Introduction
Diarrheal diseases are still among the main infectious diseases identified by the World Health Organization (WHO), as important public health problem worldwide, and is one of the causes of mortality in children under five years old. Escherichia coli is classified as a major bacterial diarrheagenic agent in developing countries and transmitted by consuming contaminated water or foods.1,2 E. coli strains causing diarrhea can be grouped into six pathotypes according to the presence of virulence factors and pathogenicity mechanisms specifics: enteropathogenic E. coli (EPEC), enterohaemorrhagic E. coli (EHEC), enterotoxigenic E. coli (ETEC), enteroinvasive E. coli (EIEC), enteroaggregative E. coli (EAEC), and diffusely adhering E. coli (DAEC).3,4,5
EPEC contains an intimin (eae) gene, is the pathotype most associated with diarrhea in infants, and is sometimes associated with vomit and fever. EPEC is classified into typical and atypical strains depending upon on the presence eae gene and bfp A gene, which is on a plasmid 90-kb called ‘EPEC adherence factor’ (pEAF) that encodes type IV-like bundle-forming pili (BFP). Typical EPEC (tEPEC) strains are eae + and bfp A +, whereas atypical EPEC (aEPEC) strains are eae + bfp A−, because lack pEAF plasmid.6 EHEC, causing abdominal pain, bloody diarrhea and low fever, is the main etiologic agent associated with hemorrhagic colitis and hemolytic uremic syndrome (HUS), characterized by renal damage. This pathogen produces two types of Shiga toxins, sxt 1 and sxt 2.7,8 ETEC pathogenicity is determined by the production of heat-stable (est) and labile-stable (elt) enterotoxins. ETEC is most common in children under two years of age, diarrhea may be accompanied by fever and sometimes vomiting, and is the main cause of traveler’s diarrhea.9 EIEC carries the gene for the invasive plasmid antigen H (ipaH), is important in six-month-old children, manifested with diarrhea with blood and mucus.10 EAEC harbors the transcriptional activator-encoding aat A gene, is a major cause of acute diarrhea in children and adults worldwide. DAEC possesses the afimbrial adhesin B (afaB) gene, and may play a role in causing sporadic diarrheal illnesses, particularly in pediatric patients.11
Diarrheagenic E. coli (DEC) is one of the most important causes of post-weaning diarrhea in pigs. This diarrhea is responsible for significant economic losses due to mortality, morbidity, and decreased growth rate.12 DEC strains in swine include EPEC, ETEC, and EHEC. ETEC infections are responsible for swine diarrhea and the severity of this infection has been associated to stress of weaning, lack of antibodies from the sow’s milk, and dietary changes.5 The objective of this study is to determine the frequency of these pathogenicity bacteria colonizing swine. A better understanding of the variety of virulence genes can provide us with important information for diagnosis and prevention of diarrheal diseases in swine, and potential transmission of these bacteria to human.
Materials and methods
Isolation and identification of E. coli
This is a transversal study based on sampling the total number of pigs in a semi-technical farm at Jiutepec, Morelos state, Mexico. This farm is dedicated to swine farming to medium scale where the swine production is for local consumption. In the range of March-April 2015, a stool sample was obtained in the total number of pigs present in the farm (280). Swine feces were collected directly in the anus by rectal swab. Rectal swabs were transported in Cary-Blair medium (DELTALAB, Spain) at 4°C., and MacConkey agar (MCD Lab, Mexico) was used for stool cultures, incubated at 37°C overnight. Three colonies with appearance of E. coli were randomly selected from each stool swabs. The identification of E. coli suggestive colonies was performed by biochemical tests, API 20E (Biomérieux, USA). API 20E strips were been incubated at 35°C for 18 h, and identified according to the manufacturer criteria for reading and interpretation.
Reference strains
A panel of E. coli reference strains was used as positive controls for the multiplex polymerase chain reaction (mPCR). Strains EPEC O127:H6 strain E2348/69 (eae and bfp A), EHEC O157:H7 (eae, stx 1, stx 2), ETEC O78:H11 strain H10407 (elt, est), EIEC O136:NM (ipaH), EAEC O44:H18 strain 042 (aggR), DAEC O75:H- strain E66438 (daa C) (kindly donated by Dr Carlos Eslava, National Autonomous University of Mexico) [Universidad Nacional Autónoma de México, UNAM]. E. coli ATCC 25922 strain served as a negative control for virulence genes in all PCRs. Bacteria were routinely spread on MacConkey agar (MCD Lab, Mexico), and incubated at 37°C overnight.
