Introduction
The world of consumer wearable electronic devices drives energy storage and augmentation research with low costs and environmentally friendly. The greater use of renewable energy will imply new technology systems and distribution, transmission, and storage systems management. Furthermore, it is necessary to advance in battery technology, electric double layer capacitors (EDLC), and for this, it is essential to advance the technology of energy storage, like batteries and electrical double-layer capacitors (EDLC’s) to make them economically viable in wide forms of application (CGEE, 2008). Prospective studies indicate that in the next three decades, renewable energy sources will have an expansion of approximately four times related to the installed capacity, implying a 50% reduction of CO2 emissions concerning the current volume. In this scenario, electricity will represent up to 50% of the world’s energy, so it is imperative to build new solutions for energy storage that are not yet available and can cope with the predicted demands (Chu, Majumdar, 2012) and with the environmental demands. The EDLC are complementary energy storage devices to batteries, occupying a niche position with high power densities (Hu et al., 2009; Lu, 2013).
New and innovative materials, such as multi-walled carbon nanotubes (MWCNT)) are used in energy-stored devices to increase energy density and lifespan (Vicentini et al., 2019). Nanomaterials, especially carbon nanotubes (CNT), are attractive in this context (Du, Pan, 2006). The nanomaterials have significant relevance due to their electrical, mechanical, and thermal properties that differ with the size of the particles that compose them (Buzea et al., 2007; Ramrakhiani, 2012).
The MWCNT constitute one of the most promising nanotechnology classes (Petersen, Henry, 2012). They are extensions of sp2 carbon atoms arranged on fused benzene rings. Their structures give exceptional material properties, having applications in composite materials, sensors, and energy storage cells, in addition to various environmental applications (Dillon et al., 1997; Snow et al., 2005; Dalton et al., 2003; Mauter, Elimelech, 2008).
Although they demonstrate great applicability, products containing nanomaterials can generate manufacturing waste and other harmful environmental factors. Due to the incorporation of nanoparticles (NP) into commercial products, on a large scale, its incorporation into environmental matrices (water, air, and earth) can occur in any stage of the life cycle of products (Lovern et al., 2007; Keller et al. 2013; Mitrano et al., 2015).
Although the MWCNT in this study are obtained by an environmentally friendly process (Vicentini et al., 2018) using manly Camphor (C10H16O) and ethanol (C2H5OH), these devices, once in the environment due to improper disposal, will be decomposed. Their constituents will have their final destination in water bodies (Ren et al., 2015; Zhao et al., 2010; Gottschalk et al., 2009; Cornelis et al., 2014), where they can be consumed by primary species and undergo magnification in the food chain (Pakarinen et al., 2013). More and more evidence has been presented which shows an association of CNT with potentially dangerous effects on cells, tissues, and organisms (Van der Zande et al., 2010; Braun et al., 2008; Johnston et al., 2010; Zhao et al., 2012; Zhao et al., 2021). However, due to different experimental conditions, including the properties of CNT, the chemistry of the test medium, and species of organisms, the results of CNT toxicity studies are often contradictory, and there is no consensus regarding their potential impacts on these ecosystems, thus the environmental safety of these devices must be guaranteed. Soil is an essential environmental sink of CNT (Gottschalk et al., 2009, Cornelis et al., 2014) through its application of sewage sludge or by the disposal of e-waste (Tourinho et al., 2012). Unfortunately, our understanding of the possible adverse effects of these NP on the environment and human health is still lagging behind their rapid incorporation into commercial products (Magdolenova et al., 2014; Kim et al., 2019).
Several studies have shown that an important mechanism among those involved in NP toxicity refers to the induction of oxidative stress (Valant et al., 2012; Hu et al., 2015; Chen et al., 2016; Waissi et al., 2017; Irazusta et al., 2018). Inflammation, immunotoxicity, and genotoxicity have also been postulated as underlying mechanisms of NP toxicity (Magdolenova et al., 2014), and the mechanism behind these toxic effects may be due to its ability to bind proteins.
