Introduction
Stress is a biological mechanism through which the body attempts to regain homeostasis when affected by stressors (Stratakis & Chrousos, 1995). Research on mental health and sexual therapy describes that the stresses of daily life can drastically affect the quality of sexual activity by decreasing sexual satisfaction (Bodenmann, Ledermann, Blattner, & Galluzo, 2006; Bodenmann, Ledermann, & Bradbury, 2007). Moreover, the psychological symptoms associated with chronic stress (e.g., depression, anxiety) are directly related to lower sexual desire, erectile problems, premature ejaculation, and hypoactive sexual disorders (Bodenmann et al., 2007).
Animal models have also shown the deleterious effects of stress on sexual behavior (Retana-Márquez, Bonilla-Jaime, Vázquez-Palacios, Martínez-García, & Velázquez-Moctezuma, 2003; Hernández-González, Guevara, Ramírez-Rentería, & Hernández-Arteaga, 2015; Hernández-González et al., 2017), which can vary with stressor type, duration, and the period when it occurs (Lapiz et al., 2003; Vetulani, 2013; Hernández-Arteaga et al., 2016; Hernández-González et al., 2017). Puberty is a critical period for sexual maturation as important hormonal changes occur to allow adequate manifestations of sexual behavior during adulthood (Duffy & Hendricks, 1973; Cooke, Chowanadisai, & Breedlove, 2000; Hernández-González, 2000; Arteaga-Silva et al., 2013). Gonadal hormones like testosterone (T) and its metabolites (Bonilla-Jaime, Vázquez-Palacios, Arteaga-Silva, & Retana-Márquez, 2006) are crucial to sexual behavior. However, some studies of adult male rats have found that after a period of stress glucocorticoid levels increase but T levels decrease, leading to the proposal that the hypothalamus-pituitary-adrenal (HPA) axis directly affects the hypothalamus-pituitary-gonad (HPG) axis to produce an antagonistic effect between the glucocorticoids and gonadal hormones (Retana-Márquez et al., 2003). Studies in men with stress-induced erectile dysfunction have similarly found lower T levels (Byun et al., 2013).
Pubertal stress in rats induced on postnatal days 25-50 by social isolation is known to increase corticosterone concentrations (Serra, Pisu, Floris, & Biggio, 2005; Perelló, Chacon, Cardinali, Esquifino, & Spinedi, 2006), but reduce T secretion during a sexually-motivated state in adulthood (Amistislavskaya, Bulygina, Tikhonova, & Maslova, 2013). Those rats also presented alterations in aromatase activity (Di Prisco, Lucarini, & Dessi-Fulgheri, 1978), which aromatizes T into estradiol (Moralí, Larsson, & Beyer, 1977; Muller, Van Den Beld, Van Der Schouw, Grobbe, & Lamberts, 2006) and activates sexual motivation. These hormones produce non-genomic effects in the neuronal membrane that modulates the electrical activity of neurons in several brain areas (del Río-Portilla, Ugalde, Juárez, Roldán, & Corsi-Cabrera, 1997; Joëls, 1997; Poblano et al., 2004; Balthazart, Baillien, Cornil, & Ball, 2004), including the medial prefrontal cortex (mPFC) and the basolateral amygdala (BLA), two cerebral structures that have androgen (Naghdi, Oryan, & Etemadi, 2003; Nuñez, Huppenbauer, McAbee, Juraska, & DonCarlos, 2003), estrogen (Montague et al., 2009; Lonc, 2012), and glucocorticoid (Pryce, 2008) receptors. The BLA has strong connections with the mPFC, especially layers II and V, in rodents (Cunningham, Bhattacharyya, & Benes, 2002). This connectivity participates in various complex processes, including emotional memory on inhibitory avoidance tasks (Cahill & McGaugh, 1991), aversive Pavlovian conditioning (Fanselow & LeDoux, 1999), and goal-oriented behaviors using motivational odors as reinforcers (Schoenbaum, Chiba, & Gallagher, 2000). Thus, they have been implicated in the processing of sexually-relevant stimuli and the regulation of sexual motivation (Fernández-Guasti, Omaña-Zapata, Luján, & Condés-Lara, 1994; Ågmo, Villalpando, Picker, & Fernández, 1995; Hernández-González, Guevara, & Ågmo, 2014a; Hernández-González, Robles Aguirre, Guevara, Quirarte, & Haro-Magallanes, 2014b). The processing of sexual stimuli and sexual motivation have been associated with electroencephalographic (EEG) activity characterized by a prevalence of slow frequencies in the 4-13 Hz (Hernández-González, Guevara, Cervantes, Moralí, & Corsi-Cabrera, 1998; Hernández-González, Prieto-Beracoechea, Arteaga-Silva, & Guevara, 2007; Hernández-González et al., 2014a) and 14-30 Hz bands (Hernández-González et al., 2017) in both the BLA and the mPFC.