DNA extraction and multiplex PCR for virulence genes
The boiling method was used to extract bacterial DNA of each E. coli isolates.13 Here we describe a mPCR that simultaneously detects nine virulence genes associated with the six DEC pathotypes. The genes selected eae, stx 1, stx 2, elt, est, ipa H, aatA, daa C were amplified using 18 specific oligonucleotides (table I) including those to differentiate typical or atypical EPEC. The previously reported a mPCR reaction for DEC detection14 was adapted to three reactions multiplex PCR each one with six primers pairs to allow the simultaneous detection of three different virulence genes in each reaction mixture. The reaction I, for tEPEC, aEPEC, ETEC, containing eae (189 pb), pEAF (107 pb), elt (440 pb); reaction II, for ETEC, EAEC, EIEC, including est (191 pb), aat (152 pb), ipa H (619 pb); reaction III, for EHEC, DAEC, including sxt 1 (418 pb), sxt 2 (255 pb), daa (146 pb). Each 50 µ l reaction mixture contained the following: 1X reaction buffer (100 mM Tris/HCl, pH 8.5; 500 mM KCl, 1 % Triton X-100), 2 mM MgCl2, 0.2 mM each dNTP (dNTP mix, 10 mM, Thermo Scientific, USA), 10 µ M each primer, 1.0 U Taq DNA polymerase (Thermo Scientific, USA), 5 µ l template DNA and nuclease-free water. The PCR conditions for all reactions were: 94 °C for 5 min, 30 cycles of 94°C for 45 s, 58°C for 45 s, and 72°C for 45 s; and a final extension at 72°C for 7 min. mPCR-amplified fragments (5 µ l) were separated in a 1.7% (wt/vol) agarose gel and visualized under UV light after staining with ethidium bromide.
Table I: Primers used in the multiplex pcr for amplification of diarrheagenic E. coli isolated of a swine farm in Jiutepec, Morelos, Mexico, March-April 2015
|
Pathotype |
Target gene |
Primer name |
Primer sequence (5’-3’) |
Amplicón size (pb) |
Annealing tem (°C) |
Reference |
|
EPEC |
eae |
eae F |
ACTGGACTTCTTATTTCCGTTCTATG |
189 |
58 |
[16] |
|
eae R |
CCTAAACGGGTATAATCACCAGA |
|||||
|
tEPEC |
bfpA |
pEAF F |
GTTCTTGGCGAACAGGCTTGTC |
107 |
58 |
[16] |
|
pEAF R |
TTAAGCCAGCTACCATCCACCC |
|||||
|
EHEC |
stx-1 |
stx-1 F |
AGTCGTACGGGGATGCAGATAAAT |
418 |
58 |
[16] |
|
stx-1 R |
CCGGACACATAGAAGGAAACTCAT |
|||||
|
stx-2 |
stx-2 F |
GGCACTGTCTGAAACTGCCC |
255 |
58 |
[16] |
|
|
stx-2 R |
TCGCCAGTTATCTGACATTCTG |
|||||
|
ETEC |
elt |
elt F |
GGCGACAGATTATACCGTGC |
440 |
58 |
[16] |
|
elt R |
CGGTCTCTATATTCCCTGTT |
|||||
|
est |
est F |
ATTTTTMTTTCTGTATTRTCTT |
191 |
58 |
[16] |
|
|
est R |
CACCCGGTACARGCAGGATT |
|||||
|
EIEC |
ipaH |
ipaH F |
GTTCCTTGACCGCCTTTCCGATACCGTC |
619 |
58 |
[16] |
|
ipaH R |
GCCGGTCAGCCACCCTCTGAGAGTAC |
|||||
|
EAEC |
aat |
aat F |
AGGTTTGATAATGATGTCCTTGAGGA |
152 |
58 |
[16] |
|
aat R |
TCAGCTAATAATGTATAGAAATCCGCTGTT |
|||||
|
DAEC |
daa |
daa F |
ATTACGTCATCCGGGAAGCACACA |
146 |
58 |
[16] |
|
daa R |
GCTTGCTCATAAAGCCGCAGACAA |
EPEC: enteropathogenic E. coli; tEPEC: Typical enteropathogenic E. coli; EHEC: enterohemorrhagic E. coli; ETEC: enterotoxigenic E. coli; EIEC: enteroinvasive E. coli; EAEC: enteroaggregative E. coli; DAEC: diffusely adhering E. coli.