The proliferation of nanotechnology-based products in recent decades has spawned a new environment-related sub-discipline, the nanotoxicology (Kahru, Dubourguier, 2010; Boyes et al., 2017). This science also considers the transfer of these nanomaterials in the food chain, causing the accumulation of non-biodegradable pollutants and may affect the bioavailability of other toxicants by facilitating their transport (Mahapatra et al., 2015; Neal, 2008). The unique properties of these NP, so widely described, such as their size, varied shape, and high surface area (Federici et al., 2007; Paschoalino et al., 2010) that make them so attractive, may also potentially be responsible for harmful effects to living organisms, as reported in toxicological studies in several species, such as algae, fish, rodents and human cells (Tong et al., 2007; Oberdörster, 2005; Gomes et al., 2018, Irazusta et al., 2018, Zhao et al., 2021).
Therefore, this work evaluated the potential impacts of MWCNT removed from EDLC using two bioindicator models, unicellular green algae of the species Raphidoceles subcapitata and oligochaetes of the species Eisenia andrei, aiming to contribute to the productive sector regarding the life cycle of new materials, especially related to electronic waste.
Materials and methods
Microporous carbon electrodes with high defective carbon nanotubes (HD-CNT)
Electric double layer capacitors (EDLC) were composed of aluminum foil as a current collector and a microporous carbon active material so that the microporous carbon adheres well to the steel sheet. In electroplating, the engraved aluminum foil is used as the cathode and the nickel rod as the anode. Electrodeposition is performed at a current density of 48 mA. cm-2 at 60 ºC for 1 minute. The aluminum foil is dried under ambient conditions and finally cut into 1 cm diameter circles (area 1.93 cm2) suitable for assembly. A stainless steel spring and separator were placed at the base of the cell as current collectors. The manufacture of EDLC and characterizations by Raman spectroscopy were performed at the Carbon Sci-Tech laboratory, Unicamp, SP, Brazil. Figure 1 shows a schematic of the composition of the EDLC.
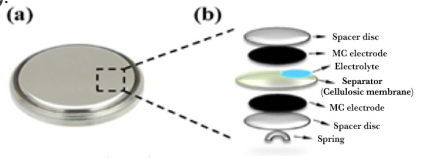
Source: Adapted from Vicentini et al. (2018).
Figure 1 Electric double layer capacitors (EDLC). In (a) top view of the coin cell and in (b) schematic diagram of the assembly.
Multi-walled carbon nanotubes (MWCNT) were removed from the EDLC and solubilized in distilled water at concentrations of 0.1; 1.0; 10.0 and 100 mg.L-1. The solutions were sonicated in an ultrasound device for 40 minutes to improve the CNT dissolution.
Bioassay with R. subcapitata
From a seven-day culture of algae of the species Raphidoceles subcapitata, an inoculum with 2.56 x 105 cels.mL-1 was performed. In a volume of 2.5 mL of each concentration of MWCNT, or of buffered water (1.5 mM NaHCO3), 100 µL of the algal inoculum was added. The samples were kept for 72 hours under agitation and constant lighting. After this time, the algal biomass was determined by counting in a Neubauer chamber (Environment Canada, 1992 EPS1/RM/25). The samples were prepared in triplicate and the averages of the counts are compared by Student’s “t” test, assuming 95% confidence interval in the Prisma 5,0 program.
Bioassay with Eisenia Andrei
Acute toxicity
The test organism matrices were kindly provided by the Environmental Sanitation Laboratory (LABSAN) of the Civil Engineering Faculty of the State University of Campinas, SP-Brazil. The organisms were placed in substrate as recommended in the standard (ABNT NBR 15537:2014) for a period of 15 to 30 days for acclimatization. The organisms after this time were classified as large (from 2 cm in length) and small (below 2 cm in length). Ninety large earthworms were used. A mixture was prepared containing 700 g of sand, 200 g of earth and 100 g of coconut fiber powder, where the worms were placed for conditioning for 24 hours. After acclimatization, the earthworms were divided into 10 pots (500 g) with the same soil composition, with MWCNT at concentrations of 0.1-1.0-10.0 and 100 mg. Kg-1, each experimental group was done in triplicate. The organisms were evaluated for mortality and body mass variation after 7 and 14 days of exposure.
