Introduction
The plants have evolved a suite of strategies for acquiring nutrients from the soil and to respond to changes in their availability in time and space. These strategies include architectural and morphological root traits, and also biotic root interactions with fungi and bacteria (Bardgett et al., 2014; Kong et al., 2017). Architectural traits determine the spatial configuration of root system of an individual plant and include root branching rate (Bardgett et al., 2014). Whereas morphological traits are features on individual roots such as total root length, specific root length, root tissue density, and diameter of first-order roots (Bardgett et al., 2014). It has been suggested that plant species with thin and thickroots represent two strategies for foraging soil mineral nutrients, but they are resource-costly (Eissenstat, 1992; Comas et al., 2014; Kong et al., 2014). Therefore, many plants do not invest resources in multiple nutrientacquisition mechanisms, because there is a trade-off between one strategy at the expense of another (Chen et al., 2016). Recent evidence also shows that thin and thickroots interact in different ways with arbuscular mycorrhizal (AM) fungi. For instance, thick root systems are not very efficient at nutrient foraging and they invest instead in mycorrhizal symbionts to meet their nutrient demands (Kong et al., 2016; Lugli et al., 2020). Additionally, thick-roots are more colonized by AM fungi because their cortex provides greater space for mycorrhizal colonization (Kong et al., 2017).
Arbuscular mycorrhizal fungi belonging to subphylum Glomeromycotina (Spatafora et al., 2016) are an ancient lineage (Strullu-Derrien et al., 2018) of obligate biotrophs that colonize the roots of most terrestrial plant species, including cultivated plants, around 80 % of plant species and 92 % of plant families (Wang and Qui, 2006). Because of its important ecological and agricultural implications, the impact of this symbiosis has been studied, not only, on the host plant’s growth and development, but also on soil adherence, soil stability, mineral redistribution, water retention and restoration of disturbed ecosystems (Álvarez-Sánchez and Peña, 2009; Gianinazzi et al., 2010; Cuenca, 2015; Pickles and Simard, 2017; Ji et al., 2019; Li et al., 2020). The relationship between AM fungi and the majority of land plants is often of a bidirectional nature, i.e., the fungi procure inaccessible minerals and water (Smith and Read, 2008) to the host plant and in return they receive up to 20 % of photosynthates produced by the host plant (Jakobsen and Rosendahl, 1990; Drigo et al., 2010). Due to the improved nutrient acquisition, mycorrhizal plants obtain other benefits; for instance, they are better at tolerating stresses, both biotic, e.g. herbivores, pathogens (Sharma et al., 2017) and abiotic drought, heavy metals, salinity (Miransari, 2010; Latef et al., 2016).
It is possible that root traits and AM symbionts play an important role in the success of plants to grow in several environments, including habitats strongly modified by humans such as cultivated land. Plant species from the same genus but that differ in their degree to which they associate with anthropogenic habitats (synanthropy index or weediness), are suitable to evaluate if there are differences in their root traits (thin vs thick) and if these types of roots differ in their association with AM fungi. Therefore, in the present study, the mycorrhizal colonization by AM fungi, and both architectural (root branching rate) and morphological (total root length, diameter of first-order root, root tissue density, and specific root length) traits were quantified in three weed species from the same genus (Melampodium) but differing in their synanthropy index (weediness).
Three Melampodium species were studied because their phylogenetic relationships are known (Stuessy et al., 2011), their synanthropy indexes were previously estimated (Hanan-A et al., 2016), and also can coexist in the same habitats (Hanan-A et al., 2016). The studied species are: (i) Melampodium divaricatum (Rich.) DC. is widespread in subtropical habitats from Mexico to Central America, Colombia, and Brazil. It has been introduced in Cuba, Burma, Puerto Rico and the Virgin Islands. This species can be found in disturbed sites, it blooms all year-round, and it has the highest synanthropy index (2.06). (ii) M. tepicense B.L. Rob. has distribution in cloud pineoak and tropical dry forests in the states of Nayarit, Jalisco, Colima and Michoacán, it blooms from August to February, and it has the lowest synanthropy index (1.67). Additionally, M. divaricatum and M. tepicense (the weediest and least weedy, respectively) differ in their life history traits (Hanan-Alipi et al., 2021). For instance, M. divaricatum has a fast germination, it produces flowers earlier, and also has a more sustained growth rate than M. tepicense. Although M. divaricatum produces more biomass, the majority is allocated to stems, leaves and flowers compared with M. tepicense which allocates more biomass to roots (Hanan-Alipi et al., 2021). These life history traits could explain the success of M. divaricatum to colonize different habitats including anthropogenic habitats. (iii) M. perfoliatum (Cav.) Kunth is found in tropical dry and pine-oak forests in Mexico, Guatemala, and Costa Rica, and it was introduced in Cuba and southern California. It blooms all year-round, and it has an intermediate synanthropy index (2.0) between M. divaricatum and M. tepicense (Hanan-A et al., 2016).