Ågmo (1999) defines sexual motivation as the process that leads an individual to search for sexual contact with another. It is measured by approaching behaviors towards the potential sexual partner (Ventura-Aquino & Paredes, 2017). These behaviors during rats’ sexual interaction satisfy the criteria of correlation and homology with human sexual motivation (Ågmo, 2017).
While many studies have reported the deleterious effects that stress exerts on sexual behavior in animals and humans (Retana-Márquez et al., 2003; Byun et al., 2013; Hernández-González et al., 2015; 2017), few have examined hormonal and cerebral changes associated with sexual motivation in male rats that are stressed during puberty (Cooke et al., 2000; Amistislavskaya et al., 2013). Thus, the aim of this study was to determine the effects of stress experienced during puberty on sexual motivation, serum T levels and mPFC, and BLA functionality in adult male rats.
Method
Animals
At age 22 days, 60 male rats were weaned and housed in groups of five per cage. On day 25, they were randomly classified into two groups (n = 30/group) called stressed (SG) and control (CG). The SG rats were housed only one per cage and remained socially-isolated until day 51, when they were re-socialized with their former mates. CG, meanwhile, remained undisturbed on all days. Figure 1 presents a timeline of the general procedure.
Sexual interaction tests
At age three months (weight = 250-350 g), the rats were subjected to three copulatory tests (every second day) between 10:00 and 13:00 h. All 60 rats presented intromission on the three tests and reached ejaculation on at least two.
Stereotaxic surgery
The rats were injected subcutaneously with atropine sulfate (.1 mg/kg) and anesthetized with sodium pentobarbital (47 mg/kg i.p.). Stainless steel electrodes (.2 mm in diameter) were implanted bilaterally into the prelimbic area of the mPFC (3.2 mm anterior to bregma, 1 mm lateral to midline, and 4.0 mm below the duramater), and the basolateral amygdala (BLA) (2.8 mm posterior to bregma, 5 mm lateral to midline, and 8.4 mm below the duramater), with the incisor bar set at -3.3 mm, following the stereotaxic atlas of Paxinos and Watson (2007). Two stainless steel screws were placed in the posterior area of the skull as reference and ground electrodes, respectively. All electrodes were attached to a miniature connector fixed to the skull with stainless steel hooks and acrylic cement. After surgery, all rats were housed in individual cages with food and water ad libitum. The post-surgical recovery period was seven days.
Experimental design
Sexual incentive motivation box
The sexual incentive motivation box was a transparent plexiglas testing chamber (64 cm × 40 cm × 34 cm) divided in two equal compartments by a transparent acrylic partition with several 7-mm diameter holes. A male rat was placed in one compartment, and a stimulus female in the other such that they could see, hear, and smell each other, but no direct contact was possible. In the male’s compartment, a line was drawn dividing it in two sections. The section closest to the female’s compartment was considered the incentive zone.