Pulsed-field gel electrophoresis
All MDR strains were genotyped using pulsed-field gel electrophoresis (PFGE).15,16 Briefly, genomic DNA was isolated in an agarose-embedded formand was subjected to enzymatic digestion with 50 U XbaI. Agarose digests were then placed in preformed wells of an agarose gel and separated by electrophoresis in the 0.5X TBE buffer using a CHEF Mapper system (Bio-Rad Laboratories,Inc.). After 23 h, gels were stained with ethidium bromide and PFGE patterns were visualized under UV light. Salmonella serotype Braenderup strain (H9812) was used as the global reference standard and Lambda PFG ladder (New England BioLabs Inc. USA) was used as a size marker. Patterns PFGE were interpreted using Tenover criteria17 and analyzed with BioNumerics 5.10 software (Applied Maths, Sint-Martens-Latem, Belgium). A dendrogram was constructed using the Dice similarity coefficient, a tolerance coefficient, and un-weighted pair group methods with the arithmetic mean algorithm (UPGMA).
The study protocol was approved by the ethics, research, and biosafety committees of National Institute of Public Health (Instituto Nacional de Salud Pública, INSP). Written informed consent was requested from the owner of the participant farm in this study.
Results
Of the 521 E. coli strains identified in this study, 50 (9.6%) strains isolated of 42 different animals were positive for at least one pathogenicity marker (table I). The highest frequency was observed in EPEC (6.5%), followed by EHEC (2.3%) (figure 1). EIEC and ETEC were the least frequent pathotypes among E. coli-positive samples (0.4%). We did not obtain EAEC and DAEC pathotypes. In order of distribution of virulence genes, the most frequent identified included eae and sxt 1 genes with a frequency of 6.5 and 1.3%, respectively. The lowest frequent virulence genes included ipa H and elt, each having a frequency of less than 1% (table II). We did not obtain amplicons for the bfp B, est, aat A and daa C genes. All EPEC strains were atypical because possess theeae gene but lack bfp A gene located in plasmid pEAF (figure 2).
Table II: Prevalence of six E. coli pathotypes and their virulence genes in a swine farm from Jiutepec, Morelos, Mexico, March-April 2015
|
Pathotype |
Virulence genes |
n |
Frequency in E. coli-positive samples* % |
|
EPEC |
eae |
34 |
6.5 |
|
EHEC |
sxt1 |
7 |
1.3 |
|
sxt2 |
5 |
1.0 |
|
|
ETEC |
elt |
2 |
0.4 |
|
EIEC |
ipaH |
2 |
0.4 |
|
EAEC |
aat |
0 |
0.0 |
|
DAEC |
daa |
0 |
0.0 |
|
Total |
50 |
9.6 |
*Frequency is calculated by dividing the numbers to the total number of E. coli-positive strains identified in sampling (N= 521).
EPEC: enteropathogenic E. coli; EHEC: enterohemorrhagic E. coli; ETEC: enterotoxigenic E. coli; EIEC: enteroinvasive E. coli; EAEC: enteroaggregative E. coli; DAEC: diffusely adhering E. coli.
DEC: diarrheagenic E. coli; EPEC: enteropathogenic E. coli; EHEC: enterohemorrhagic E. coli; ETEC: enterotoxigenic E. coli; EIEC: enteroinvasive E. coli; EAEC: enteroaggregative E. coli; DAEC: diffusely adhering e. coli.
