Brandão et al. (2020) | Sol-gel | 25 | Lung cells (A549) Liver cells (HepG2) Glial cells (A172) Neurons (SH-SY5Y) |
L |
| Madhubala et al. (2019) | 30 | Human monocytic leukemia cell (THP-1) Human mast cell (HMC-1) |
L: 1 - 10 μg/ml M: 20 - 50 μg/ml |
|
| Wang et al. (2022) | 18-24 | Lung epithelial cells (A549) | L | |
| Wang et al. (2022) | 100 | Corneal endothelial cells | H | |
| Prokopiuk et al. (2023) | Hydrolysis | 5 | Primary fibroblast cultures | L |
Obs.: Low-L; moderate-M; high-H.
Source: Author’s elaboration.
Discussion
In the present study, we evaluated the antimicrobial and cytotoxic effects of TiO2-HOCl nanodispersions. Currently, there is not enough scientific evidence of a nanodispersion that combines HOCl with TiO2 NPs with the characteristics evaluated in this study. The discussion of this study is as follows:
• Reactive oxygen species (ROS) production:
One of the major characteristics of both TiO2 NPs and HOCl is their antimicrobial action mechanism. Leung et al., explain that TiO2 NPs exhibit a high oxidative potential, leading to the production of ROS and affecting bacterial cells by different mechanisms, leading to their death (Leung et al., 2016). Priyanka et al. also explained that the generation of ROS has a greater advantage when things are at the nanoscale level because its nanometric nature implies a significant increase in the surface area/volume ratio, which provides maximum contact with water and oxygen in the environment. As nanomaterials can easily penetrate the cell wall, which is the first defense barrier against oxidative damage, they are first affected by ROS and later by the cell membrane, which allows an increase in intracellular oxidation. It was verified in the bacterial MTT and antifungal XTT experiments that the experimental TiO2 NPs managed to have a greater effect, due to their size and less aggregation, which allows it to easily penetrate the cell wall and with the synergistic effect of HOCl, which also has the ability to release ROS, intensifies the possibilities affecting the cell wall on a larger scale, thereby disrupting nucleic acids, proteins, and synthesis (Priyanka et al., 2016).
• Microbial cell membrane damage:
In contrast, Maher et al. (2023) demonstrated that HOCl is capable of penetrating bacterial cell membranes, leading to intracellular damage and cell death, is effective at low concentrations, has a short contact time, and is effective against a variety of bacteria, including resistant strains as well as fungal pathogens. From our experiment, the nanodispersion of TiO2-HOCl, an inhibitory effect was observed with the strains of S. aureus, E. faecalis, and S. mutans, but was more evident with C. albicans, since they are the most common pathogens in the oral cavity. In addition, at very low concentrations, we observed excellent antibacterial and antifungal effects that were not cytotoxic using the MTT assay with a certain rate of SCAPs proliferation.
• In a study by Argueta-Figueroa et al., (2018) TiO2 NPs at low and intermediate concentrations were not cytotoxic against some of the cell lines derived from the oral cavity. It has also been considered by several researchers that TiO2 NPs can act as a potential candidate to stimulate cell proliferation. Thus, they are currently used in bone and dental implant coatings in dentistry.
• Non-cytotoxic effect:
Therefore, we verified the biocompatibility of the nanodispersion using an MTT assay by incubating the cells for 24 h. This confirmed that TiO2-HOCl was non-cytotoxic at low and intermediate concentrations. Another study by Sismanoglu et al. compared the effects of various endodontic irrigants, such as chlorhexidine and sodium hypochlorite, on dental mesenchymal stem cells. As a result, HOCl was demonstrated to have greater viability, which was verified by the TiO2-HOCl nanodispersion cytotoxicity assay, providing guidelines for its use as an irrigant in endodontics (Argueta-Figueroa et al., 2018; Sismanoglu & Ercal, 2022).
• Synergistic effect:
Thus, from our study, it is very clear that the synergistic antimicrobial effect of TiO2-HOCl nanodispersion can be potentially used in the field of dentistry.
Conclusions
Summing up, the nanodispersion of the experimental TiO2 NPs with HOCl exhibited high stability and enhanced antimicrobial effects. Characterization analysis confirmed that the effect was due to the NPs size that allowed the release of ions and the synergistic effect of HOCl. Thus, experimental NPs against the pathogens were confirmed by a microdilution assay and evaluated using bacterial MTT and fungal XTT assays. The cytotoxicity assay showed negligible toxicity in SCAPs cells exposed to a nanodispersion concentration of 100 µg/mL. At lower concentrations, a significant finding was observed in the proliferation of cells that exceeded that of the positive control.
Therefore, based on this investigation suggest that TiO2 NPs can enhance the antibacterial and antifungal activity of a non-antibiotic antimicrobial agent, such as HOCl, through nanodispersion and can be used as a promising antimicrobial agent in the treatment of various dental pathogens. Thus, non-toxic nanodispersion can be used as an effective alternative because it does not cause antimicrobial resistance, similar to other antibiotics or antimicrobial agents for dental use.











 nueva página del texto (beta)
nueva página del texto (beta)