Comet test
The comet test evaluates genomic lesions that result in non-reversible mutations in DNA (Singh et al., 1988). The assay consists of cell lysis, DNA relaxation and electrophoresis. After being stained, it is possible to analyze the running pattern of the DNA fragments that form a “tail”. The size of the tail allows classifying the level of damage caused by the analyzed compound or environmental sample.
The assay followed the protocol of Singh et al. (1988). To perform the comet assay, 3 organisms from each treatment were euthanized by exposure to cold (4 hours at 4-5 ºC). For cell extraction, the organisms were immersed in 10% ethanol for 1 hour. It was then centrifuged at 3000 RPM for 10 minutes, the supernatant was removed and the pellet was resuspended with 500 µL of Phosphate Saline Buffer (0.137mM NaCl, 27mM KCl, 10mM Na2HPO4, 1.8mM KH2PO4). Cell viability assessment was performed with Trypan Blue (0.4% in PBS). For the comet, samples were prepared by mixing 80 µL of the cell suspension with 120 µL of Low Melting agarose (Sigma-Aldrich A9414) and applied on slides previously prepared with a layer of 1% agarose (Sigma-Aldich A9539) and, then they were covered with coverslips. The slides remained in the refrigerator for 15 minutes and then the coverslips were gently removed, taking care to not remove the material. The hydrolysis was then carried out with a lysis solution (25mM NaCl, 100mM EDTA, TRIS10mM, 1% N-lauroyl-sarcosine, 0.1% TRITON-X100, 10% Dimethylsulfoxide), for 60 minutes in the refrigerator, followed by washing for remove excess solution. The slides were immersed in electrophoresis buffer (1.5 mM EDTA, 30 mM NaOH) in an ice bath for 20 minutes, followed by running for 30 minutes (300 mA and 26 V). After washing and neutralizing with 0.4M TRIS buffer, the slides were dried in an oven at 37 ºC for 2 hours and fixed with a fixative solution (0.92M Trichloroacetic acid, 0.043M ZnSO4.7H2O, 5% Glycerol), and dried in an oven at 37 ºC. After rehydration, they were stained with DAPI (4’,6-diamidino-2-phenylindole) for 30 minutes and observed under a fluorescence microscope.
Cytotoxicity
A drop of the sediment obtained in the previous step was deposited and spread on a slide. After complete drying of the smear, staining with Leishman’s stain was performed. After drying the cells were observed for cytoplasmic or nuclear changes, as well as the presence of micronuclei in the optical microscopy.
Results and discussion
Carbon-based nanomaterials, one of the attractive nanomaterials with various shapes, covering fullerenes, single- and multi-walled carbon nanotubes, carbon nanoparticles, graphene, among others, are at the forefront of the rapidly developing field of nanotechnology (Zhang et al., 2013). In addition to the influence of charge transfer, the interaction of carbon nanomaterials and cellular compounds, like proteins are also related to Van der Waals force and Coulomb force (Hou et al., 2015).
The Raman scattering spectroscopy technique has been widely used in the characterization of carbonaceous materials. With microfocusing resources, the investigations are very precise, identifying the different crystalline and amorphous forms that can compose the samples. According to their possible applications, a precise characterization of carbonaceous materials is necessary, preferably by non-destructive methods, with analyzes not only regarding their heterogeneity, but also regarding their structural form (Lobo et al., 2012).
In this study, carbon nanotubes were characterized by Raman spectroscopy (Figure 2A), scanning electron microscopy (SEM) (Figure 2B), transmission electronic microscopy (TEM) (Figure 2C). The peak observed at 1300 cm-1, referring to the D band, and the peak at 1500 cm-1, referring to the G band, are characteristic of sp2 carbon bonds. The peak at 2620 cm-1 represents the D+G sum, confirming the carbon nanostructured nature of the material studied. The nano dimentions of the material was confirmed, as can be seen by SEM and TEM characterizations.
