Antecedents
In the current context of the COVID-19 pandemic, a disease caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), sanitary measures such as washing surfaces and equipment to reduce the risk of contamination by pathogens are crucially important (Avila-Quezada et al., 2010; Nussbaumer-Streit et al., 2020; Avila-Quezada et al., 2008; Gil et al., 2015). One of the most widely used sanitary products, due to its affordability, is chlorine, usually sold as sodium hypochlorite and calcium hypochlorite (OIRSA, 2020). The use of sodium hypochlorite (NaClO) as a disinfectant has increased during the COVID-19 pandemic (Patel et al., 2020), since it is a commonly used antiseptic for cleaning environmental surfaces in the healthcare sector, without the flammability and rapid evaporation that characterizes ethanol. An additional advantage of chlorine is that it can easily cover large surfaces (Hulkower et al., 2011). Some studies have shown that chlorine can inhibit fungal growth at a concentration of 75 ppm (Zoffoli et al., 2005), while Owoseni and Okoh (2017) reported that the lethal dose against bacteria was 1 ppm. But is chlorine effective against viruses? This review aims to describe the mode of action of chlorine when used as a disinfectant.
Persistence of SARS-CoV-2 on surfaces
The speed at which SARS-CoV-2 has spread throughout the world is alarming, as is the long period during which the virus remains latent outside the host (Riddell et al., 2020). Laboratory studies have assessed the persistence of the virus on different surfaces (Table 1) but there are still no studies of the persistence of SARS-CoV-2 on surfaces from agricultural environments, such as machinery and tools used in the preharvest and postharvest stages. Having this information would be useful to establish procedures for sanitizing and disinfecting surfaces as a preventive measure against contagion by SARS-CoV-2.
Table 1 Persistence of SARS-CoV-2 on different inert surfaces.
| Superficie | Permanencia | Referencia |
|---|---|---|
| Ropa y madera | ≤ 1 d | Chin et al., 2020 |
| Plástico | 4 d | Chin et al., 2020; van Doremalen et al., 2020 |
| Mascarilla médica | ≤ 7 d | Chin et al., 2020 |
| Cobre | 4 h | van Doremalen et al., 2020 |
| Cartón | 24 h | van Doremalen et al., 2020 |
| Superficies no porosas (vidrio, acero inoxidable, billetes y papel) | > 28 d | Riddell et al., 2020 |
How does chlorine destroy bacteria?
The concentration of commercial sodium hypochlorite products is usually between 3 and 6%, which is equivalent to 30,000-60,000 ppm of free chlorine. Free chlorine refers to the amount of hypochlorous acid and hypochlorite in the water. Hypochlorous acid (HClO) is electrically neutral, while hypochlorite ions (ClO-) are electrically negative. These ions constitute free chlorine, which, when in contact with bacteria, oxidizes them in a process known as chemical disinfection (Lafaurie et al., 2015).
When chlorine is diluted in water, an aqueous solution is formed in which undissociated HClO becomes activated, penetrating the cell walls and membranes of bacteria by passive diffusion due to the negative charge of these structures (da Cruz Nizer et al., 2020; Radovic-Moreno et al., 2012). ClO- ions have a negative charge too, so they can hardly pass through the bacterial wall since the charges repel each other (da Cruz Nizer et al., 2020). Thus, as the pH of the solution increases, hypochlorite ions become the predominant ones and the microbicidal activity decreases (Figure 1).
Figure created in BioRender.com
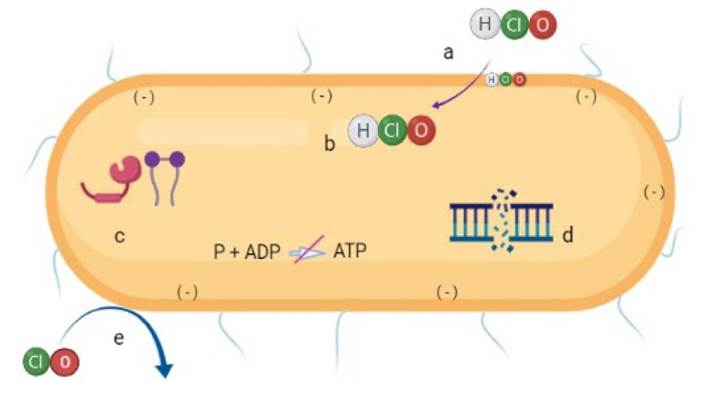
Figure 1 Diagram of the mechanism of action of hypochlorous acid (HClO) in bacterial cells. HClO penetrates the bacterial cell due to its neutral charge (a), affecting membrane components (b) such as transporters, proteins, and ATPase; it also degrades lipids and proteins (c) and interrupts DNA synthesis (d). Bacterial oxidation can also occur from contact with hypochlorite ions (ClO)- (e).
It has also been proven that the antimicrobial activity of HClO works by causing respiratory loss in bacterial membranes as a result of an irreversible reaction with structural, proteins and sulfur- and heme-containing membrane enzymes, causing cell death (Sam and Lu, 2009; Ghernaout, 2017). Damage to membrane proteins has a negative effect on energy transduction and transport that can lead to ATP hydrolysis (Barrette et al., 1987). Protein fragmentation also disrupts DNA synthesis (Kiamco et al., 2019). The reaction of HClO with the amino groups of nucleotides generates reactive chloramines, which break the hydrogen bonds that hold the two DNA strands together (Fukuzaki, 2006). HClO reacts with unsaturated fatty acids to form chlorohydrins, and lipid chlorohydrins cause cell lysis and toxicity (Dever et al., 2006; Spickett et al., 2000) (Figure 1).