The specific aims of this study were: (i) to quantify the arbuscular mycorrhizal colonization of Melampodium species with different synanthropy index, (ii) to determine the correlations between AM colonization and the architectural and morphological root traits. Given that mycorrhizal plants often obtain benefits such as greater amount of mineral nutrients, water and buffering to biotic and abiotic stresses, a range from highest to lowest AM colonization proportional to that of synanthropy index (M. divaricatum - M. perfoliatum - M. tepicense) was predicted. Additionally, it was expected that AM colonization would correlate positively with diameter of first-order root and root tissue density, but negatively with total root length, specific root length and root branching rate in agreement with the root economic spectrum (Kong et al., 2017), i.e., maximizing the benefits and minimizing costs for soil resource acquisition.
Materials and methods
Study site
Roots from Melampodium species and soil sampling were carried out along a stretch of the highway San Blas-Tepic (Federal highway Mexico 76), heading northeast, past the locality of Jalcocotán, in the municipality of San Blas, in the state of Nayarit, Mexico, where individuals of the three species have been previously located. We sampled the roots and the soil in three sites (Table 1) along a stretch of the highway, which measures a total of ~8.6 km, and the distance between two sampled points (sites) was of 2 km and 4.6 km approximately.
Table 1 Localization, number of plant species sampled per site, and soil chemistry parameters in the three sites
| Sites | |||
| 1 | 2 | 3 | |
| Coordinates | 21° 31’ 44.4” N, 105° 02’ 09.3” W | 21° 29’ 46.6” N, 105° 04’ 59.0” W | 21° 31’ 14.2” N, 105° 02’ 59.5” W |
| Sampled plants | 25 M. tepicense,
7 M. divaricatum |
18 M. divaricatun, 14 M. perfoliatum |
11 M. perfoliatum |
| MO (%) | 8.76 | 3.11 | 4.52 |
| N (kg ha-1) | 258 | 85 | 141 |
| P (mg kg-1) | 115.3 | 225.7 | 49.6 |
| K (Cmol kg-1) | 0.7 | 0.87 | 1.57 |
| Ca (Cmol Kg-1) | 15.7 | 3.31 | 11.81 |
| Mg (Cmol Kg-1) | 7.16 | 2.85 | 4.91 |
| pH | 6.03 | 5.94 | 6.45 |
MO = organic matter, N = total nitrogen, P = total phosphorus, K = potassium, Ca = calcium, Mg = magnesium.
The vegetation along the sampling sites is tropical sub-deciduous (semi-deciduous) forest, however, there is high fragmentation because of extensive crops such as mango, coffee, avocado and banana. The climate is warm sub-humid with rainfalls in summer (INEGI, 2017), the minimum average precipitation (10 mm) and temperature (10.5 ºC) occur in May and January respectively, while the maximum average precipitation (943.5 mm) and temperature (39.8 ºC) occur in August and October, respectively (Servicio Meteorológico Nacional, 2020).
Soil chemistry
In October 2016, we collected soil samples from all sites. The soil samples were taken using a metal core with 2.5 cm of diameter and 10 cm from the top. Three samples from each site were collected and homogenized them before chemical analyses were done. The following chemical soil parameters were determined according to the Mexican Official Standard NOM-021-PROY-NOM-021-RECNAT-2000 (DOF, 2000): soil pH in water (1:2), organic matter (OM %) by Walkey-Black, total nitrogen (N kg ha-1) by Kjeldahl, total phosphorus (mg kg-1) and potassium (K Cmol kg-1) by flammometry, calcium (Ca Cmol kg-1) and mag-nesium (Mg Cmol kg-1) by spectrophotometry (Table 1). All analyses were carried out in the “Laboratorio de Análisis de Suelo, Agua y Plantas” of the “Unidad Académica de Agricultura”, Autonomous University of Nayarit, Mexico.