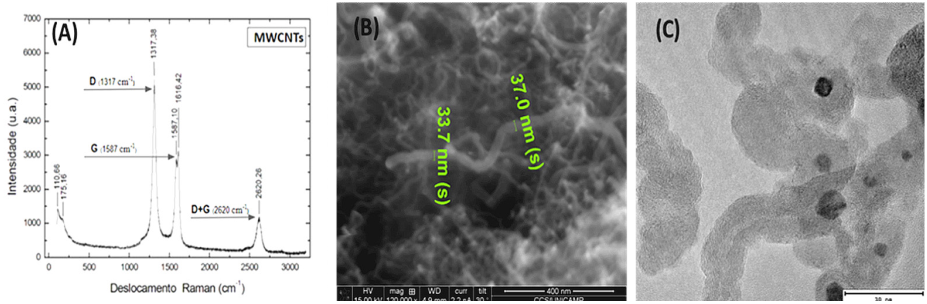
Source: Adapted from Vicentini et al. (2018).
Figure 2 (A) Raman spectrum of MWCNT, ƛ 485nm, showing the D, G and D+G bands, (B) SEM and (C) TEM of the multi-walled carbon nanotubes electrodes supported onto the Ni x Al current collector.
Algae are primary producers in the aquatic environment and, therefore, are an important tool in monitoring studies (Saxena et al., 2020). These organisms present a rapid physiological response, which allows the detection of deleterious effects caused by toxic compounds in the short term (Nogueira et al., 2015; Lu et al., 2021). Our results showed that the MWCNT removed from the EDLC inhibited the growth of algal biomass, in a concentration-dependent manner at concentrations higher than 10mg.L-1 (Figure 3), the EC50 was 0.73 mg.L-1.
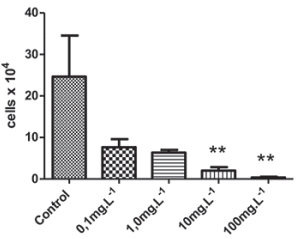
Source: Author’s elaboration.
Figure 3 Raphidoceles subcapitata toxicity bioassay; algal biomass growth inhibition test (p < 0.05).
Raman spectroscopy also showed spctra confiming an interaction between CNT and algae cells. In Figure 4A it is shown the spectrum corresponding to algae in the culture medium with and without CNT at a concentration of 10mg.L-1. The Raman spectrum characteristic of the freshwater algae Raphidocelis subcapitata shows bands between 1000 and 2000 cm-1 corresponding to the cellular structure of plants (Figure 4A), as the band 1555.27 cm-1 corresponds to the clear carotenoid (Parab, Tomar, 2012; Reynolds, Giltrap, Chambers, 2021), the band at 1337.96 cm-1 corresponds to the elongation mode of the C-C bond characteristic of type a chlorophyll biomolecules (Chl a) (Parab, Tomar, 2012). In addition there is the band 1019.51 cm-1, which corresponds to the common carotenoid of algae, this biomolecule exerts the protective action of chlorophyll (Gall, Pascal, Robert, 2015; Parab, Tomar, 2012; Reynolds et al., 2021). The Raman spectrum for the algae culture exposed to CNT is shown in Figure 4B, where it is observed distint characteristics in the spectrum of the algae cells , in the spectrum it is observed a decrease in the intensity of the bands that are structural features of algae. However, it is possible to observe the D band around 1320 cm-1, the G and D bands around 1580 and 1610 cm-1 , as well as the D+G band around 2620 cm-1.