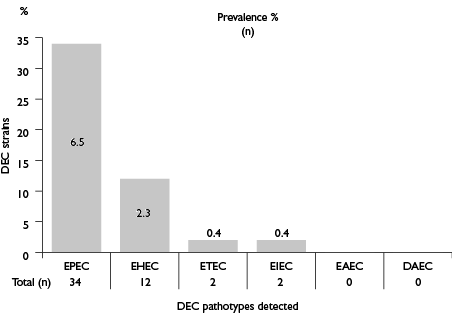
Figure 1: Prevalence of diarrheagenic E. coli detected in feces samples. Jiutepec, Morelos, Mexico, March-April 2015
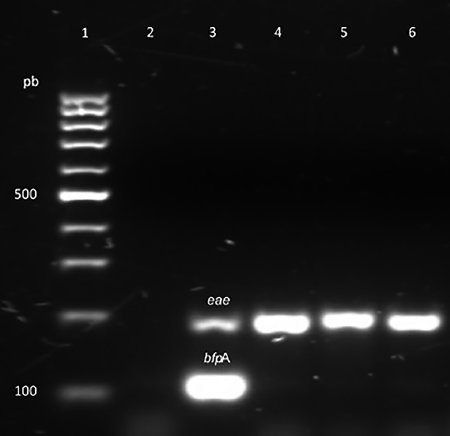
Figure 2: Multiplex polymerase chain reaction (mPCR) analysis of three representative atypical E. coli (aEPEC) strains. Agarose gel electrophoresis of mPCR reaction showing presence of eae and bfpA genes. Lane 1, molecular size marker (100 pb DNA ladder); lane 2, negative control; lane 3, enteropathogenic E. coli (EPEC) strain e2348/69 positive control (eae gene= 189 bp and bfp gene= 107 bp); lane 4-6 swine samples with eae gene. Jiutepec, Morelos, Mexico, March-April 2015
Age-specific prevalence of E. coli pathotypes
The highest frequency of pathogenic E. coli infections was observed in suckling and weaned piglets (14.8 and 12.6%, respectively). EPEC was highly frequent in suckling (12%) and weaned (9.8%). EHEC had a very similar infection frequency in both types of piglets (2.8%). Although the frequency of EPEC and EHEC was higher in piglets, these pathotypes were also common in faecal samples of adult females and males. While EIEC and ETEC only appeared in adult females (table III).
Table III: Age-specific frequency of E. coli pathotypes in 521 strains isolated from 275 swines faecal samples in a farm from Jiutepec, Morelos, Mexico, March-April 2015
|
Age group |
All pathotypes |
EPEC |
EHEC |
ETEC |
EIEC |
EAEC |
DAEC |
Total strains |
|
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
N |
|
|
Suckling piglets (14-21 days) |
16 (14.8) |
13 (12) |
3 (2.8) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
108 |
|
Weaning piglets (>21 days) |
22 (12.6) |
17 (9.8) |
5 (2.9) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
174 |
|
Sow (gender) |
11 (4.7) |
4 (1.7) |
3 (1.3) |
2(0.9) |
2(0.9) |
0 (0.0) |
0 (0.0) |
233 |
|
Boar (gender) |
1(16.7) |
0 (0.0) |
1(16.7) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
6 |
|
Total |
50 (9.6) |
34 (6.5) |
12 (2.3) |
2 (4) |
2 (4) |
0 (0.0) |
0 (0.0) |
521 |
EPEC: enteropathogenic E. coli; EHEC: enterohemorrhagic E. coli; ETEC: enterotoxigenic E. coli; EIEC: enteroinvasive E. coli; EAEC: enteroaggregative E. coli; DAEC: diffusely adhering E. coli.
EPEC: enteropathogenic E. coli; EHEC: enterohaem orrhagic E. coli; EIEC: enteroinvasive E. coli; ETEC: enterotoxigenic E. coli.
Genetic diversity of diarrheagenic E. coli strains. PFGE was conducted to determine the clonal-relatedness among diarrheagenic E. coli strains from swine. A representative group of 40 pathogenic E. coli strains, randomly selected of the 50 E. coli strains included in the study, was analyzed by PFGE. The PFGE results showed 25 different pulsotypes that is showed in the similarity dendogram (figure 3), where it is noted a high polymorphism considering as significative a diference of a single band. In the dendogram produced by UPGMA algorithm, the isolates were clustered in 21 clones (A to U, 1 to 16 isolates per clone) of 80% similarity according to the Dice similarity index, with 21 isolates clustering in two clones of closely related (similarity >95%) PFGE profiles. The highest homogeneity (similarity >95%) was observed among a group of 16 isolates (clone A) belonging to pathotype EPEC and a group of five isolates (clone G) with pathotype EHEC (figure 3). The remaining patterns were associated with different enteropathotypes (EPEC, EHEC, ETEC and EIEC).