Root trait measurements
For each weed species, also in October 2016, roots from a total of 25 flowering individuals (with the aim of distinguishing between species) per species were sampled. Some species sampled coexisted in two sites (Table 1). The soil was carefully excavated 0-20 cm deep. Once that part of root system was exposed, root branches (including first and second-order roots) were cut from the main roots and transported within a few hours to the laboratory in plastic bags with soil. In the case of M. tepicense, all individual plants as a whole because of their small size were collected, in order to have enough root for root traits measurements and AM colonization. In the laboratory, the soil was removed and the roots were washed with enough tap water. Then, a subsample of each plant’s roots was studied, including first and second-order roots, which were photographed alongside a 20 cm ruler to serve as a reference scale. Subsequently, these subsamples were dried in an oven at 60˚C over a period of three days and then weighed on an analytical balance to determine the root’s dry weight. The images were analyzed utilizing a plugin for the image processing program ImageJ (Rasband, 2016) called SmartRoot (Lobet et al., 2011). Utilizing SmartRoot it was possible to estimate total root length (TRL, cm), volume (cm3), and diameter of first-order roots (FOR, cm). These parame-ters were utilized to then calculate root tissue density (RTD), specific root length (SRL) and root branching rate (RBR). Root tissue density (g cm-3) was calculated by dividing the root dry weight by the volume. Specific root length (m g-1) was calculated by dividing the total length of the root by the root’s dry weight of the subsample. The root branching rate was calculated by dividing the number of first-order roots by the number of second-order roots. In addition, the basal diameter of stem (BDS) from all sampled plants was measured before collecting their roots. Under field conditions the size of flowering plants is heterogeneous, therefore, the BDS can be used as covariable.
Arbuscular mycorrhizal measurements
In the field, a subsample of fine roots from all sampled individuals was taken and stored in ethanol and distilled water (1:1, respectively). Roots were processed according to the method of Koske and Gemma (1989) and stained with trypan blue (0.05 %). The colonization of roots was quantified by AM fungal structures (hyphae, vesicles and arbuscules) in 15 root segments of approximately 15 mm long per plant, and they were fixed in polyvinyllacto-glycerol (PVLG). Each root fragment was scanned at three equally spaced points under a light microscope (ZEISS Primo Star) at 100x total magnification, using the cross-hair intersection method (McGonigle et al., 1990). This method consists of scanning the presence or absence of fungal structures across the equator of each field of view (45 fields of view) under the microscope. The AM hyphae were identified as smooth and coenocytic hyphae (aseptate) stained blue by trypan blue. To estimate the percentage of hyphae, vesicles and arbuscules, positive counts were summed and weighted by the total number of fields of view analyzed. Given that the field roots have different thickness it is difficult to properly observe the presence or absence of fungal structures, therefore, these roots were not taken into consideration when calculating percentages. Therefore, the number of fields of view varied from 16 to 45.
Statistical analyses
All statistical tests were done using the R statistical programming language (R Core Team, 2020). Before running the tests, we performed a graphic data exploration to check for outliers, homogeneity of variance, and normality, and to evaluate the best potential models with which to analyze each type of response variable (Zuur et al., 2010). For all the models, we verified whether the residuals were normally distributed, the variances were homogeneous, and when it was necessary the variables were transformed.
To test for differences in the BDS between species, we used one-way ANOVA. To meet the model’s assumptions, the Neperian logarithmic transformation was applied to the BDS. To test whether the variables TRL, RTD, SRL, FOR, RBR and the colonization by hyphae differed between species, ANCOVA was used; the BDS was used as a covariable. To meet the model’s assumptions, the SRL and RBR were transformed with Neperian logarithm. The square root transformation was applied to TRL, RTD, FOR, and the percentage of hyphae.
Vesicles and arbuscules were analyzed as binary variables (presence/absence) because several individuals did not show these fungal structures. For these binary variables, generalized linear models (GLM) with a qua-sibinomial error structure and the logit link function were used. For both ANCOVA and GLM, when there were significant differences between species these were explored using a posteriori contrast based on t-tests (Warnes et al., 2018).
Spearman correlation coefficients were employed to determine the relationship between AM colonization by hyphae and root traits (TRL, RTD, SRL, FOR and RBR), first for all the species together and then for each one of them. The correlations were calculated separately for each root variable and the hypothesis of “true rho is not equal to cero” was used. Given that vesicles and arbuscules were binary variables, these data were not used in the Spearman correlation.
Results
Root trait measurements
The weed species differed significantly in the BDS (F 2,72 =33.38, P < 0.001), Melampodium divaricatum and M. perfoliatum had the higher BDS (mean ± standard error: 1.472 ± 0.137 cm and 2.116 ± 0.313 cm, respectively), and differed significantly with M. tepicense (0.524 ± 0.026 cm).