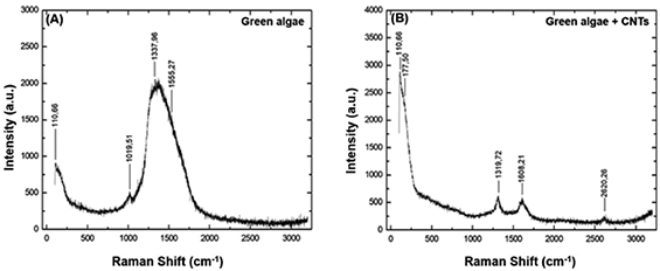
Source: Author’s elaboration.
Figure 4 Raman spectrum referring to the algae Raphidocelis subcapitata before and after exposure to CNT.
These characteristic bands of the CNT also showed a reduction in intensity and an increase in the width of the bands, indicating component overlaps. Thus, it is possible to affirm that the structural modification of both algae and CNT occurred, due to their physical interaction (Zhang et al., 2015).
In A, the bands referring to the algal are observed; in B. the bands corresponding to algae exposed to MWCNT are presented, where the D band is observed around 1320 cm-1, the G and D’ bands around 1580 and 1610 cm-1 and also the D+ band. G around 2620 cm-1. The characteristic bands of the CNT showed a reduction in intensity and an increase in the width of the bands, indicating the overlapping of components.
Toxicity analyzes have already demonstrated inhibition of algal growth after exposure to CNT (Patel et al., 2019). The reduction in growth would be due to physical interactions or agglomeration of nanoparticles with the cell surface. The opacity of CNT and the agglomeration next to the algal cells cause a “shading” that could explain the inhibition of their growth (Wick et al., 2007; Schwab et al., 2011), although for some authors, this effect is insignificant (Kwok et al., 2010; Long et all., 2012), attributing the inhibition to the physical interaction with the cell surface (Saxena et al., 2020), destruction of the plasma membrane (Hu et al., 2015) and internalization (Gomes et al., 2018; Irazusta et al., 2021), all of them also leading to metabolic changes resulting from the production of reactive oxygen species (ROS) and oxidative stress (Long et al., 2012; Nogueira et al., 2015). In fact, it was previously stated that the interaction of CNT with cellular proteins may be a common path to their biological effects (Wang et al., 2019).
Algae Pseudokirchneriella subcapitata (R. subcapitata) and Chlorella vulgaris exposed to SWCNT showed decreased growth rate, with EC50 of 29.99 and 30.96 mg.L-1, respectively (Sohn et al., 2015), cellular damage and oxidative stress (Hu et al., 2015). In another study, the inhibition concentration (IC25) of 1.04 mg.L-1 was determined in 72 h for P. subcapitata (Blaise et al., 2008). In our study the EC50 was 0.73mg.L-1, it is observerd a great variation in these values, probably due CNT characteristics.
Thus, the algae Pseudokirchneriella subcapitata, renamed Raphidocelles subcapitata constitute one of the most sensitive organisms of the aquatic biota in relation to the toxicity of these nanoparticles and are adversely affected (Blaise et al., 2008). Reduced graphene oxide (rGO), another carbonaceous nanoparticle, also inhibited the growth of R. subcapitata algae and was internalized by algal cells, causing metabolic and structural changes related to structural proteins (Irazusta et al., 2021). Carbon nanomaterial agglomerated and/or internalized by cells not only reduces the availability of light, but also interrupts the provision of sufficient nutrition. This restricted supply leads to inhibition of algal growth and viability. Exposure also results in the generation of stress within the algal cells, production of reactive oxygen species and elevation of stress enzymes, revealing an adverse condition within the algal cells. Despite this, contrasting results of increased algal growth have also been reported recently (Zhang et al., 2018a, b, c). This is a general impact, but not the same for all algae species.
Earthworms are widely studied among soil invertebrates, as they play a key role in most terrestrial ecosystems and represent a significant portion of soil macrofauna (Courtois et al., 2021). They are also regarded as “soil engineers” involved in maintaining soil structure and fertility (Carbonell et al., 2009), are also widely used as bioindicator organisms in toxicity in studies to measure the effects of soil contaminants such as pesticides, heavy metals, pathogens, microplastics and nanomaterials (Gu et al., 2017; Xiao et al., 2020; Sun et al., 2021; Forbes et al., 2021; Bourdineaud et al., 2021). Increased production and environmental release of multi-walled carbon nanotubes (MWCNTs) increase soil exposure and potential risk to earthworms. Besides, CNT toxicity to earthworms remains unclear, with some studies identifying negative effects and others negligible effects.