Discussion
Suckling and post-weaning diarrheal (PWD) disease affecting pigs during the first weeks after birth result in significant economic losses for the pig industry due to mortality and decreased weight gain.18,19,20 However, none of the animals colonized with these diarrheagenic E. coli presented diarrhea in the sampling period of this study maybe due to the limitation of a descriptive-transversal study in the farm.
Most samples (76%) were obtained from pigs during suckling and weaning period, and showed to be significantly associated with EPEC (6.5%, 34/521), which was the most frequent pathotype in our study. This is consistent because porcine EPEC is the second type of pathogenic E. coli involved in PWD.21 These results are compared to two studies in swine, where only 3.9% (8/206) and 3.3% (15/455) of pathogenic E. coli isolates were carriers of the eae gene.22,23 In another study on the prevalence of virulence genes in E. coli of pigs in Mexico, the frequency of the eae gene was 18.3%.21
The estimated frequency for EHEC pathotype was 2.3%, where E. coli strains isolated from swine fecal samples were positive for the stx 1 and sxt 2 genes with an average rate of 1% for both genes. Our results confirm the observation made by other authors that pigs, mainly piglets, are not a potential EHEC reservoir.24 A study conducted in Mexico identified Shiga toxins (sxt 1 and sxt 2) with a frequency of 0.4 and 1% respectively in suckling piglets and weaned.21 Another study in northern Italy showed that EHEC strains were present in 7.8% (19/242) of faecal samples obtained from healthy pigs.25,26 In addition to these two types of diarrheagenic E. coli (EPEC and EHEC), EIEC and ETEC showed a frequency of less than 1%. Although, ETEC is reported as the most important of enteric colibacillosis in pigs, mainly in suckling and weaned piglets. However, the distribution and frequency of the pathologies and virulence genes can vary considerably from one region to another.21,27
The frequency of different pathotypes was variable in this study. EPEC and EHEC were found more frequently in suckling and weaned piglets. This can be explained because piglets in this period lack maternal immunity, which may make them non-immune to pathogenic E. coli. In the same way, the variation in environmental temperature and the stress associated with changes in both accommodation and diet may be important factors that trigger the proliferation of pathogenic E. coli in the intestine.23,24,28 The presence of Shiga toxins in the population of E. coli isolates studied indicates a risk among these strains because E. coli strains that possess sxt 2 are potentially more virulent than those with sxt 1. It should be noted that most EHEC associated with HUS produce sxt 2 and rarely sxt 1.29
On the other hand, none of the EPEC strains analyzed presented the plasmid pEAF, which codes for virulence factors that allow the bacteria to adhere locally, so all EPEC strains were atypical EPEC (aEPEC). Studies of EPEC strains from animals have found that none of the isolates contain the plasmid (aEPEC) and that it is only found in human strains (typical EPEC, tEPEC).6,30,31 In studies to determine the frequency of tEPEC and aEPEC, it has been observed that aEPEC is the most frequent. It is know that when a community begins to develop better distribution systems for drinking water and drainage, a transition occurs and atypical strains begin to be more frequent.32 We might think this is happening in the community studied, so it is an urban environment that has relatively well-established drinking water and drainage systems, since all EPEC strains isolated from farm animals were atypical.
To better understand the genetic relatedness of these pathogenic E. coli isolates, PFGE were performed. It has been shown that animal pathogenic E. coli strains share common genetic backgrounds.23 Pulsed-field gel electrophoresis demonstrated that EPEC and EHEC isolates from swine tend to be closely related.33,34 This is consistent with our results; however also we found that PFGE patterns were heterogeneous among EPEC, EHEC, ETEC, EIEC strains. Taken together, our data suggest that PFGE patterns of diarrheagenic E. coli are not highly correlated with pathotype. This very interesting finding confirms the presence in Mexico of different clones among one of the most prevalence pathotypes isolated from swine.
Conclusion
In this study were identified four E. coli pathotypes among swine colonized by these bacteria. Thus, these swine are potential reservoirs of EPEC, but not of EHEC and ETEC. Pulsotypes analysis showed that, although isolates with the same virulence markers share the same PFGE group, there is a high genetic variation, among EPEC, EHEC, ETEC and EIEC. The findings of our study are important for public health and veterinary medicine because there is a potential risk of causing diarrhea on swine and in the population consuming the meat.











 nueva página del texto (beta)
nueva página del texto (beta)