For all root traits there were statistically significant differences between the weed species (Table 2). Melampodium divaricatum was the weed species with the highest values of RTD (0.350 ± 0.018 g cm3), compared with M. perfoliatum (0.291 ± 0.028 g cm3) and M. tepicense (0.238 ± 0.056 g cm3), which did not differ. TRL, FOR, and RBR showed significant differences between species in agreement with a posteriori contrast, the values matched with the synanthropy index from highest to lowest: M. divaricatum (TRL: 84.180 ± 7.429 cm, FOR: 1.955 ± 0.157 cm, RBR: 30.304 ± 2.625), M. perfoliatum (TRL: 69.030 ± 10.799 cm, FOR: 1.693 ±0.292 cm, RBR: 19.790 ± 3.825), and M. tepicense (TRL: 22.198 ± 2.332 cm, FOR: 0.267 ± 0.030 cm, RBR: 7.877 ± 1.832). But in contrast, M. divaricatum was one of the weed species with low SRL (151.117 ± 78.428 m g-1), and there was no significant difference with M. perfoliatum (64.213 ± 31.328 m g-1), while M. tepicense had the highest SRL (922.273 ± 191.996 m g-1).
Table 2 Summary of statistical results of ANCOVA for root traits and mycorrhizal colonization by hyphae, BDS (basal diameter of stem) was used as covariate
| TRL (cm) | RTD (g cm3) | SRL (m g-1) | FOR (cm) | RBR | Hyphae % | ||||||||
| Source of variation | df | F | P | F | P | F | P | F | P | F | P | F | P |
| Species | 2 | 44.875 | <0.001 | 5.709 | 0.005 | 19.669 | <0.001 | 79.817 | <0.001 | 34.38 | <0.001 | 9.734 | <0.001 |
| BDS | 1 | 58.619 | <0.001 | 0.204 | 0.652 | 3.863 | 0.053 | 57.663 | <0.001 | 5.166 | 0.026 | 2.258 | 0.137 |
| Species:BDS | 2 | 0.022 | 0.977 | 2.094 | 0.130 | 1.007 | 0.370 | 0.093 | 0.911 | 0.892 | 0.414 | 1.949 | 0.150 |
| Residuals | 69 | ||||||||||||
TRL = total root length, RTD = root tissue density, SRL = specific root length, FOR = diameter of first-order root, RBR = root branching rate. Significant values are shown in bold.
Except for RTD and SRL, root traits were found to be related with the covariable (Table 2). In general, larger plants developed higher TRL, FOR and RBR (Figure 1 a, b and c), but M. divaricatum with the intermediate BDS and highest synanthropy index had the greatest values of TRL, FOR and RBR compared with M. perfoliatum and M. tepicense. Melampodium tepicense had the lowest values of TRL, FOR and RBR (Figure 1 a, b and c), and differed significantly with the other two weed species. M. tepicense is the weed with the lowest size (BDS), and also lowest synanthropy index. While M. perfoliatum had intermediate values of TRL, FOR and RBR, it is the weed with the greatest size but it has an intermediate synanthropy index, and it differed significantly of M. divaricatum and M. tepicense(Figure 1 a, b and c). Finally, we did not find significant differences in the interaction species:covariable (Table 2).
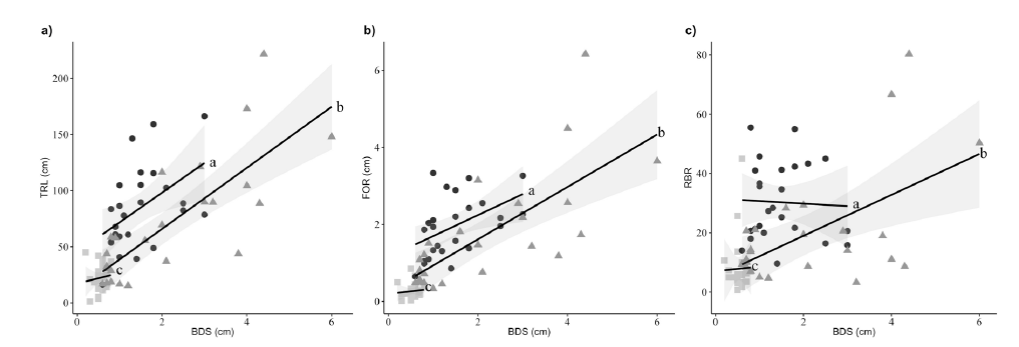
Figure 1 Interactive effects of basal diameter of stem (BDS, covariate). a: Total root length (TRL). b: Diameter of first-order root (FOR). c: Root branching rate (RBR) for Melampodium divaricatum (black circles), M. perfoliatum (grey triangles), and M. tepicense (grey squares). The lines represent the regression lines with the respective confidence intervals (shaded). Different letters beside the lines indicate statistically significant differences between the weed species according to a posteriori contrast P (<0.05). Data are given without transformation.