In this study, after 14 days of exposure of the organisms, there was 5% (1/20) mortality at concentrations of 1.0 and 10 mg. Kg-1 and there was no significant mass variation in relation to the control, except for 1.0 mg. Kg-1 concentration (Figure 5).
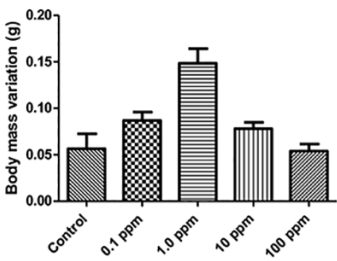
Source: Author’s elaboration.
Figure 5 Body mass variation (g) after 14 days of exposure of Eisenia andrei earthworms to MWCNT.
The comet assay did not detect significant damage at any of the concentrations (Figure 6), but a subletal effect was demonstrated by cytotoxicity analysis, by identify intense cytoplasmic vacuolization (Figure 7), witch is a indicative of a metabolic diversion for detoxification. Sublethal toxic effects have also been reported, both in metabolism, due to the increase in ROS production, and in histopathological alterations, with malformations in E. andrei oligochaetes exposed to PdCu/MWCNand PdCu/MWCNT at a concentration of 2000 mg.k-1 (Köktürk et al., 2021). Recent study suggests that toxicity of MWCNTs to earthworms is associated with reduced detoxification capacity, excessive oxidative stress, and disturbance of multiple metabolic pathway, corroboranting with the probably interaction with cellular proteins (Wang et al., 2019). These effects can be amplified by the characteristics of bioaccumulation of MWCNT in earthworms, as demonstrated by Petersen et al. (2010) and Li et al. (2013).
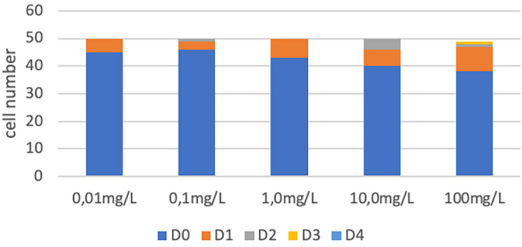
Source: Author’s elaboration.
Figure 6 Quantification of DNA damage by the Comet assay, considering damage levels from 0-4. “D” means damage level, rang 0 to 4.
Conclusion
As already mentioned, nanomaterials have a wide variety of applications and due to this broad distribution, the understanding of their pottential contaminant effects on the environment is crucial. The nanotechnology advances in recent decades has enabled a great development in the production of synthetic nanoparticles, whose wide application also guarantees the release of relevant amounts of them into the environment from industrial areas, water treatment plants, etc. (Nowack et al., 2012) and raises concerns regarding the safety of these nanoparticles in relation to their toxicity and the potential risks resulting from their presence in the ecosystems (Unrine et al., 2010; Cornelis et al., 2014). Furthermore, MWCNT have been shown to tend to adsorb a wide variety of toxic substances, which can increase the toxicity of chemicals in organisms (Braun et al., 2008). As demonstrated in this work, MWCNT showed aquatic toxicity in algae of the species R. subcapitata. In the terrestrial environment, no acute toxicity was observed, such as mortality in the bioindicator E. andrei, nor chronic effects, such as genotoxicity, but a sublethal effect, such as cellular suffering, was demonstrated by the intense cytoplasmic vacuolization, indicating a cellular response to stress, diverting the energy necesserary to the body methabolism for the detoxification process (Fernandes et al., 2021).











 nueva página del texto (beta)
nueva página del texto (beta)