Recording conditions
On the day of the experiment, each male was placed in the sexual incentive motivation box and connected to the polygraph to record EEG activity under two behavioral conditions: without (WSM) and with sexual motivation (SM). In the WSM condition, they were stimulated for five minutes with an ovariectomized (non-receptive) female in the other compartment, while in the SM condition they were allowed one intromission with a receptive female to induce a sexually-motivated state. Immediately afterwards, the female was transferred to the other compartment for five minutes to stimulate the male. The time in seconds that the males in both groups spent in the incentive zone was recorded.
Testosterone sample
Half of the males from CG and SG were decapitated immediately after EEG-recording in each condition (WSM and SM). Blood samples measuring 8.5 ml per subject were collected in test tubes (BD Vacutainer, ref 367988) containing gel to separate the blood serum. The samples were centrifuged at 3000 rpm for 15 minutes and then the supernatant was recovered and stored in Eppendorf tubes at -4°C for further analysis.
EEG recording
The bilateral mPFC and BLA electrodes were connected to a Model 7B GRASS polygraph with a recording window of 1-75 Hz to record EEGs during the awake-quiet state in the WSM and SM conditions. The polygraph was attached to an analogue-digital converter (CAD, Advantech Co., Mod. PCL-812). The sample rate was 1024 Hz. All EEGs were stored on a PC for offline analysis.
EEG analysis
Only the EEG recordings that were free of noise or movement artifacts were included in data analysis. The Absolute Power (AP, defined as the power density of each frequency expressed in microvolts2) of four EEG bands (4-7, 8-13, 14-30, 31-50 Hz) was calculated using Fast Fourier Transformation (FFT). To approximate a normal distribution, the AP values were transformed into natural logarithms.
Testosterone analysis
Serum T concentrations were obtained using the ELISA technique with the commercial preparation called the “Testosterone enzyme immunoassay” kit (catalog # 611CH; estimated sensitivity = .05 ng/mL). With all reagents and samples at room temperature, 10 μL of the standards, the target and one sample were pipetted into each well of the kit and 50 μL of rabbit anti-T reagent were added. This mixture was then homogenized for 30 seconds. Next, 100 μL of HRP T conjugate reagent were added and the mixture was incubated for 90 minutes at 37°C. The microplate was washed and rinsed five times with wash solution; then 100 μL of TMB reagent were added to each well and mixed for 10 seconds. This mixture was incubated at room temperature for 20 minutes, before adding 100 μL of stop solution. This was mixed for 30 seconds and read at an optical density of 450 nm. Finally, the data obtained from the standards were used to build a calibration curve that was applied to calculate the T concentration of each sample (ng/ml).
Histology
The rats’ brains were fixed by an intracardial infusion of isotonic saline (.9%) followed by a 5.0% buffered paraformaldehyde solution. They were then removed and stored in formyl for at least two weeks. Sections 50 µ-thick were cut with a microtome and stained with cresyl violet. Inspection under a stereoscopic microscope to trace the stereotaxic coordinates allowed us to reconstruct the path followed by the recording electrode.
Statistical analyses
The serum T concentrations from the four sub-groups (SG-WSM, SG-SM, CG-WSM, and CG-SM) were compared by a two-way ANOVA. The time spent in the incentive zone was compared between CG-SM and SG-SM using a Student T test, followed by a Cohen d test to calculate effect size. For the EEG data, a two-way ANOVA (groups × condition) was used to compare the AP of each frequency band recorded in the control and stressed sub-groups (n = 15/sub-group) under the WSM and SM conditions. A Tukey HDS test was performed for a posteriori comparisons, followed by a Cohen d test to calculate effect size. We calculated the effect size, η2, for the effect of experimental condition and interaction. If only one or two factors were significant in the ANOVA, an exploratory analysis was performed using a Student-t test with Bonferroni correction in all comparisons, followed by a Cohen d test to calculate effect size.
Ethical considerations
Adequate care was taken to minimize the animals’ pain and discomfort throughout the experiment. All procedures were carried out following a protocol approved by the local Animal Ethics Committee (ET062017-250) in compliance with national (NOM-062-ZOO-1999) and international (NIH) regulations on the care and use of laboratory animals.