Arbuscular mycorrhizal measurements
The arbuscular mycorrhizal colonization showed significant differences between weed species (Table 2). The percentage of hyphae was higher in M. divaricatum and M. perfoliatum, and there were no differences between them, while M. tepicense had the lowest percentage of hyphae (Figure 2a). The same pattern was observed in the vesicles (Deviance2 = 13.944, P = 0.001, Figure 2 b) and arbuscules (Deviance2 = 12.657, P = 0.002, Figure 2 c).
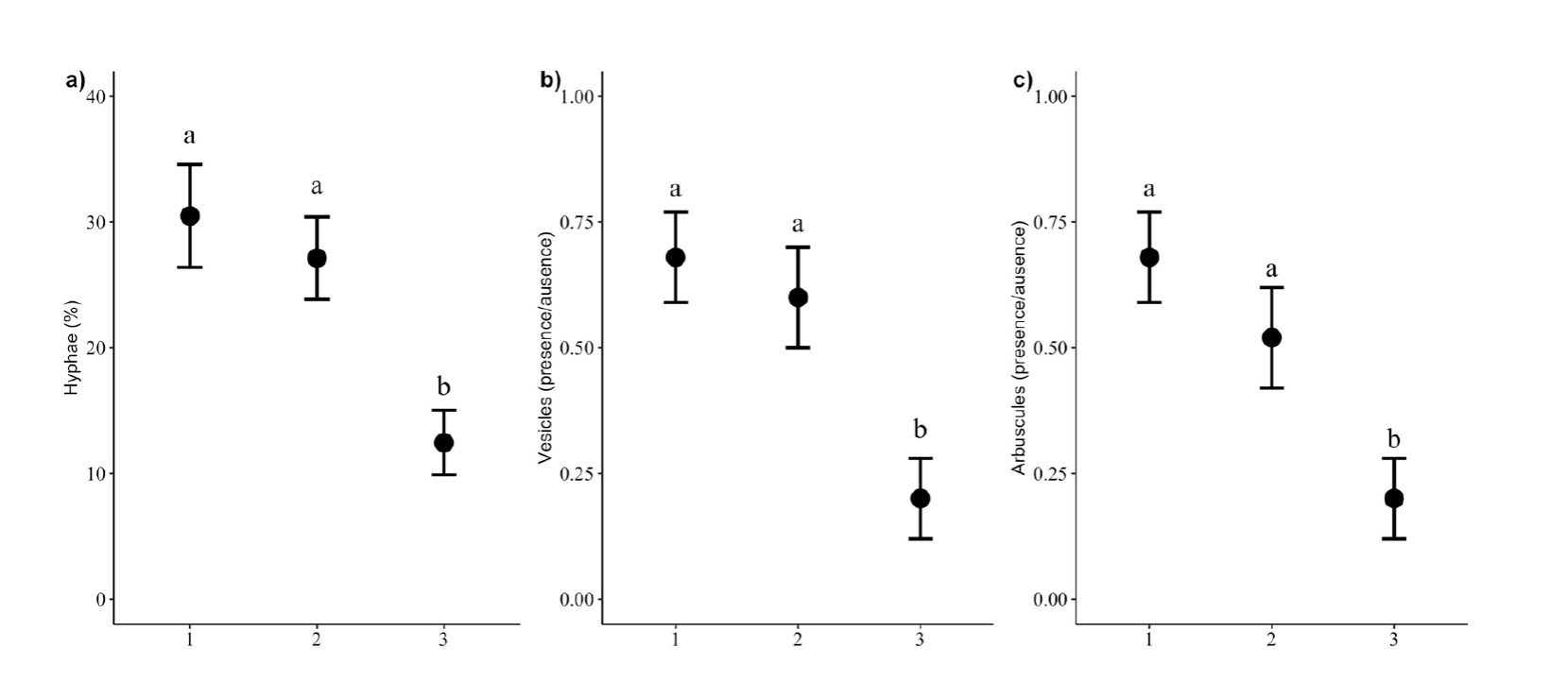
Figure 2 a: Percentage of root colonized by hyphae. b: Presence/absence of vesicles. c: Presence/absence of arbuscules in the weed species. The numbers in the axis y indicate the species: 1 = Melampodium divaricatum, 2 = M. perfoliatum, and 3 = M. tepicense. Means ± standard errors are given without data transformation by hyphae. Different letters indicate significant differences between species according to a posteriori contrast P (<0.05).