A study by Chen et al. (2016) showed that, at a concentration of 180 ppm, HClO eliminated Gram-negative bacteria (Escherichia coli and Porphyromonas gingivalis) and Gram-positive bacteria (E. faecalis and Streptococcus sanguinis) on titanium surfaces contaminated with biofilms of these microorganisms. The antibacterial efficacy of HClO increased as the treatment time increased. Owoseni and Okoh (2017) found that chlorine doses of 0.75 to 1.0 ppm, although very low, reduced the tolerance to HClO of Enterococcus species isolated from two wastewater treatment plants.
However, eliminating bacteria organized into biofilms is difficult due to the protection provided by polysaccharides (Torres-Armendariz et al., 2015). Williams and Braun-Howland (2003) found that the commonly recommended dose of HClO (1 ppm) is not sufficient to inactivate bacteria in biofilms, specifically Legionella pneumophila, E. coli, and β and δ proteobacteria.
Virus inactivation on surfaces
Regarding the effectiveness of chlorine in the inactivation of viruses, various studies have obtained controversial results. Some authors mention that coronaviruses can be inactivated with a 1000 ppm NaClO solution due to its interaction with the external lipid envelope of the virus (Campagna et al., 2016; Kampf et al., 2020). A study by Lin et al. (2020) concluded that, at a concentration of 100 ppm, NaClO can effectively disinfect a surface contaminated with the HIV-1 virus in 30 s. However, NaClO is very sensitive to the presence of organic matter (e.g. plasma and blood) on inert smooth surfaces, so significantly higher concentrations are required to maintain its disinfecting efficacy. Hulkower et al. (2011), using the mouse hepatitis virus (MHV) and the transmissible gastroenteritis virus (TGEV) as coronavirus models, determined that, after 1 min of contact with 1:100 hypochlorite (~ 600 ppm), there was a reduction of 0.62 and 0.35 log10 in viral load, respectively. However, a log10 viral reduction factor> 3 has previously been suggested as a benchmark for the effective virucidal activity against coronaviruses and other surface viruses (Sattar, 2004).
Virus inactivation in plant tissue and other samples
When the virus is inside a tissue or sample, it is not easy to remove it with chlorine; therefore, alternative techniques are required to inactivate it. Molina-Chavarria et al. (2020) reported that a 200 ppm NaClO dose was not efficient in reducing human norovirus (Human norovirus-HuNoV) in a stool sample. Kingsley et al. (2014) treated a stool filtrate containing 10% HuNoV with free chlorine at 189 ppm. This treatment reduced the viral load by 4 Log10, whereas a concentration of 350 ppm of ClO2 dissolved in water did not inactivate HuNoV after 1 min but reduced the viral load by 2.8 Log10 after 60 min. The authors concluded that chlorine dioxide has limited activity against HuNoV. Hirneisen and Kniel (2013) reported that chlorine treatment was one of the least effective in inactivating viruses such as murine norovirus (MNV), hepatitis A virus (HAV), and human adenovirus type 41 (Ad41) in onion tissue. In a study by Duizer et al. (2004), two types of calicivirus and one norovirus were inactivated with a dose of NaClO greater than 300 ppm. The high doses of chlorine that are effective against some viruses confirm that the concentrations used to disinfect fruit are insufficient to prevent viral transmission. Based on these results, it can be deduced that chlorine must be in direct contact with the virus, not only with the material, which may contain a large amount of organic matter.
Prospects for the inactivation of SARS-CoV-2
To reduce the persistence of SARS-CoV-2 on the surfaces of doors and window handles, kitchens, toilets, and faucets, touch screens, and work furniture and tools, various health organizations have recommended the use of NaClO at a concentration of 0.05% to 0.1% (500 to 1000 ppm) (WHO, 2020a). The high concentrations recommended can affect SARS-CoV-2, since this is a virus that, as the influenza and other coronaviruses, has a lipoprotein coating, which makes it much more vulnerable to chemical disinfectants than other viruses without that property. This is confirmed by the works of Maris (1989) and Lai et al. (2020), who required disinfectant solutions 20 to 500 times denser to kill viruses without a lipoprotein coating (parvovirus) than those required to eliminate coronaviruses (WHO, 2020b). It is necessary to consider that hypochlorite solutions should be prepared with water free of organic matter. This is important because, after washing fruits and vegetables, soil and plant material residues remain in the water, reducing the effectiveness of NaClO (Weng et al., 2016). Furthermore, the bad performance of hypochlorite may be due to its absorption by proteins and other organic compounds (e.g. amino acids), which limits its availability for disinfection (Hulkower et al., 2011). Moreover, halomethanes, which are potential carcinogens, are formed when chlorine comes into contact with organic matter in water (Kingsley et al., 2014). Due to the importance of coronaviruses in various fields, further studies are required to investigate the effect of hypochlorite against SARS-CoV-2, considering both the concentration and the time of contact, in order to determine if these factors can improve the virucidal activity of hypochlorite on inanimate surfaces after treatment.











 texto en
texto en 