Results
Testosterone levels
The ANOVA showed only a significant difference for the factor groups, with a medium effect size [F(A) = 4.07, p(FA) = .0485; η2 = .065]. SG had lower serum T levels than CG (between-groups comparison with both conditions as a factor in the ANOVA; Figure 2). No differences were found in any of the comparisons in the exploratory analysis (mean ± 2 S.E. of serum T levels (ng/mL) measured in CG-WSM: 4.12 ± 1.12; CG-SM: 4.44 ± .87; SG-WSM: 2.74 ± .87; SG-SM: 3.67 ± .86).
Time spent in the incentive zone
The SG-SM subjects remained in the incentive zone (i.e., in the presence of a receptive female) for less time (99.067 ± 17.825 seconds) than the CG-SM rats (155.867 ± 18.616 seconds). This produced a medium effect size of (t[28] = 2.20, p[t] = .0359; d = .756).
Histological analysis
The electrode tips in the mPFC were placed between 3.7-3.2 mm anterior to bregma, .5-1.5 mm lateral to midline, and 3.8-4.2 below the dura mater (in the prelimbic region of the PFC), while in the BLA they were placed between 2.6-2.7 posterior to bregma, 4.2-5.0 mm lateral to midline, and 8.0-9.0 mm below the dura mater (Figure 3).
Figure 3 Schematic representation of electrode tip placement in the mPFC (A-B) and BLA (C-D). Anterior-posterior coordinates are given with respect to bregma following the stereotaxic atlas of Paxinos & Watson (2007). Dots represent the electrode tips implanted in each rat of sub-groups CG-SM (n = 15) and SG-SM (n = 15).
Absolute power
Left medial prefrontal cortex (mPFC)
The ANOVA only showed significant differences for the factor condition for all frequency bands [4-7 Hz F(B) = 6.68, p(FB) = .01455; η2 = .040 (small effect size); 8-13 Hz:F(B) = 15.75, p(FB) = .00072; η2 = .076 (medium effect size); 14-30 Hz: F(B) = 17.79, p(FB) = .00044; η2 = .076 (medium effect size); 31-50 Hz: F(B) = 18.29, p(FB) = .00039; η2 = .040 (small effect size)]. In all bands, the rats (between-conditions comparison with both groups as a factor in the ANOVA) presented higher AP under the SM condition than WSM (Table 1).
In the exploratory analysis (Student’s t-test comparing both conditions in each group separately), CG showed a higher AP of the 8-13 Hz [t(28) = 2.887, p(t) = .01194; d = .634 (medium effect size)]; 14-30 Hz [t(28) = 2.956, p(t) = .01042; d = .572 (medium effect size)]; and 31-50 Hz [t(28) = 4.249, p(t) = .00081; d = .398 (small effect size)] during SM compared to WSM (Figure 4A). SG only showed a higher AP of the 8-13 Hz [t(28) = 2.965, p(t) = .01024; d = .448 (small effect size)], and 14-30 Hz bands [t(28) = 3.105, p(t) = .0076; d = .529 (medium effect size)] (Figure 4B).
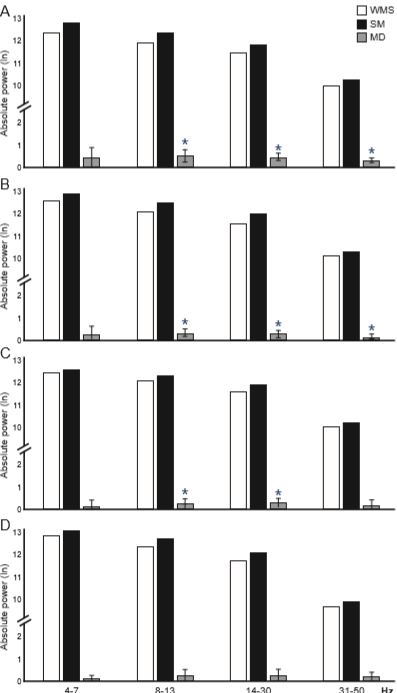
Figure 4 Mean of the absolute power (AP, transformed into logarithms) of the EEG bands recorded in the left mPFC of CG (A) and SG (B) and right mPFC of CG (C) and SG (D) during the two conditions: without (WSM, white bars) and with sexual motivation (SM, dark bars). Gray bars represent the mean difference ± 2 S.E. (n = 15/ group). *p(T) ≤ .0125 SM compared to WSM.