Only for the vesicles the covariable was significant (Deviance1 = 4.482, P = 0.042), M. tepicense showed the lowest colonization by the vesicles and it was also the species with the lowest BDS. We did not find significant differences in the interaction of the species with the covariable explaining the AM colonization by hyphae (Table 2), vesicles (Deviance2 = 0.579, P = 0.766) and arbuscules (Deviance2 = 1.966, P = 0.405).
Spearman correlation analyses showed, considering the three species, a positive correlation between mycorrhizal colonization by hyphae, and TRL, FOR and RBR (Figure 3 a, d, e). Yet, there was no correlation between mycorrhizal colonization by hyphae and RTD (Figure 3b), and SRL (Figure 3c). But when the weed species were analyzed separately there were no correlations between mycorrhizal colonization by hyphae and the root traits (Table 3).
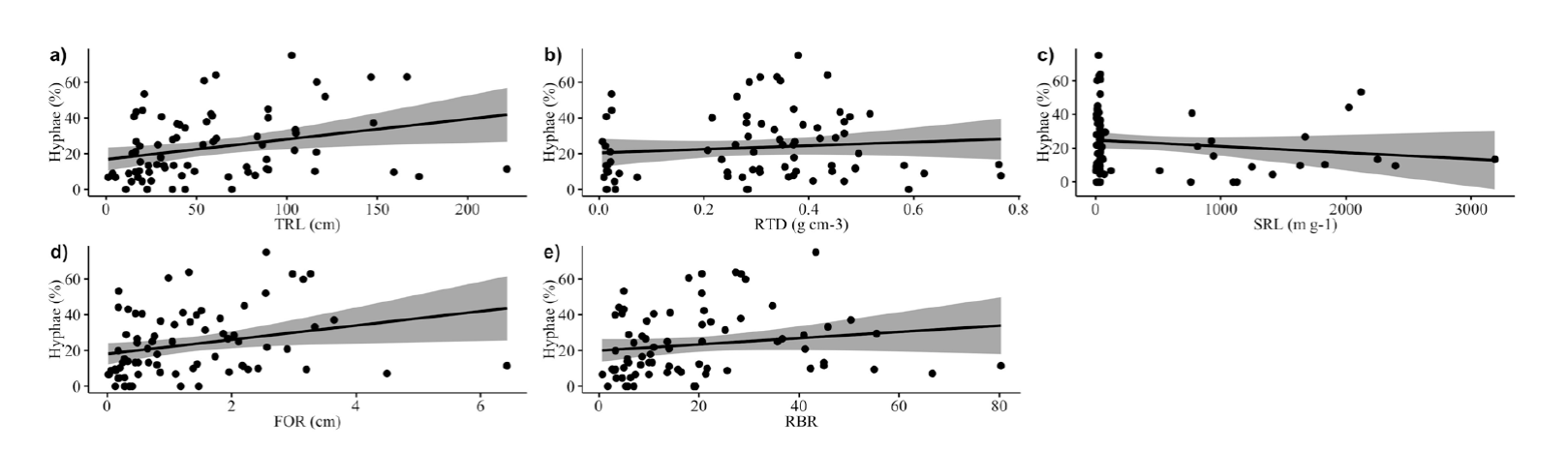
Figure 3 Relationships between mycorrhizal colonization by hyphae and root traits for the three species. Scatterplots show the relationship between mycorrhizal colonization and a: Total root length (TRL). b: Root tissue density (RTD). c: Specific root length (SRL). d: Diameter of first-order root (FOR). e: Root branching rate (RBR). Data are given without transformation.
Table 3 Spearman correlation coefficients between arbuscular mycorrhizal colonization by hyphae and root traits
| Hyphae (%) | ||||||||
| All species | M. divaricatum | M. perfoliatum | M. tepicense | |||||
| Variables | rho | P | rho | P | rho | P | rho | P |
| TRL (cm) | 0.329 | 0.004 | 0.049 | 0.816 | -0.069 | 0.742 | 0.226 | 0.276 |
| FOR (cm) | 0.359 | 0.002 | 0.037 | 0.859 | -0.096 | 0.646 | 0.116 | 0.580 |
| RTD (g cm-3) | 0.139 | 0.235 | 0.090 | 0.667 | 0.152 | 0.467 | 0.065 | 0.755 |
| SRL (m g-1) | 0.123 | 0.293 | 0.145 | 0.487 | 0.105 | 0.617 | 0.198 | 0.341 |
| RBR | 0.271 | 0.019 | 0.061 | 0.774 | -0.040 | 0.846 | 0.076 | 0.716 |
TRL = total root length, FOR = diameter of first-order roots, RTD = root tissue density, SRL = specific root length, RBR = root branching rate. Significant values are shown in bold.
Discussion
In this study, we observed mycorrhizal colonization by hyphae, vesicles and arbuscules in the three weeds, our finding is consistent with previous studies, which showed mycorrhizal colonization in weeds (Baumgartner et al., 2005; Vatovec et al., 2005; Daisog et al., 2012; Li et al., 2016). The arbuscules are the specific fungal structures that perform the exchange of nutrients between fungi and plants, therefore, this could indicate a functional symbiosis in the three species. However, the weed species could obtain different benefits from their mycorrhizal symbionts in agreement with their synanthropy index (i.e., the degree to which a species associates with anthropogenic habitats) and their root traits.
Melampodium tepicense is the weed with the lowest synanthropy index and it also had the lowest arbuscular mycorrhizal colonization. Lower AM colonization in this species was consistent with its smallest FOR and greatest SRL, compared with M. divaricatum and M. perfoliatum. Our findings agree with other studies showing the same pattern (Comas et al., 2014; Vega-Frutis et al., 2015; Kong et al., 2016, Wen et al., 2019) . But in contrast, it has been suggested that species with high SRL produce a higher TRL and RBR (Liu et al., 2015; Kramer-Walter et al., 2016). However, M. tepicense had the lowest values of these root parameters. A previous experiment, under common-garden, showed that this weed produced lower total dry biomass, but used a relatively larger part of its biomass for roots than M. divaricatum(Hanan-Alipi et al., 2021). These findings suggest that roots of M. tepicense could have pheno-typic plasticity and can respond to soil conditions (field vs experiment). Additionally, M. tepicense with a lower RTD, and highest SRL may increase the volume of soil explored and enable faster acquisition of mineral nutrients, hence, this could explain our results (Comas and Eissenstat, 2004; Lugli et al., 2020).
Melampodium divaricatum and M. perfoliatum had the greater values of the FOR. Thick-root species have higher construction cost per unit root length, i.e., low SRL, as it was observed. Also, species with thick-root allocate more carbon to AM fungi to increase absorptive surfaces by trusting more on finely structural fungal hyphae, thus, often show lower values of TRL, RBR and RTD, but greater root lifespan (Liu et al., 2015; Kramer-Walter et al., 2016). Our findings are consistent with several studies (Liu et al., 2015; Chen et al., 2016) showing the same pattern, but opposite with the greater values observed for TRL, RBR and RTD. It is probable that M. divaricatum and M. perfoliatum would benefit by investing in both strategies at the same time, i.e., smaller diameter of hyphae could efficiently explore large soil volumes and the roots could obtain mineral nutrients around (Lugli et al., 2020). Species that have higher RBR may be able to proliferate into localized patches of nutrients in the soil (Kramer-Walter et al., 2016). A previous experiment showed that M. divaricatum had a fast germination and sustained growth rate than M. tepicense(Hanan-Alipi et al., 2021), these life history traits along with the greater mycorrhizal colonization, RBR and TRL observed in this study might be key to explain the success of this species to colonize numerous habitats compared with M. tepicense. Opposite to our hypothesis, RTD was also significantly highest in M. divaricatum. It has been suggested that the plants could have high RTD and slow SRL, as in M. divaricatum, especially in soils where nutrients are not available. However, some studies have shown a relation between RTD and SRL (Kong et al., 2014; Kramer-Walter et al., 2016). In our study, M. tepicense, which had highest SRL and lower RTD seems to benefit in terms of nutrient acquisition as it was previously mentioned.