Right medial prefrontal cortex (mPFC)
The ANOVA showed only significant differences for the factor condition for all frequency bands [4-7 Hz F(B) = 8.64, p(FB) = .00656; η2 = .030 (small effect size)]; [8-13 Hz F(B) = 19.84, p(FB) = .00028; η2 = .062 (medium effect size); 14-30 Hz F(B) = 17.92, p(FB) = .00042; η2 = .053 (small effect size); 31-50 Hz F(B) = 9.28, p(FB) = .00516; η2 = .025 (small effect size)]. In all cases, the rats (between-conditions comparison with both groups as a factor in the ANOVA) presented higher AP during SM than WSM (Table 1).
Table 1 Means of the absolute power (transformed into logarithms) of the EEG bands recorded in the left and right medial prefrontal cortex (mPFC) and basolateral amygdale (BLA) during the conditions without (WSM) and with sexual motivation (SM)
| 4-7 Hz | 8-13 Hz | 14-30 Hz | 31-50 Hz | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WSM | SM | MD | WSM | SM | MD | WSM | SM | MD | WSM | SM | MD | ||||
| Left mPFC | 12.4 | 12.7 | .3 ± .2* | 12.1 | 12.4 | .4 ± .2* | 11.5 | 12.0 | .4 ± .2* | 10.0 | 10.3 | 1.1 ± .3* | |||
| Right mPFC | 12.6 | 12.9 | .3 ± .2* | 12.2 | 12.5 | .3 ± .1* | 11.7 | 12.0 | .3 ± .1* | 10.2 | 10.4 | .2 ± .1* | |||
| Left BLA | 12.7 | 13.0 | .3 ± .2* | 12.5 | 12.8 | .2 ± .1* | 12.5 | 12.4 | .2 ± .1* | 10.2 | 10.4 | .2 ± .1* | |||
| Right BLA | 12.7 | 12.8 | .1 ± .1 | 12.5 | 12.6 | .2 ± .1* | 12.2 | 12.3 | .2 ± .1* | 10.6 | 10.5 | .1 ± .2 | |||
Note: The mean difference between conditions (MD) ± 2 S.E. is also indicated. It represents the factor ‘conditions’, so comparisons were performed with n = 30 for both groups and in each condition.
*p(FB) ≤ .05, SM as compared to WSM.
In the exploratory analysis (Student’s t-test comparing both conditions in each group separately), CG showed a higher AP of frequency bands 8-13 Hz [t(28) = 4.043, p(t) = .00121; d = .498 (small effect size)]; 14-30 Hz [t(28) = 3.793, p(t) = .00198; d = .411 (small effect size)]; and 31-50 Hz [t(28) = 2.958, p(t) = .01038; d = .294 (small effect size)] during SM compared to WSM (Figure 4C). SG showed no significant differences between conditions (Figure 4D).
Left basolateral amygdala
The ANOVA only showed significant differences for the factor condition for all frequency bands. All analyses reached only a small effect size: 4-7 Hz [F(B) = 7.84, p(FB) = .00897; η2 = .013. 8-13 Hz F(B) = 14.07, p(FB) = .00112; η2 = .013]; 14-30 [Hz F(B) = 11.56, p(FB) = .00236; η2 = .010]; and 31-50 Hz [F(B) = 9.88, p(FB) = .00415; η2 = .010]. In all cases, the rats (between-conditions comparison with both groups as a factor in the ANOVA) presented a higher AP during SM than WSM (Table 1).
In the exploratory analysis (Student’s t-test comparing both conditions in each group separately), CG showed no significant differences in the AP of the EEG bands between conditions (Figure 5A). SG had higher AP during SM than WSM in the 8-13 [t(28) = 2.873, p(t) = .001228; d = .303] and 14-30 Hz [t(28) = 3.038, p(t) = .00887; d = .322] bands, both with a small effect size (Figure 5B).
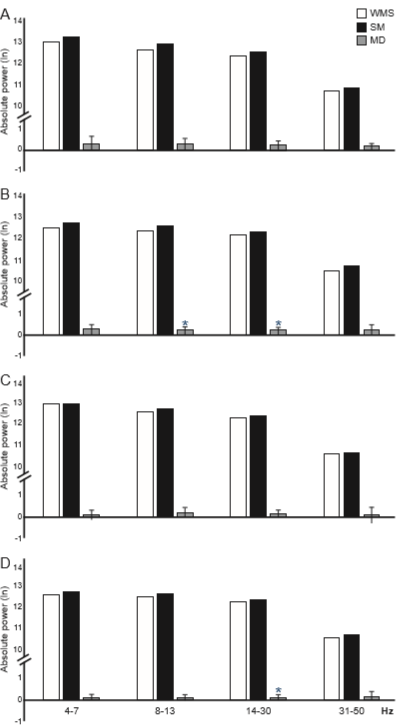
Figure 5 Mean of the absolute power (AP, transformed into logarithms) of the EEG bands recorded in the left BLA of CG (A) and SG (B) and the right BLA of CG (C) and SG (D) during the two conditions: without (WSM, white bars), and with sexual motivation (SM, dark bars). Gray bars represent the mean difference ± 2 S.E.(n = 15/ group). *p(T) ≤ .0125 SM compared to WSM.
Right basolateral amygdala
The ANOVA showed only significant differences for the factor condition for the 8-13 [F(B) = 5.62, p(FB) = .02352; η2 = .010] and 14-30 Hz [F(B) = 4.17, p(FB) = .04813; η2 = .010] bands, both with a small effect size. In both cases, the rats (between-conditions comparison with both groups as a factor in the ANOVA) had higher AP during SM than WSM (Table 1).
In the exploratory analysis (Student’s t-test comparing both conditions in each group separately), CG showed no significant differences between conditions (Figure 5C), while SG had higher AP during SM than WSM in the 14-30 Hz band, with a small effect size [t(28) = 3.001, p(t) = .00508; d = .283] (Figure 5D).
Discussion and conclusion
By taking advantage of the benefits of the rat as a model of sexual behavior, and using an ad hoc experimental stress paradigm, this study found that the effects of pubertal stress prevail into adulthood by altering brain functionality, serum T levels, and sexual motivation in male rats. The experiment evaluated the time spent in the sexual incentive zone to measure sexual motivation, finding that the stressed rats spent significantly less time there, which demonstrates that sexual motivation was affected by the stress induced during puberty, as other studies have reported (Gerall, Ward, & Gerall, 1967; Duffy & Hendricks, 1973; Cooke et al., 2000; Hernández-González et al., 2015). Also, the rats presented lower serum T levels, which confirm the deleterious effects of stress during puberty on the activation of the HPG axis in male rats (Amistislavskaya et al., 2013).
Other studies have shown that sexual motivation in male rats is related to increased serum T levels (Bonilla-Jaime et al., 2006). During puberty, this hormone plays an organizational role that facilitates the morphological and behavioral changes associated with sexual maturation (Hernández-González, 2000; Schulz et al., 2004; Arteaga-Silva et al., 2013; Hernández-González et al., 2015). As mentioned previously, corticosterone exerts a negative effect on T levels (Retana-Márquez et al., 2003), so it is likely that the stress paradigm used herein increased corticosterone levels (Serra et al., 2005; Perelló et al., 2006; Amistislavskaya et al., 2013) during puberty and so exerted a long-term negative effect on T levels that led the stressed rats to manifest lower levels than controls in adulthood.