We also hypothesized that AM colonization would correlate positively with FOR and RTD, but negatively with TRL, SRL and RBR. However, our hypothesis was partially supported. Specifically, AM colonization by hyphae showed a positive correlation with FOR, thick-root species are generally more colonized by AM fungi, because their cortices are thicker and can provide greater space for intraradical fungal structures, and hence a higher absorption rate (Guo et al., 2008; Bardgett et al., 2014; Kong et al., 2014; Liu et al., 2015). This finding is consistent with several investigations, including plant species with different life histories and phylogenetic relationships. For instance, Liu et al. (2015) evaluated the root traits of 14 subtropical tree species, Kong et al. (2014) in 96 subtropical plant species, and Vega-Frutis et al. (2015) in 15 rainforest tree and palm species. RTD is considered as an important predictor of plant strategies (Birouste et al., 2013; Kramer-Walter et al., 2016) and has been associated with slow-growing species, although it is variable among woody species differing in moisture availability (Comas and Eissenstat, 2004). Also, RTD has been associated with AM fungi, although we observed the highest mycorrhizal colonization and RTD in M. divaricatum, we did not find a correlation between these variables, as we hypothesized. Liu et al. (2015) also did not find a correlation, they evaluated 14 subtropical tree species in China, and Lugli et al. (2020) found the same pattern in Central Amazonia using roots from 32 plots with similar soil and vegetation.
Regarding TRL, SRL and RBR, some studies have shown that species with higher AM colonization show a negative correlation with these variables. In this study, there was no correlation between AM fungi and SRL, this is consistent with Liu et al. (2015) and Maherali (2014). We observed a positive correlation between AM fungi and both TRL and BRB, these findings were opposite to our initial hypothesis. Other reports have demonstrated that mycorrhizal plants often have thinner roots and greater branching intensity (Comas et al., 2014), there-fore suggesting that the plants could use both foraging strategies, i.e., thin roots with more branching and mycorrhizas for the acquisition of mineral soil nutrients, thus maximizing the benefits and minimizing costs for soil resource acquisition (Bardgett et al., 2014; Liu et al., 2015). Additionally, TRL and RBR are key traits in determining root plastic response to nutrient patches, hence they may have high values in soils with low fertility (Kong et al., 2014). Consequently, weed species could be more competitive in environments where nutrients are heterogeneous in space and time (Chen et al., 2016) such as anthropogenic habitats.
It is important to mention that we did not find any correlation between AM colonization by hyphae and root traits when the species were analyzed separately. In a meta-analysis used to evaluate the relationships between root architectural and mycorrhizal growth response, Maherali (2014) did not detect any relationship between the variables, probably because the plants have the ability to respond plastically to fungal colonization. Therefore, studies comparing inoculated and non-inoculated plants are necessary to test whether root traits per se display phenotypic plasticity to colonization by AM fungi (Comas et al., 2014; Maherali, 2014).
The weed plants were collected in three sites, but in the first site coexisted M. tepicense and M. divaricatum, i.e., the species with lowest and highest weediness and also, they are the species which are closer phylogenetically. These species had access to the same pool of nutrients, but they showed different strategies to explore soil mineral nutrients independently of their phylogenetic relationship, i.e., the weeds have different foraging strategies, probably via natural selection (Comas et al., 2014) given that there was no phylogenetic signal (“tendency of related species to resemble each other more than species drawn at random from the same tree”, Münkemüller et al., 2012). Although the amount of nitrogen and phosphorus (the phosphorus is used in an array of plant processes such as photosynthesis and respiration) were high in the sites in agreement with the Mexican Official Standard (DOF, 2000), we did not quantify the amount available to the plants, but the total amount. In the second site M. divaricatum and M. perfoliatum coexisted, these species have similar synanthropy index and there were no significant differences in AM colonization and SRL between them. Regarding the TRL, RTD, FOR and RBR M. perfoliatum showed values more closer to M. divaricatum than M. tepicense and this is consistent with their synanthropy index.
Finally, roots perform multiple pivotal functions in plants and the ecosystem functions. For instance, absorption of both mineral nutrients and water from the soil, physical anchoring, resource storage, and vegetative reproduction. Additionally, they are the main point of interface with soil symbiotic organisms. However, the role of root traits is not well understood compared with leaf and stem traits (Kramer-Walter et al., 2016). Future studies may incorporate more weed species to understand how root traits, AM fungi and soil conditions mediate nutrient-acquisition mechanisms.
Conclusions
The three Melampodium species showed AM colonization, and the weediest (M. divaricatum) and least weedy (M. tepicense) species had the highest and lowest AM colonization, respectively. These species differed also in their morphological and architectural root traits, suggesting that they have different foraging strategies even though phylogenetically they are closer, whereas M. perfoliatum and M. divaricatum had similar values regarding AM colonization and root traits, and also a similar synanthropy index. Our findings suggest that proximate causes are operating.











 nueva página del texto (beta)
nueva página del texto (beta)