We decided to evaluate prefrontal and amygdaline functionality because these two areas are interconnected in rodents (Cunningham et al., 2002) and participate in various complex processes, including goal-oriented behaviors using motivational odors as reinforcers (Schoenbaum et al., 2000). This means that they are implicated, as well, in the processing of sexually-relevant stimuli (Fernández-Guasti et al., 1994; Ågmo et al., 1995; Hernández-González et al., 2014a; 2014b). In addition, both regions contain androgen receptors (Naghdi et al., 2003; Nuñez et al., 2003). In this context, the study evidenced that the CG and SG rats had distinct activations of these structures during the perception and processing of the stimuli emitted by the receptive and non-receptive females. In male rats, the 4-7 and 8-13 Hz EEG bands are associated with approach behaviors to incentive stimuli, attention (Vanderwolf, 1969; Bland & Whishaw, 1976; Hernández-González et al., 1998), and motivated states (Hernández-González et al., 2014a), while the fast frequencies (14-50 Hz) – in both animal and human models – have been related to the higher processing that underlies stimuli selection (Başar, Başar-Eroğlu, Karakaş, & Schürmann, 2000; Engel & Singer, 2001).
Only CG showed a higher AP in almost all bands in the left and right mPFC when a receptive female was present, compared to a non-receptive one. The mPFC is involved in attention, the processing of sensory stimuli emitted by a potential partner, and the assigning of incentive value, three processes that are necessary for the onset of sexual behavior (Ågmo et al., 1995; Hernández-González et al., 2014a; Hernández-González et al., 2017). Thus, it is probable that the higher activation of the mPFC in CG is associated with a greater degree of attention given to the sexually-receptive female and, hence, greater sexual motivation, as evidenced by the longer time they spent near the receptive female.
SG only showed EEG changes in the left mPFC in the presence of the receptive female, but with lower sexual motivation. Thus, it is likely that their lower T levels altered the mPFC activity, leading to inadequate processing of the sexually-relevant stimuli. Moreover, the stressed males had a higher AP of the EEG bands in the left and right BLA. Pubertally-stressed rats exhibit higher amygdaline activity (Wang, Ho, Ko, Liao, & Lee, 2012) associated with anxiety-like behaviors (Zhang & Rosenkranz, 2012), together with higher concentrations of dopaminergic receptors (D2) in the BLA (Djouma, Card, Lodge, & Lawrence, 2006). It is well-known that D2 activation inhibits sexual behavior (Balthazart, Castagna, & Ball, 1997). Higher amygdaline activity has also been observed in patients with social anxiety disorder in response to socially-aversive stimuli (Kraus et al., 2018), so it may be that as a result of greater amygdaline activation our pubertally-stressed male rats had difficulty in processing and assigning incentive value to the stimuli emitted by the female.
Lower sexual motivation in non-human animals can be operationalized as a feature of hypoactive sexual desire disorder in humans. Patients with this disorder report that sexual partners can seem aversive (for a discussion, see Ågmo, Turi, Ellingsen, & Karpersen, 2004). Leussis and Andersen (2008) demonstrated that rats stressed by social isolation during puberty show depression-like behaviors, so it may be that the generation of depression-like effects in these rats means they process the female’s stimuli as socially-aversive instead of sexually-relevant. However this affirmation will require further analyses with rats.
It is likely that pubertal stress decreased T levels and exerted long-term effects on the functionality of prefrontal and amygdaline areas. Hence, the stressed males failed to perceive the receptive female as a pleasant, rewarding stimuli. However, this hypothesis also needs to be explored in future clinical and animal research.











 nueva página del texto (beta)
nueva página del texto (beta)




